Intrathecal/Intraventricular Colistin for Antibiotic-Resistant Bacterial CNS Infections in Pediatric Population: A Systematic Review
Abstract
1. Introduction
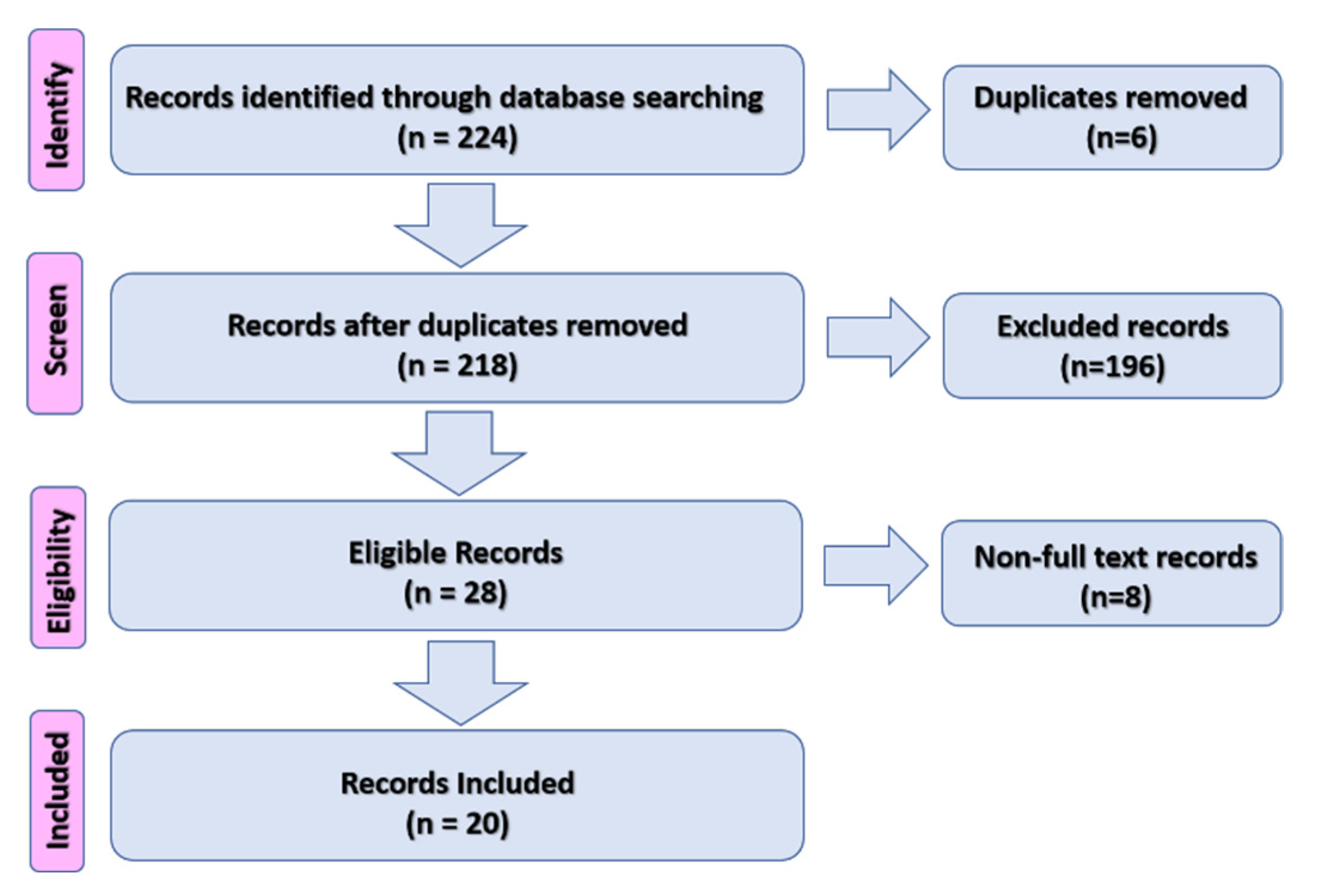
2. Materials and Methods
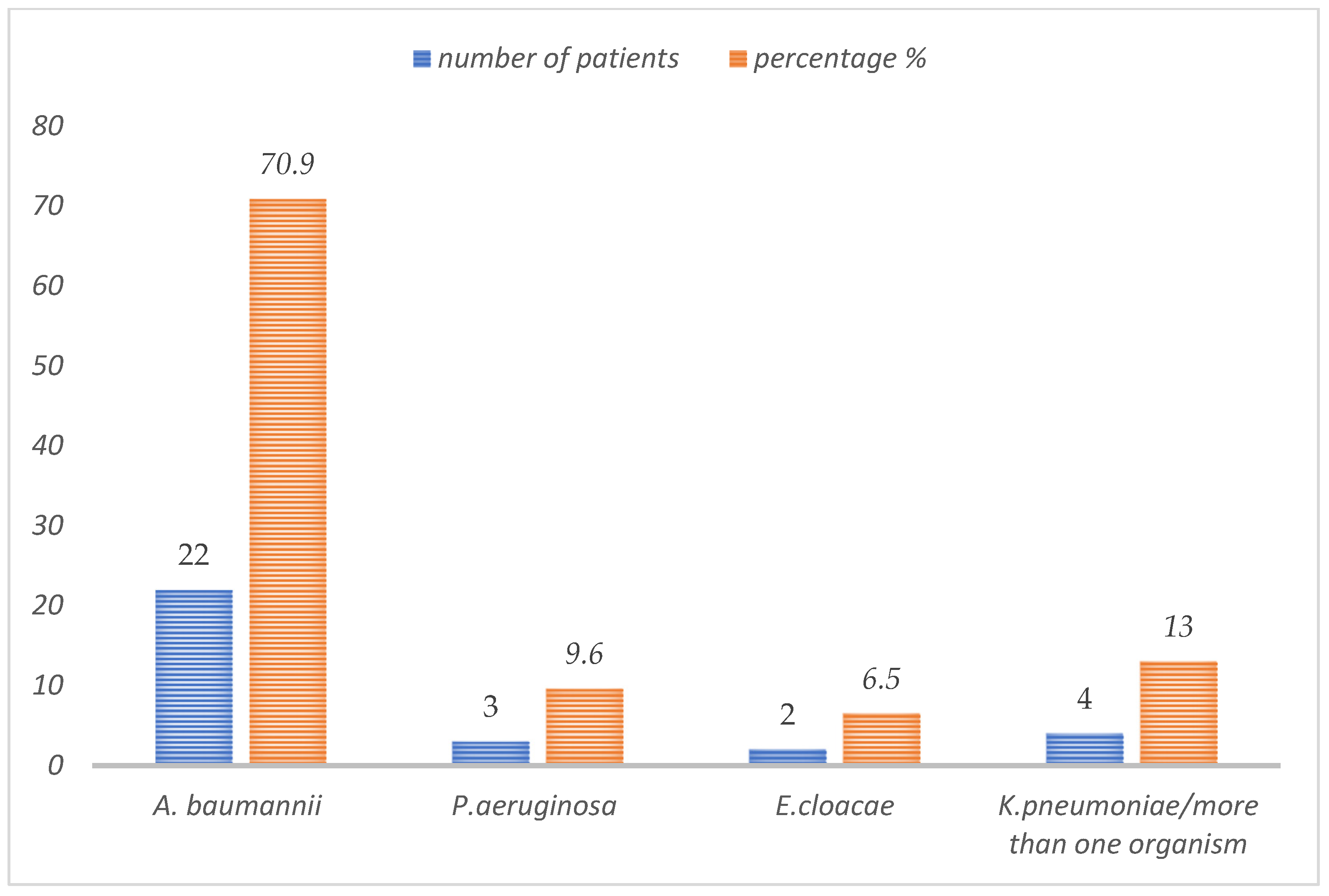
3. Results
4. Discussion
5. Limitations of the Review
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Imberti, R.; Iotti, G.A.; Regazzi, M. Intraventricular or intrathecal colistin for the treatment of central nervous system infections caused by multidrug-resistant Gram-negative bacteria. Expert Rev. Anti-Infective Ther. 2014, 12, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, M.; van de Beek, D. Management of bacterial central nervous system infections. In Critical Care Neurology. Part I. Handbook of Clinical Neurology, 1st ed.; 3rd Series; Wijdicks, E.F.M., Kramer, A.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 140. [Google Scholar] [CrossRef]
- De Pascale, G.; Pompucci, A.; Maviglia, R.; Spanu, T.; Bello, G.; Mangiola, A.; Scoppettuolo, G. Successful treatment of multidrug-resistant Acinetobacter baumannii ventriculitis with intrathecal and intravenous colistin. Minerva Anestesiol. 2010, 76, 957–960. [Google Scholar] [PubMed]
- Tartor, Y.H.; Gharieb, R.M.A.; El-Aziz, N.K.A.; El Damaty, H.M.; Enany, S.; Khalifa, E.; Attia, A.S.A.; Abdellatif, S.S.; Ramadan, H. Virulence Determinants and Plasmid-Mediated Colistin Resistance mcr Genes in Gram-Negative Bacteria Isolated from Bovine Milk. Front. Cell. Infect. Microbiol. 2021, 11, 761417. [Google Scholar] [CrossRef] [PubMed]
- Tartor, Y.H.; El-Aziz, N.K.A.; Gharieb, R.M.A.; El Damaty, H.M.; Enany, S.; Soliman, E.A.; Abdellatif, S.S.; Attia, A.S.A.; Bahnass, M.M.; El-Shazly, Y.A.; et al. Whole-Genome Sequencing of Gram-Negative Bacteria Isolated from Bovine Mastitis and Raw Milk: The First Emergence of Colistin mcr-10 and Fosfomycin fosA5 Resistance Genes in Klebsiella pneumoniae in Middle East. Front. Microbiol. 2021, 12, 770813. [Google Scholar] [CrossRef]
- Karaiskos, I.; Galani, L.; Baziaka, F.; Giamarellou, H. Intraventricular and intrathecal colistin as the last therapeutic resort for the treatment of multidrug-resistant and extensively drug-resistant Acinetobacter baumannii ventriculitis and meningitis: A literature review. Int. J. Antimicrob. Agents 2013, 41, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Markantonis, S.L.; Markou, N.; Fousteri, M.; Sakellaridis, N.; Karatzas, S.; Alamanos, I.; Dimopoulou, E.; Baltopoulos, G. Penetration of Colistin into Cerebrospinal Fluid. Antimicrob. Agents Chemother. 2009, 53, 4907–4910. [Google Scholar] [CrossRef]
