Antimicrobial Prescribing Patterns in Patients with COVID-19 in Russian Multi-Field Hospitals in 2021: Results of the Global-PPS Project
Abstract
:1. Introduction
2. Materials and Methods
- Compliance with local hospital guidelines,
- Documentation of indications for prescription of antimicrobial therapy,
- Documentation of stop/review dates,
- Targeted treatment based upon microbiological results,
- Treatment based upon the use of biomarker data (C-reactive protein, procalcitonin, or others).
3. Results
3.1. Characteristics of the Hospitals and Study Population
3.2. AMD Prescribing Patterns in COVID-19 Wards
3.3. Key Patterns and Quality Indicators of Systemic AMD Prescribing for “COVID-19/Pneumonia”
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 31 March 2022).
- Mahoney, A.R.; Safaee, M.M.; Wuest, W.M.; Furst, A.L. The silent pandemic: Emergent antibiotic resistances following the global response to SARS-CoV-2. iScience 2021, 24, 102304. [Google Scholar] [CrossRef] [PubMed]
- Living Guidance for Clinical Management of COVID-19: Living Guidance, 23 November 2021–World Health Organization (WHO). Available online: https://apps.who.int/iris/rest/bitstreams/1394399/retrieve (accessed on 31 March 2022).
- Ministry of Healthcare of Russian Federation. Temporary Guidelines on COVID-19 Prophylaxis, Diagnostics and Management. Version 15 (22 February 2022). Available online: https://static-0.minzdrav.gov.ru/system/attachments/attaches/000/059/392/original/%D0%92%D0%9C%D0%A0_COVID-19_V15.pdf (accessed on 31 March 2022).
- Chong, W.H.; Saha, B.K.; Ananthakrishnan, R.; Chopra, A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection 2021, 49, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Seaton, R.A.; Gibbons, C.L.; Cooper, L.; Malcolm, W.; McKinney, R.; Dundas, S.; Griffith, D.; Jeffreys, D.; Hamilton, K.; Choo-Kang, B.; et al. Survey of antibiotic and antifungal prescribing in patients with suspected and confirmed COVID-19 in Scottish hospitals. J. Infect. 2020, 81, 952–960. [Google Scholar] [CrossRef]
- Xu, X.W.; Wu, X.X.; Jiang, X.G.; Xu, K.J.; Ying, L.J.; Ma, C.L.; Li, S.B.; Wang, H.Y.; Zhang, S.; Gao, H.N.; et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-CoV-2) outside of Wuhan, China: Retrospective case series. BMJ 2020, 368, m606. [Google Scholar] [CrossRef] [Green Version]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and Fungal Coinfection in Individuals With Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin Infect Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef]
- Pshenichnaya, N.Y.; Kareva, E.N.; Leneva, I.A.; Bulgakova, V.A.; Kravchenko, I.E.; Nikolaeva, I.V.; Grekova, A.I.; Ivanova, A.P.; Puzyreva, L.V.; Khasanova, G.M.; et al. Pharmacoepidemiological research of COVID-19 in the Russian Federation EGIDA-2020. Ter. Arkh. 2021, 93, 1306–1315. [Google Scholar] [CrossRef]
- Pauwels, I.; Versporten, A.; Vermeulen, H.; Vlieghe, E.; Goossens, H. Assessing the impact of the Global Point Prevalence Survey of Antimicrobial Consumption and Resistance (Global-PPS) on hospital antimicrobial stewardship programmes: Results of a worldwide survey. Antimicrob. Resist. Infect. Control 2021, 10, 138. [Google Scholar] [CrossRef]
- Global Point Prevalence Survey of Antimicrobial Consumption and Resistance (2021 GLOBAL-PPS). Available online: https://www.global-pps.com/ (accessed on 24 February 2022).
- WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC Classification and DDD Assignment 2022; WHO Collaborating Centre for Drug Statistics Methodology: Oslo, Norway, 2021. [Google Scholar]
- WHO AWaRe Classification Database of Antibiotics for Evaluation and Monitoring of Use. 2019. Available online: https://www.who.int/publications/i/item/WHOEMPIAU2019.11 (accessed on 1 April 2022).
