Antileishmanial Efficacy of the Calpain Inhibitor MDL28170 in Combination with Amphotericin B
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasites and Culture
2.2. Antileishmanial Compounds
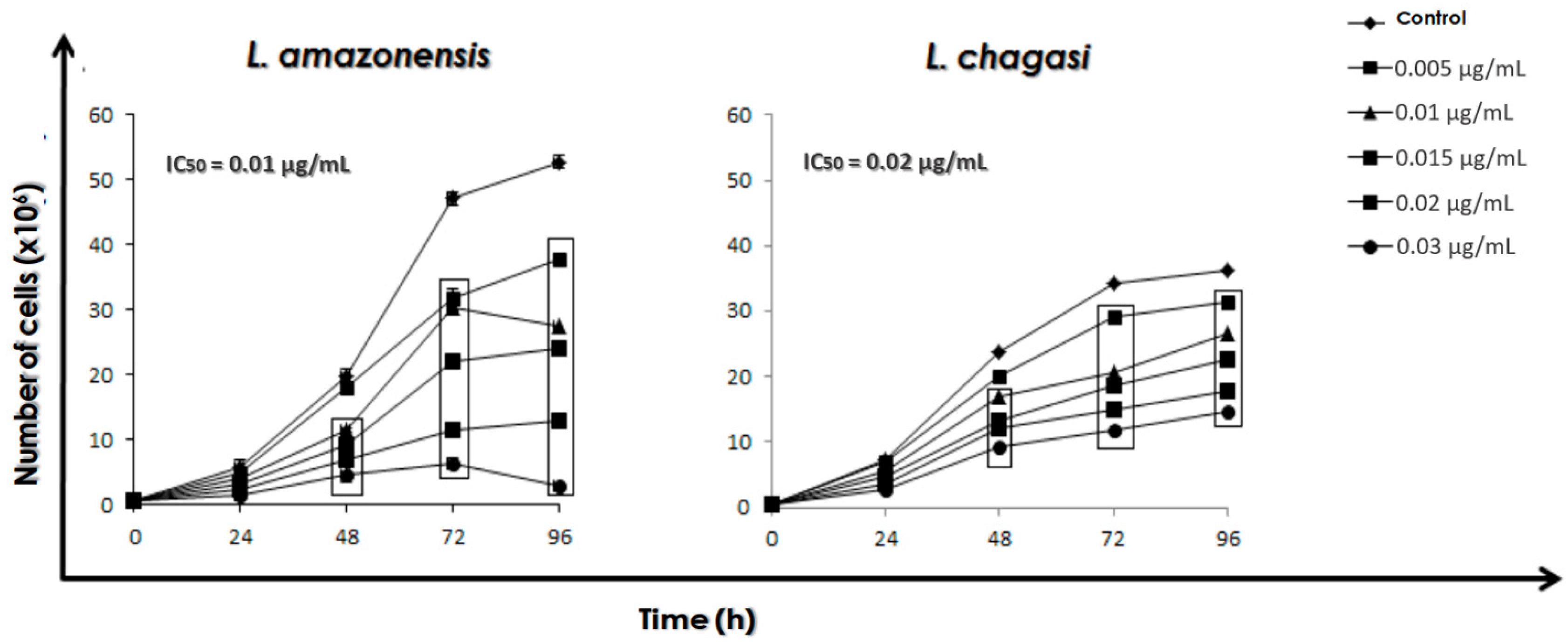
2.3. Effect of AmB on the Growth Rate of Promastigotes
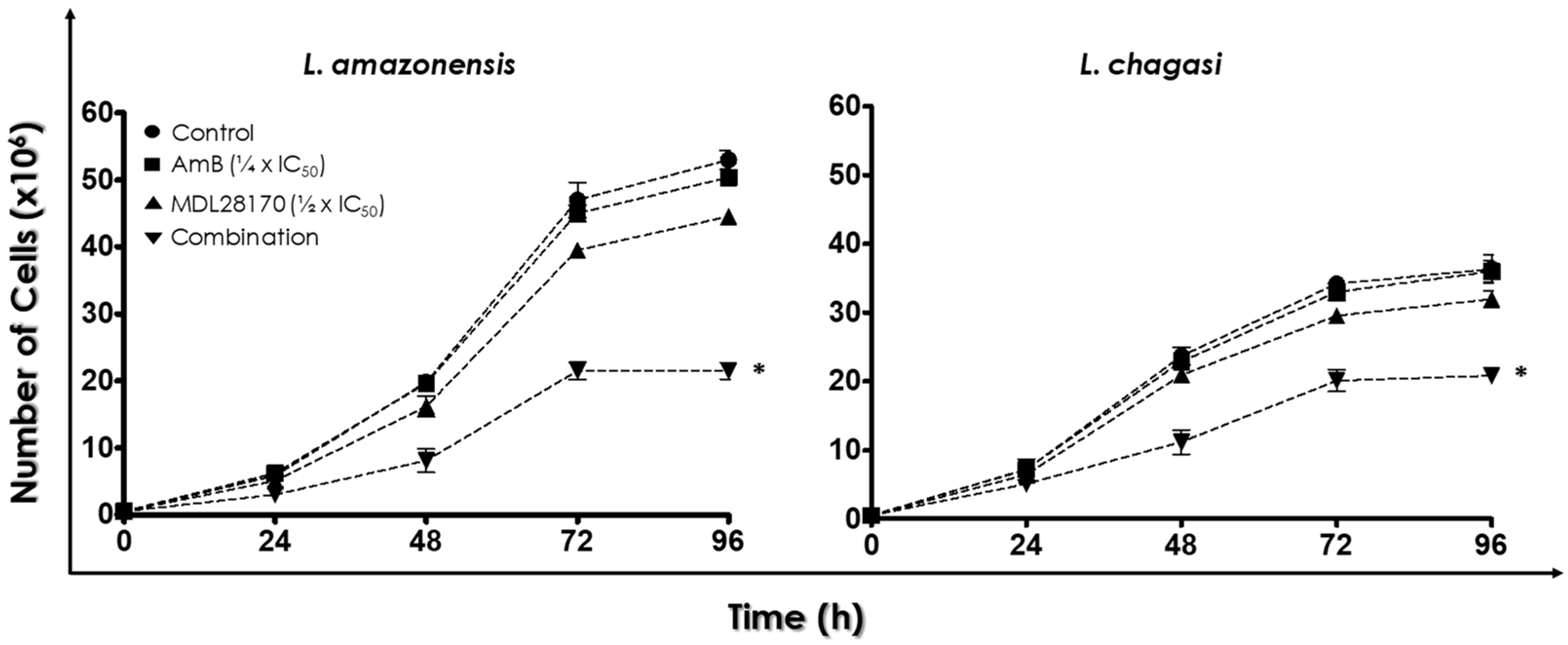