- Kaplan, S.L.; Patrick, C.C. Cefotaxime and aminoglycoside treatment of meningitis caused by Gram-negative enteric organisms. Pediatr. Infect. Dis. J. 1990, 9, 810–814. [Google Scholar] [CrossRef]
- Fernandez-Viladrich, P.; Corbella, X.; Corral, L.; Tubau, F.; Mateu, A. Successful Treatment of Ventriculitis Due to Carbapenem-ResistantAcinetobacter baumanniiwith Intraventricular Colistin Sulfomethate Sodium. Clin. Infect. Dis. 1999, 28, 916–917. [Google Scholar] [CrossRef][Green Version]
- Ng, J.; Gosbell, I.B.; Kelly, J.A.; Boyle, M.J.; Ferguson, J.K. Cure of multiresistant Acinetobacter baumannii central nervous system infections with intraventricular or intrathecal colistin: Case series and literature review. J. Antimicrob. Chemother. 2006, 58, 1078–1081. [Google Scholar] [CrossRef]
- Yagmur, R.; Esen, F. Intrathecal colistin for treatment of Pseudomonas aeruginosa ventriculitis: Report of a case with successful outcome. Crit. Care 2006, 10, 428. [Google Scholar] [CrossRef][Green Version]
- Dalgic, N.; Ceylan, Y.; Sancar, M.; Telhan, L.; Kafadar, I.; Cavusoglu, H.; Ceylan, O.; Hasim, O. Successful treatment of multidrug-resistant Acinetobacter baumannii ventriculitis with intravenous and intraventricular colistin. Ann. Trop. Paediatr. 2009, 29, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, H.; Tapısız, A.; Çiftçi, E.; Ince, E.; Mokhtari, H.; Güriz, H.; Aysev, A.D.; Doğru, Ü.; Tapisiz, A. Successful treatment of three children with post-neurosurgical multidrug-resistant Acinetobacter baumannii meningitis. Infection 2010, 38, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Cascio, A.; Conti, A.; Sinardi, L.; Iaria, C.; Angileri, F.F.; Stassi, G.; David, T.; Versaci, A.; Iaria, M.; David, A. Post-neurosurgical multidrug-resistant Acinetobacter baumannii meningitis successfully treated with intrathecal colistin. A new case and a systematic review of the literature. Int. J. Infect. Dis. 2010, 14, e572–e579. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.F.; Shah, M.S.; Shaikh, A.S.; Mir, F.; Zaidi, A.K.M. Acinetobacter species meningitis in children: A case series from Karachi, Pakistan. J. Infect. Dev. Ctries. 2011, 5, 809–814. [Google Scholar] [CrossRef]