- Versporten, A.; Zarb, P.; Caniaux, I.; Gros, M.F.; Drapier, N.; Miller, M.; Jarlier, V.; Nathwani, D.; Goossens, H. Global-PPS network. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. Lancet Glob. Health 2018, 6, e619–e629. [Google Scholar] [CrossRef] [Green Version]
- Zarb, P.; Goossens, H. European Surveillance of Antimicrobial Consumption (ESAC): Value of a point-prevalence survey of antimicrobial use across Europe. Drugs. 2011, 71, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Bengoechea, J.A.; Bamford, C.G. SARS-CoV-2, bacterial co-infections, and AMR: The deadly trio in COVID-19? EMBO Mol. Med. 2020, 12, e12560. [Google Scholar] [CrossRef] [PubMed]
- Nieuwlaat, R.; Mbuagbaw, L.; Mertz, D.; Burrows, L.L.; Bowdish, D.M.E.; Moja, L.; Wright, G.D.; Schünemann, H.J. Coronavirus Disease 2019 and Antimicrobial Resistance: Parallel and Interacting Health Emergencies. Clin. Infect. Dis. 2021, 72, 1657–1659. [Google Scholar] [CrossRef] [PubMed]
- Van Duin, D.; Barlow, G.; Nathwani, D. The impact of the COVID-19 pandemic on antimicrobial resistance: A debate. JAC Antimicrob. Resist. 2020, 2, dlaa053. [Google Scholar] [CrossRef] [PubMed]
- Romashov, O.M.; Ni, O.G.; Bykov, A.O.; Kruglov, A.N.; Protsenko, D.N.; Tyurin, I.N. Antimicrobial resistance and antimicrobial therapy modification during COVID-19 pandemic in large tertiary hospital. Clin. Microbiol. Antimicrob. Chemother. 2021, 23, 293–303. [Google Scholar] [CrossRef]
- Lavrinenko, A.V.; Kolesnichenko, S.I.; Akhmaltdinova, L.L.; Klodzinskii, A.A.; Turgunova, L.G. Increase of antibacterial resistance levels in a hematological ward of a central Kazakhstan during the COVID-19 pandemic. Clin. Microbiol. Antimicrob. Chemother. 2021, 23, 24. [Google Scholar]
- Sandhu, A.; Tillotson, G.; Polistico, J.; Salimnia, H.; Cranis, M.; Moshos, J.; Cullen, L.; Jabbo, L.; Diebel, L.; Chopra, T. Clostridioides difficile in COVID-19 Patients, Detroit, Michigan, USA, March-April 2020. Emerg. Infect. Dis. 2020, 26, 2272–2274. [Google Scholar] [CrossRef]
- Lewandowski, K.; Rosołowski, M.; Kaniewska, M.; Kucha, P.; Meler, A.; Wierzba, W.; Rydzewska, G. Clostridioides difficile infection in coronavirus disease 2019 (COVID-19): An underestimated problem? Pol. Arch. Intern. Med. 2021, 131, 121–127. [Google Scholar] [CrossRef]