2.4. Combination of the Calpain Inhibitor MDL28170 and AmB
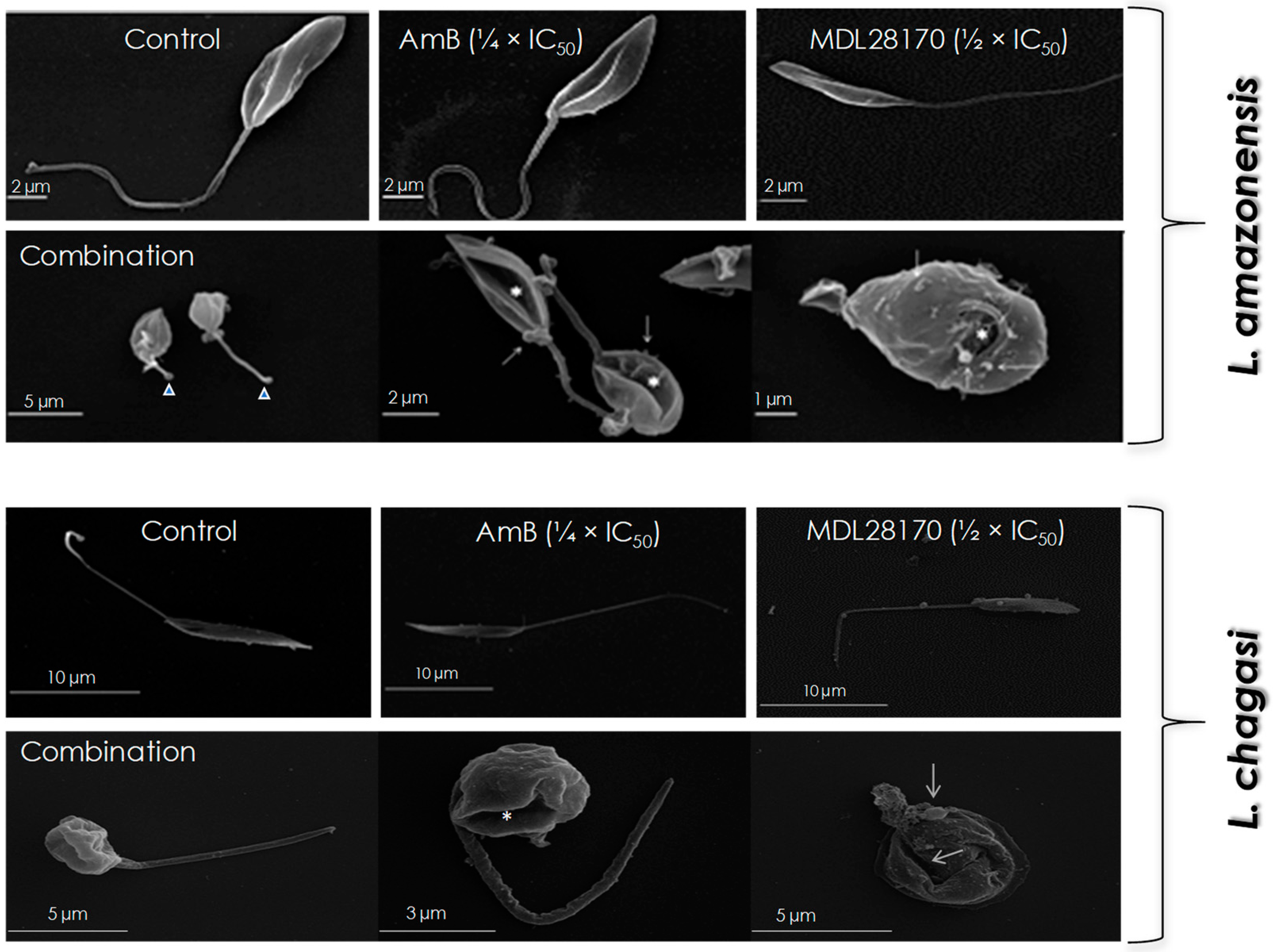
2.5. Scanning Electron Microscopy (SEM)