- Wang, J.-H.; Lin, P.-C.; Chou, C.-H.; Ho, C.-M.; Lin, K.-H.; Tsai, C.-T.; Wang, J.-H.; Chi, C.-Y.; Ho, M.-W. Intraventricular antimicrobial therapy in postneurosurgical Gram-negative bacillary meningitis or ventriculitis: A hospital-based retrospective study. J. Microbiol. Immunol. Infect. 2014, 47, 204–210. [Google Scholar] [CrossRef]
- Bargiacchi, O.; Rossati, A.; Car, P.; Brustia, D.; Brondolo, R.; Rosa, F.; Garavelli, P.L.; DE Rosa, F.G. Intrathecal/intraventricular colistin in external ventricular device-related infections by multi-drug resistant Gram negative bacteria: Case reports and review. Infection 2014, 42, 801–809. [Google Scholar] [CrossRef]
- Tekgunduz, K.S.; Kara, M.; Caner, I.; Demirelli, Y. Safety and Efficacy of Intravenous Colistin in Neonates with Culture Proven Sepsis. Iran. J. Pediatr. 2015, 25, e453. [Google Scholar] [CrossRef]
- Santos, A.S.; Iraneta, A.; Matos, M.; Brito, M.J. Intraventricular colistin in Gram-negative ventriculoperitoneal shunt infection in two pediatric patients. Acta Neurochir. 2015, 157, 2219–2220. [Google Scholar] [CrossRef][Green Version]
- Tekgündüz, K.S.; Demirelli, Y.; Caner, I.; Kara, M. Intraventricular colistin use in a premature infant with cerebral abscess and ventriculitis. J. Clin. Neonatol. 2015, 4, 132–134. [Google Scholar] [CrossRef]
- Mahabeer, P.; Mzimela, B.W.; Lawler, M.A.; Singh-Moodley, A.; Singh, R.; Mlisana, K.P. Colistin-resistantAcinetobacter baumanniias a cause of neonatal ventriculitis. S. Afr. J. Infect. Dis. 2018, 33, 1–3. [Google Scholar] [CrossRef]
- Rao, K.; Rangappa, P.; Jacob, I.; Hiremath, P. Cerebrospinal fluid lactate as a prognostic indicator in postneurosurgical bacterial meningitis and use of intrathecal colistin. Indian J. Crit. Care Med. 2018, 22, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Abad-Restrepo, J.; Díaz-Díaz, A.; Osorio-Cadavid, N. Post-surgical ventriculitis due to extensively resistant Pseudomonas aeruginosa treated with intrathecal colistin: Pediatric case report and literature review. Rev. Chil. Infectología 2018, 35, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Alzailaie, A.K.; Aljarie, A.A.; Abdelwahab, H.M. Successful Outcome in Treating Ventriculitis with Intrathecal Colistin in a Child. Bahrain Med. Bull. 2018, 40, 59–60. [Google Scholar] [CrossRef]
- Al Yazidi, L.S.; McMullan, B.; Kohan, S.; Palasanthiran, P. Persistent Gram-negative Neurosurgical Meningitis in a Neonate, Successfully Treated with Intraventricular Colistin: Case Report and Review of the Literature. Pediatr. Infect. Dis. J. 2018, 37, e79–e81. [Google Scholar] [CrossRef]