- Granata, G.; Bartoloni, A.; Codeluppi, M.; Contadini, I.; Cristini, F.; Fantoni, M.; Ferraresi, A.; Fornabaio, C.; Grasselli, S.; Lagi, F.; et al. The Burden of Clostridioides Difficile Infection during the COVID-19 Pandemic: A Retrospective Case-Control Study in Italian Hospitals (CloVid). J. Clin. Med. 2020, 9, 3855. [Google Scholar] [CrossRef]
- Rozanova, S.M.; Perevalova, E.I.; Kyrf, M.V.; Sheveleva, L.V.; Lazareva, O.N. Clostridium difficile toxin-producing strains prevalence during the COVID-19 pandemic. Clin. Microbiol. Antimicrob. Chemother. 2021, 23, 35. [Google Scholar]
- Levitan, A.I.; Titarenko, D.O.; Zubriichuk, A.S.; Ardentova, N.N.; Reshetko, O.V. Analysis of antiviral therapy in patients with COVID-19 infection. Clin. Microbiol. Antimicrob. Chemother. 2021, 23, 25. [Google Scholar]
- Mason, C.Y.; Kanitkar, T.; Richardson, C.J.; Lanzman, M.; Stone, Z.; Mahungu, T.; Mack, D.; Wey, E.Q.; Lamb, L.; Balakrishnan, I.; et al. Exclusion of bacterial co-infection in COVID-19 using baseline inflammatory markers and their response to antibiotics. J. Antimicrob. Chemother. 2021, 76, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Guo, S.; He, Y.; Zuo, Q.; Liu, D.; Xiao, M.; Fan, J.; Li, X. COVID-19 Is Distinct From SARS-CoV-2-Negative Community-Acquired Pneumonia. Front. Cell. Infect. Microbiol. 2020, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Rachina, S.; Belkova, Y.; Kozlov, R.; Versporten, A.; Pauwels, I.; Goossens, H.; Bochanova, E.; Domanskaya, O.; Elokhina, E.; Ezhova, L.; et al. Longitudinal Point Prevalence Survey of Antimicrobial Consumption in Russian Hospitals: Results of the Global-PPS Project. Antibiotics 2020, 9, 446. [Google Scholar] [CrossRef]
- Shek, E.A.; Sukhorukova, M.V.; Edelstein, M.V.; Skleenova, E.Y.; Ivanchik, N.V.; Shajdullina, E.R.; Mikotina, A.V.; Kuzmenkov, A.Y.; Dekhnich, A.V.; Kozlov, R.S.; et al. Antimicrobial resistance, carbapenemase production, and genotypes of nosocomial Acinetobacter spp. isolates in Russia: Results of multicenter epidemiological study ”MARATHON 2015–2016”. Clin. Microbiol. Antimicrob. Chemother. 2019, 21, 171–180. [Google Scholar] [CrossRef]
- Sukhorukova, M.V.; Edelstein, M.V.; Skleenova, E.Y.; Ivanchik, N.V.; Shajdullina, E.R.; Mikotina, A.V.; Azyzov, I.S.; Shek, E.A.; Kuzmenkov, A.Y.; Dekhnich, A.V.; et al. Antimicrobial resistance of nosocomial Enterobacterales isolates in Russia: Results of multicenter epidemiological study “MARATHON 2015–2016”. Clin. Microbiol. Antimicrob. Chemother. 2019, 21, 147–159. [Google Scholar] [CrossRef]
- Edelstein, M.V.; Shek, E.A.; Sukhorukova, M.V.; Skleenova, E.Y.; Ivanchik, N.V.; Shajdullina, E.R.; Mikotina, A.V.; Kuzmenkov, A.Y.; Dekhnich, A.V.; Kozlov, R.S.; et al. Antimicrobial resistance, carbapenemase production, and genotypes of nosocomial Pseudomonas aeruginosa isolates in Russia: Results of multicenter epidemiological study “MARATHON 2015–2016”. Clin. Microbiol. Antimicrob. Chemother. 2019, 21, 160–170. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Site #1 | Site #2 | Site #3 | Site #4 | Site #5 | Site #6 | Total/Average |
|---|---|---|---|---|---|---|---|
| COVID-19 wards surveyed, n | 1 | 3 | 2 | 4 | 8 | 1 | 19 |
| medical wards | 0 | 1 | 0 | 2 | 5 | 1 | 9 |
| ICU | 0 | 0 | 0 | 2 | 3 | 0 | 5 |
| mixed wards (medical and intensive care beds) | 1 | 2 | 2 | 0 | 0 | 0 | 5 |
| Patients at the COVID-19 wards on the day of PPS, n | 110 | 67 | 313 | 133 | 306 | 70 | 999 |
| Patients receiving antimicrobials on the day of PPS, n | 70 | 35 | 106 | 116 | 161 | 24 | 512 |
| Sex, % of male | 35.7 | 68.6 | 34 | 39.7 | 36 | 45.8 | 39.1 |
| Average age, years | 61.6 ± 16.1 | 49 ± 16.5 | 53.2 ± 16.8 | 69 ± 15.6 | 70.8 ± 13.6 | 60.5 ± 12.9 | 61.7 ± 17 |
| Previous hospitalization, % | |||||||
| yes, ICU | 0 | 2.9 | 5.7 | 0 | 1.2 | 0 | 1.8 |
| yes, other | 0 | 31.4 | 23.6 | 0 | 13.7 | 8.3 | 11.7 |
| no | 0 | 65.7 | 4.7 | 81 | 83.2 | 83.3 | 53.9 |
| unknown | 100 | 0 | 66 | 19 | 1.9 | 8.3 | 32.6 |
| Previous antibiotic treatment, % | |||||||
| yes | 14.3 | 20 | 39.6 | 6 | 30.4 | 16.7 | 23.2 |
| no | 10 | 57.1 | 58.5 | 89.7 | 60.9 | 79.2 | 60.5 |
| unknown | 75.7 | 22.9 | 1.9 | 4.3 | 8.7 | 4.2 | 16.2 |
| Surgery during current admission in hospital, % | |||||||
| yes | 0 | 51.4 | 8.5 | 0.9 | 1.2 | 4.2 | 6.1 |
| no | 100 | 48.6 | 91.5 | 97.4 | 98.8 | 91.7 | 93.4 |
| unknown | 0 | 0 | 0 | 1.7 | 0 | 4.2 | 0.6 |
| Invasive device present on the day of PPS *, % | |||||||
| indwelling urinary catheter | 20 | 28.6 | 11.3 | 14.7 | 18 | 16.7 | 16.8 |
| peripheral vascular catheter | 54.3 | 31.4 | 12.3 | 65.5 | 60.2 | 100 | 50.6 |
| central vascular catheter | 0 | 25.7 | 19.8 | 19.8 | 13 | 4.2 | 14.6 |
| non-invasive ventilation (CPAP, BiPAP, etc.) ** | 11.4 | 2.9 | 14.2 | 7.8 | 14.9 | 4.2 | 11.3 |