- Falagas, M.E.; Bliziotis, I.A.; Tam, V.H. Intraventricular or intrathecal use of polymyxins in patients with Gram-negative meningitis: A systematic review of the available evidence. Int. J. Antimicrob. Agents 2007, 29, 9–25. [Google Scholar] [CrossRef]
- Giamarellou, H.; Antoniadou, A.; Kanellakopoulou, K. Acinetobacter baumannii: A universal threat to public health? Int. J. Antimicrob. Agents 2008, 32, 106–119. [Google Scholar] [CrossRef]
- Tunkel, A.R.; Hartman, B.J.; Kaplan, S.L.; Kaufman, B.A.; Roos, K.L.; Scheld, W.M.; Whitley, R.J. Practice Guidelines for the Management of Bacterial Meningitis. Clin. Infect. Dis. 2004, 39, 1267–1284. [Google Scholar] [CrossRef]
- Hussain, K.; Salat, M.S.; Ambreen, G.; Iqbal, J. Neurodevelopment Outcome of Neonates Treated with Intraventricular Colistin for Ventriculitis Caused by Multiple Drug-Resistant Pathogens—A Case Series. Front. Pediatr. 2021, 8, 843. [Google Scholar] [CrossRef]
| Reference | Age | Gender | Route of Infection | EVD | CSF Culture | Colistin (Duration) | Other Antibiotics | Outcome |
|---|---|---|---|---|---|---|---|---|
| Kaplan & Patrick, 1990 [8] | 4 years | NR | CSF leak after trauma | Yes | A. baumannii | IV, IVT, and IT (20 days) | -- | Recovered |
| Fernandez-Viladrich et al., 1999 [9] | 16 years | Male | After neurosurgical intervention | Yes | A. baumannii | IVT and IT (20 days) | Meropenem, Tobramycin, Sulbactam | Severe disability |
| Ng et al., 2006 [10] | 4 years | Male | After neurosurgical intervention | No | A. baumannii | IV, IVT, and IT (24 days) | Amikacin | Severe disability |
| Yagmur & Esen, 2006 [11] | 16 years | Male | After neurosurgical intervention | Yes | P. aeruginosa | IVT and IT (21 days) | IV amikacin | Recovered |
| Dalgic et al., 2009 [12] | 2 months | Female | After neurosurgical intervention | Yes | A. baumannii | IV, IVT, and IT (27 days) | -- | Recovered |
| Dalgic et al., 2009 [12] | 2 months | Female | After neurosurgical intervention | Yes | K. pneumoniae | IV, IVT, and IT (14 days) | Ciprofloxacin | Recovered |
| Özdemir et al., 2010 [13] | 3 years | Female | After neurosurgical intervention | Yes | A. baumannii | IV, IVT, and IT (35 days) | Meropenem, amikacin, ampicillin | Recovered |
| Cascio et al., 2010 [14] | 5 years | Male | After neurosurgical intervention | Yes | Enterobacter cloacae | IV, IVT, and IT (14 days) | Teicoplanin, Rifampin, cefazidime | Moderate disability |