| invasive mechanical ventilation | 4.3 | 2.9 | 4.7 | 4.3 | 4.3 | 0 | 4.1 |
| inserted tubes and drains | 0 | 11.4 | 2.8 | 0.9 | 0 | 0 | 1.6 |
| Characteristics | Site #1 | Site #2 | Site #3 | Site #4 | Site #5 | Site #6 | Total/Average |
|---|---|---|---|---|---|---|---|
| Patients in the COVID-19 wards on the day of PPS, n | 110 | 67 | 313 | 133 | 306 | 70 | 999 |
| medical beds | 100 | 67 | 284 | 108 | 251 | 70 | 880 |
| intensive care beds | 10 | 0 | 29 | 25 | 55 | - | 119 |
| Antimicrobial prevalence, % | 63.6 | 52.2 | 33.9 | 87.2 | 52.6 | 34.3 | 51.3 |
| medical beds | 60 | 52.2 | 28.2 | 84.3 | 51 | 34.3 | 47.5 |
| intensive care beds | 100 | - | 89.7 | 100 | 60 | - | 79 |
| Antiviral prevalence, % | 42.7 | 44.8 | 17.9 | 56.4 | 31.7 | 7.1 | 31 |
| medical beds | 44 | 44.8 | 17.3 | 58.3 | 35.5 | 7.1 | 31.8 |
| intensive care beds | 30 | - | 24.1 | 48 | 14.5 | - | 25.2 |
| Antibiotic prevalence, % | 36.4 | 32.8 | 25.6 | 69.2 | 31.4 | 30 | 35.1 |
| medical beds | 30 | 32.8 | 19 | 62 | 26.7 | 30 | 29.7 |
| intensive care beds | 100 | - | 89.7 | 100 | 52.7 | - | 75.6 |
| Combination of antivirals and antibiotics, % | 15.5 | 25.4 | 9.9 | 38.3 | 7.8 | 1.4 | 14.1 |
| medical beds | 14 | 25.4 | 8.1 | 36.1 | 7.6 | 1.4 | 12.8 |
| intensive care beds | 30 | - | 27.6 | 48 | 9.1 | - | 23.5 |
| Indication | Site #1 | Site #2 | Site #3 | Site #4 | Site #5 | Site #6 | Average |
|---|---|---|---|---|---|---|---|
| Pneumonia or lower respiratory tract infection | 92.9 | 69.6 | 85.7 | 41.5 | 33 | 100 | 59.3 |
| COVID-19 infection | 0 | 0 | 0 | 41.5 | 47.6 | 0 | 25.1 |
| C. difficile-associated infection | 0 | 4.3 | 3.8 | 5.7 | 8.7 | 0 | 5 |
| Upper urinary tract infection | 2.4 | 0 | 2.9 | 0 | 8.7 | 0 | 2.8 |
| Skin and soft tissue infection | 0 | 13 | 1.9 | 0 | 0 | 0 | 1.1 |
| Sepsis/bacteremia with no clear anatomic site | 4.8 | 0 | 1 | 8.8 | 0 | 0 | 3.7 |
| Bronchitis | 0 | 0 | 0 | 1.9 | 0 | 0 | 0.7 |
| Intra-abdominal infection | 0 | 0 | 1.9 | 0 | 0 | 0 | 0.4 |
| Obstetric/gynecological infection | 0 | 0 | 1.9 | 0 | 0 | 0 | 0.4 |
| Lower urinary tract infection | 0 | 0 | 1 | 0 | 1 | 0 | 0.4 |
| Other | 0 | 13 | 0 | 0.6 | 1 | 0 | 1.1 |
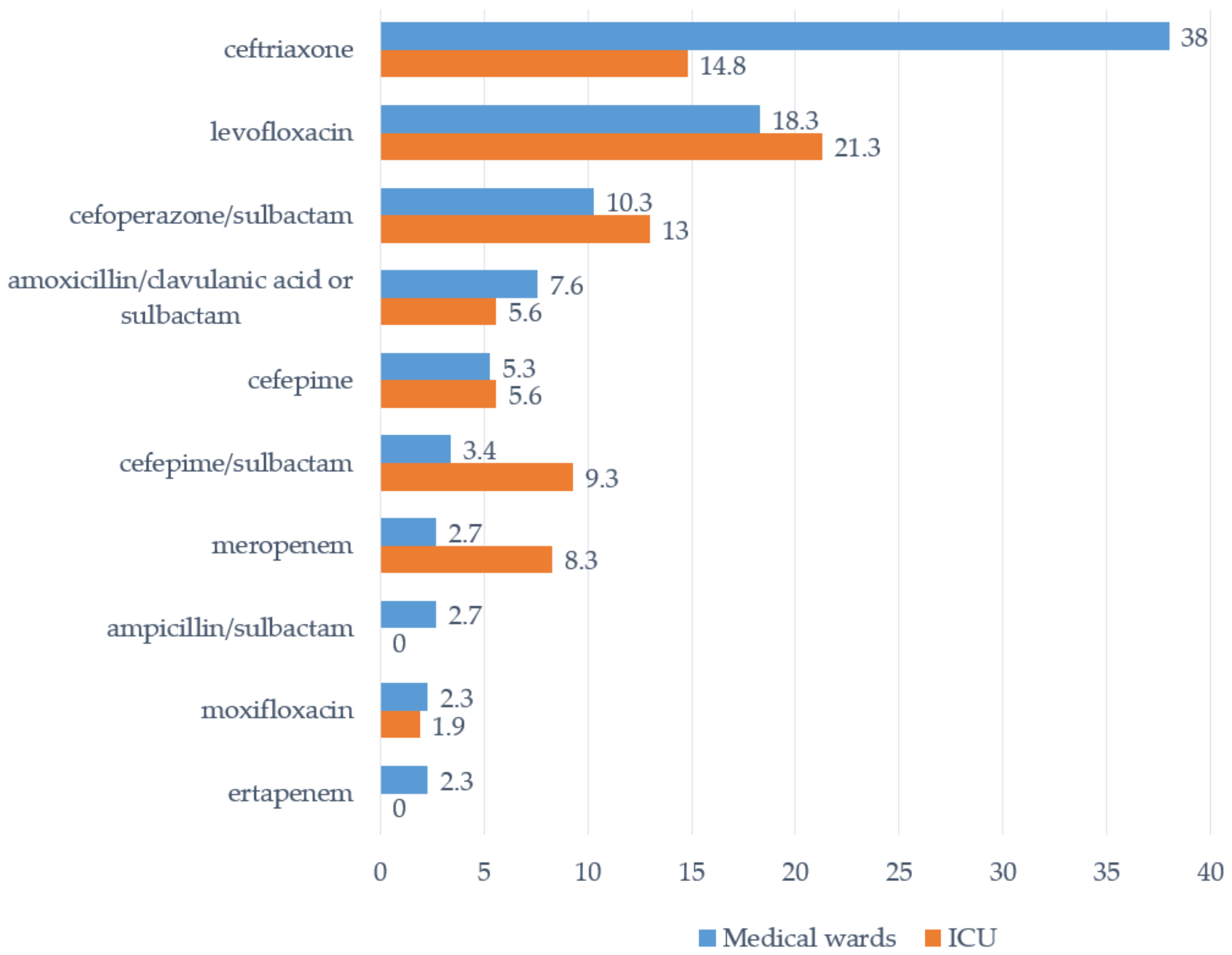
| Antibacterials | Site #1 | Site #2 | Site #3 | Site #4 | Site #5 | Site #6 | Average |
|---|---|---|---|---|---|---|---|
| Antivirals, % | |||||||
| favipiravir | 100 | 40 | 78.6 | 69.3 | 42.6 | 40 | 65 |
| remdesivir | 0 | 0 | 0 | 0 | 57.4 | 60 | 19.2 |
| umifenovir | 0 | 60 | 21.4 | 30.7 | 0 | 0 | 15.8 |
| Antibiotics, % | |||||||
| ceftriaxone | 38.5 | 17.6 | 54.4 | 35.6 | 0,0 | 36.4 | 31.5 |
| levofloxacin | 0.0 | 0.0 | 25.6 | 22.9 | 14.0 | 40.9 | 19.1 |
| cefoperazone/sulbactam | 23.1 | 0.0 | 5.6 | 17.8 | 2.3 | 18.2 | 11.0 |
| amoxicillin/clavulanic acid + amoxicillin/sulbactam | 17.9 | 52.9 | 3.3 | 0.8 | 1.2 | 0.0 | 5.6 |
| cefepime | 10.3 | 5.9 | 0.0 | 0.0 | 17.4 | 0.0 | 5.4 |
| cefepime/sulbactam | 0.0 | 0.0 | 0.0 | 0.0 | 22.1 | 0.0 | 5.1 |
| meropenem | 7.7 | 11.8 | 3.3 | 1.7 | 5.8 | 4.5 | 4.3 |
| ampicillin/sulbactam | 0.0 | 0.0 | 0.0 | 0.0 | 14.0 | 0.0 | 3.2 |
| imipenem | 0.0 | 0.0 | 0.0 | 7.6 | 0.0 | 0.0 | 2.4 |
| moxifloxacin | 0 | 0 | 0 | 1.7 | 7 | 0 | 2.2 |
| ertapenem | 0.0 | 0.0 | 0.0 | 0.8 | 5.8 | 0.0 | 1.6 |
| amikacin | 0.0 | 0.0 | 2.2 | 0.0 | 3.5 | 0.0 | 1.3 |
| linezolid | 0.0 | 5.9 | 0.0 | 2.5 | 1.2 | 0.0 | 1.3 |
| other | 2.6 | 5.9 | 5.6 | 8.5 | 5.8 | 0.0 | 5.9 |
| Patterns | Site #1 | Site #2 | Site #3 | Site #4 | Site #5 | Site #6 | ICU Wards | Medical Wards | Average |
|---|---|---|---|---|---|---|---|---|---|
| Treatment based on biomarker data, % of patients | 52.2 | 80.8 | 40.4 | 56.5 | 17.4 | 75 | 58.6 | 39.2 | 42.7 |
| C-reactive protein * | 34.8 | 65.4 | 35.4 | 38.3 | 2 | 0 | 32.2 | 24.1 | 25.5 |
| white blood cells | 17.4 | 3.8 | 0 | 8.7 | 8.7 | 33.3 | 13.8 | 8.1 | 9.1 |
| procalcitonin | 0 | 3.8 | 5.1 | 9.6 | 6.7 | 41.7 | 12.6 | 6.6 | 7.7 |
| Culture test performed, % of patients | 0 | 92.3 | 5.1 | 2.6 | 24.2 | 0 | 11.5 | 14.7 | 14.1 |
| Quality indicators, % of prescriptions | |||||||||
| Targeted therapy | 10.5 | 32.4 | 44.5 | 7.8 | 53.9 | 0 | 29 | 29.8 | 29.6 |
| antivirals | 19.1 | 40 | 96.4 | 17.3 | 94.7 | 0 | 86.7 | 55.1 | 58.2 |
| antibiotics | 0 | 23.5 | 12.2 | 1.7 | 9.3 | 0 | 13 | 4.2 | 6.7 |
| Compliance with the hospital guidelines | 81.4 | 97.3 | 100 | 82.8 | 66.7 | 96.3 | 79.0 | 84.5 | 83.4 |
| antivirals | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| antibiotics | 59 | 94.1 | 100 | 71.8 | 30.2 | 95.5 | 73.1 | 68.8 | 70.1 |
| Indication for treatment was recorded | 96.5 | 97.3 | 100 | 81.3 | 79.4 | 96.3 | 85.5 | 89.1 | 88.3 |
| antivirals | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| antibiotics | 92.3 | 94.1 | 100 | 69.2 | 57 | 95.5 | 81.5 | 77.9 | 79.0 |