| Saleem et al., 2011 [15] | 5 months | Male | After neurosurgical intervention | Yes | A. baumannii | IV, IVT, and IT (24 days) | -- | Died |
| Saleem et al., 2011 [15] | 9 months | Male | After neurosurgical intervention | No | A. baumannii | IV, IVT, and IT (6 days) | -- | Recovered |
| Saleem et al., 2011 [15] | 3 months | Female | After neurosurgical intervention | Yes | A. baumannii | IV, IVT, and IT (11 days) | -- | Recovered |
| Saleem et al., 2011 [15] | 9 years | Female | After neurosurgical intervention | Yes | A. baumannii | IV, IVT, and IT (21 days) | -- | Recovered |
| Wang et al., 2012 [16] | 15 years | Male | After neurosurgical intervention | Yes | A. baumannii | IV, IVT, and IT (24 days) | Meropenem | Recovered |
| Karaiskos et al., 2013 [6] | 18 years | Female | After neurosurgical intervention | Yes | A. baumannii | IV, IVT, and IT (17 days) | Carbapenem, Sulbactam. | Recovered |
| Bargiacchi et al., 2014 [17] | 18 years | Male | After neurosurgical intervention | Yes | P. aeruginosa | IV, IVT, and IT (18 days) | Ciprofloxacin | Recovered |
| Tekgündüz et al., 2015 [18] | <1 month | Male | After neurosurgical intervention | Yes | A. baumannii | IV, IVT, and IT (9 days) | Vancomycin | Recovered |
| Santos et al., 2015 [19] | 15 months | Male | After neurosurgical intervention | Yes | E. coli, K. pneumoniae | IV, IVT, and IT | Meropenem | Recovered |
| Santos et al., 2015 [19] | 11 months | Male | After neurosurgical intervention | Yes | A. baumannii | IV, IVT, and IT | Meropenem, Amikacin | Recovered |
| Tekgunduz et al., 2015 [20] | 2 months | Male | After neurosurgical intervention | Yes | A. baumannii | IV, IVT, and IT | Gentamicin, Sulbactam | Moderate disability |
| Mahabeer et al., 2018 [21] | 1 month | Male | Healthcare-associated infection | Yes | A. baumannii | IV, IVT, and IT | Gentamicin | Recovered |
| Hiremath et al., 2018 [22] | 17 years | Female | After neurosurgical intervention | Yes | A. baumannii | IVT and IT (11 days) | IV meropenem and teicoplanin | recovered |
| Abad-Restrepo et al., 2018 [23] | 11 years | Female | After neurosurgical intervention | Yes | P. aeruginosa | IV, IVT, and IT (42 days) | Vancomycin | Recovered |
| AlZailaie et al., 2018 [24] | 5 years | Female | After neurosurgical intervention | Yes | A. baumannii | IV, IVT, and IT (49 days) | -- | Recovered |
| Al Yazidi et al., 2018 [25] | <1 month | Male | After neurosurgical intervention | Yes | Enterobacter cloacae | IV, IVT, and IT (9 days) | Meropenem, Ciprofloxacin | Died |