| Stop/review date documented | 66.3 | 100 | 93.2 | 90.1 | 92.2 | 0 | 87.7 | 84.5 | 85.2 |
| antivirals | 97.9 | 100 | 100 | 100 | 95.7 | 0 | 100 | 96.3 | 96.6 |
| antibiotics | 28.2 | 100 | 88.9 | 83.8 | 88.4 | 0 | 84.3 | 72.6 | 76 |
| Prescribed antibiotics according to AWaRe classification, % of prescriptions | |||||||||
| access | 20.5 | 58.8 | 6.7 | 0.9 | 18.6 | 0 | 10.2 | 11.4 | 11.1 |
| watch | 56.4 | 35.3 | 86.7 | 71.8 | 74.4 | 81.8 | 70.4 | 74.5 | 73.3 |
| reserve | 0 | 5.9 | 0 | 9.4 | 4.7 | 0 | 5.6 | 3.8 | 4.3 |
| not recommended | 23.1 | 0 | 6.7 | 17.9 | 2.3 | 18.2 | 13.9 | 10.3 | 11.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avdeev, S.; Rachina, S.; Belkova, Y.; Kozlov, R.; Versporten, A.; Pauwels, I.; Goossens, H.; Bochanova, E.; Elokhina, E.; Portnjagina, U.; et al. Antimicrobial Prescribing Patterns in Patients with COVID-19 in Russian Multi-Field Hospitals in 2021: Results of the Global-PPS Project. Trop. Med. Infect. Dis. 2022, 7, 75. https://doi.org/10.3390/tropicalmed7050075
Avdeev S, Rachina S, Belkova Y, Kozlov R, Versporten A, Pauwels I, Goossens H, Bochanova E, Elokhina E, Portnjagina U, et al. Antimicrobial Prescribing Patterns in Patients with COVID-19 in Russian Multi-Field Hospitals in 2021: Results of the Global-PPS Project. Tropical Medicine and Infectious Disease. 2022; 7(5):75. https://doi.org/10.3390/tropicalmed7050075
Chicago/Turabian StyleAvdeev, Sergey, Svetlana Rachina, Yuliya Belkova, Roman Kozlov, Ann Versporten, Ines Pauwels, Herman Goossens, Elena Bochanova, Elena Elokhina, Ulyana Portnjagina, and et al. 2022. "Antimicrobial Prescribing Patterns in Patients with COVID-19 in Russian Multi-Field Hospitals in 2021: Results of the Global-PPS Project" Tropical Medicine and Infectious Disease 7, no. 5: 75. https://doi.org/10.3390/tropicalmed7050075
APA StyleAvdeev, S., Rachina, S., Belkova, Y., Kozlov, R., Versporten, A., Pauwels, I., Goossens, H., Bochanova, E., Elokhina, E., Portnjagina, U., Reshetko, O., Sychev, I., Strelkova, D., & On behalf of Russian Global-PPS Project Study Group. (2022). Antimicrobial Prescribing Patterns in Patients with COVID-19 in Russian Multi-Field Hospitals in 2021: Results of the Global-PPS Project. Tropical Medicine and Infectious Disease, 7(5), 75. https://doi.org/10.3390/tropicalmed7050075