| Hussain et al., 2021 [26] | 1 month | Female | After neurosurgical intervention | Yes | E.coli, K. pneumoniae | IV, IVT, and IT (7 days) | IV meropenem, vancomycin | Recovered |
| Hussain et al., 2021 26] | <1 month | Male | After neurosurgical intervention | Yes | A. baumannii | IV, IVT, and IT (7 days) | IV meropenem, vancomycin | Recovered |
| Hussain et al., 2021 [26] | < 1 month | Male | Healthcare-associated infection | Yes | A. baumannii | IV, IVT, and IT (5 days) | IV meropenem, vancomycin | Recovered |
| Hussain et al., 2021 [26] | <1 month | Female | After neurosurgical intervention | Yes | A. baumannii | IV, IVT, and IT (8 days) | IV cefotaxime, Meropenem, amikacin, colistin | Recovered |
| Hussain et al., 2021 [26] | <1 month | Male | Healthcare-associated infection | Yes | A. baumannii | IV, IVT, and IT (7 days) | IV cefotaxime, Meropenem, amikacin | Moderate disability |
| Hussain et al., 2021 [26] | <1 month | Male | Healthcare-associated infection | Yes | A. baumannii | IV, IVT, and IT (3 days) | IV cefotaxime, Meropenem, amikacin, vancomycin | Died |
| Hussain et al., 2021 [26] | <1 month | Male | After neurosurgical intervention | Yes | K pneumoniae, A. baumannii | IV, IVT, and IT (8 days) | IV meropenem, Ceftazidime, amikacin | Recovered |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnaami, I.; Alahmari, Z. Intrathecal/Intraventricular Colistin for Antibiotic-Resistant Bacterial CNS Infections in Pediatric Population: A Systematic Review. Trop. Med. Infect. Dis. 2022, 7, 41. https://doi.org/10.3390/tropicalmed7030041
Alnaami I, Alahmari Z. Intrathecal/Intraventricular Colistin for Antibiotic-Resistant Bacterial CNS Infections in Pediatric Population: A Systematic Review. Tropical Medicine and Infectious Disease. 2022; 7(3):41. https://doi.org/10.3390/tropicalmed7030041
Chicago/Turabian StyleAlnaami, Ibrahim, and Zubaidah Alahmari. 2022. "Intrathecal/Intraventricular Colistin for Antibiotic-Resistant Bacterial CNS Infections in Pediatric Population: A Systematic Review" Tropical Medicine and Infectious Disease 7, no. 3: 41. https://doi.org/10.3390/tropicalmed7030041
APA StyleAlnaami, I., & Alahmari, Z. (2022). Intrathecal/Intraventricular Colistin for Antibiotic-Resistant Bacterial CNS Infections in Pediatric Population: A Systematic Review. Tropical Medicine and Infectious Disease, 7(3), 41. https://doi.org/10.3390/tropicalmed7030041