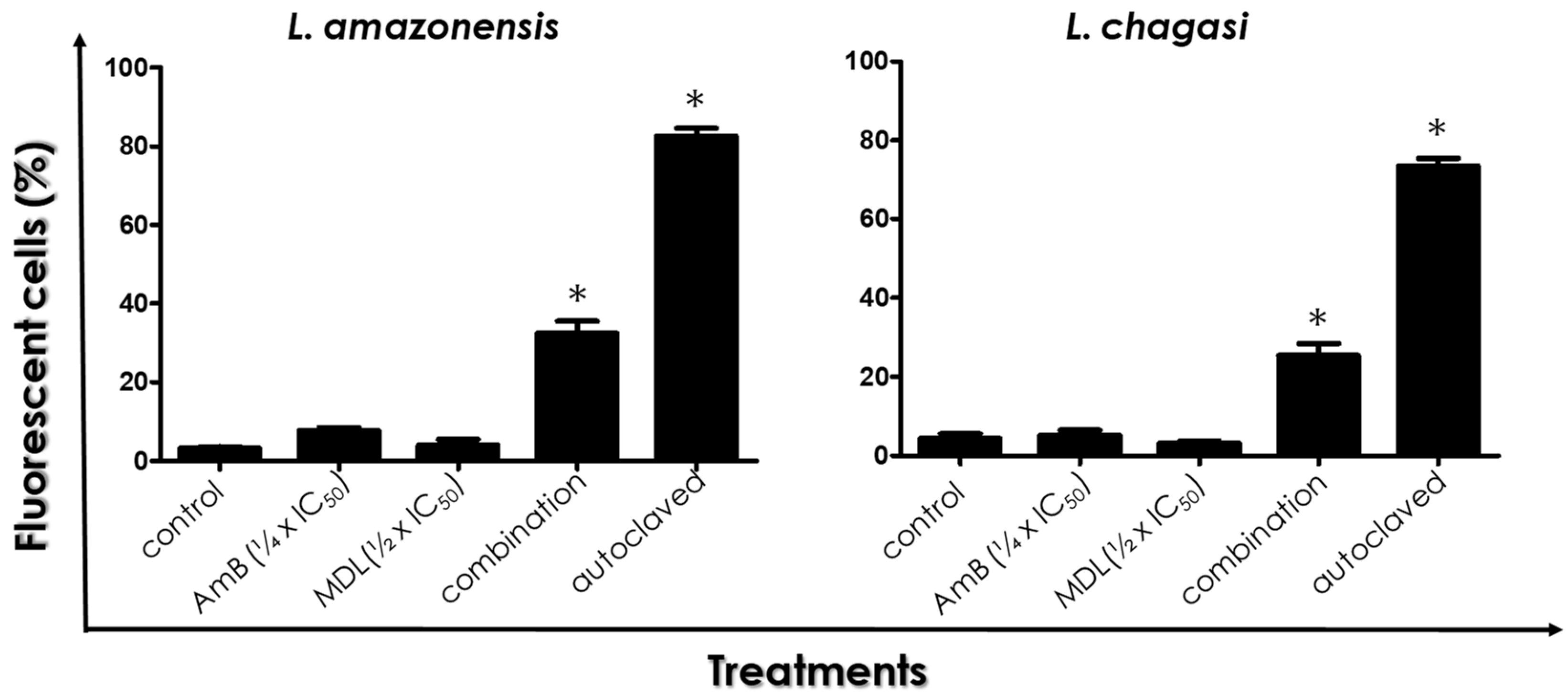
2.6. Cell Membrane Integrity
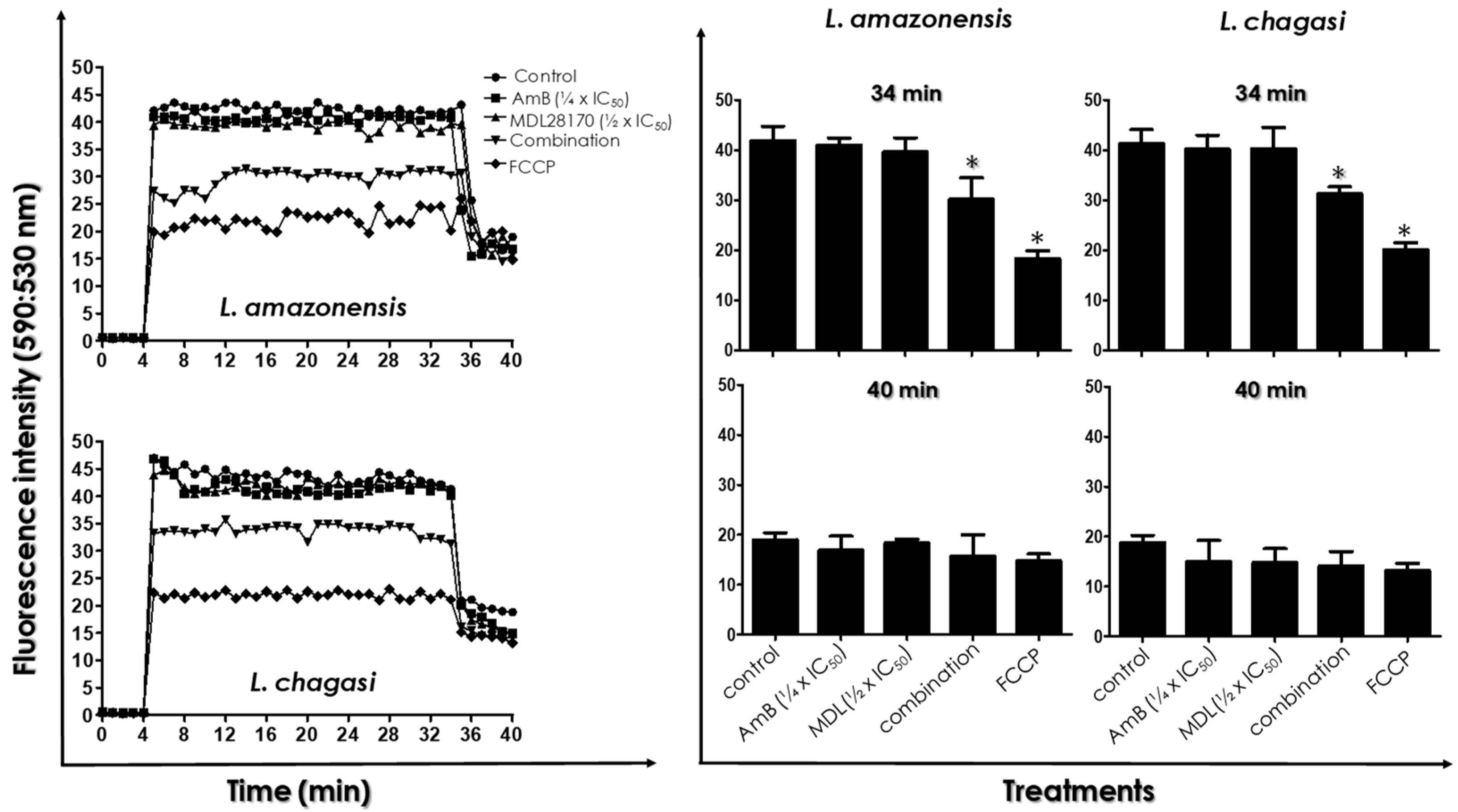
2.7. Determination of Mitochondrial Transmembrane Electric Potential
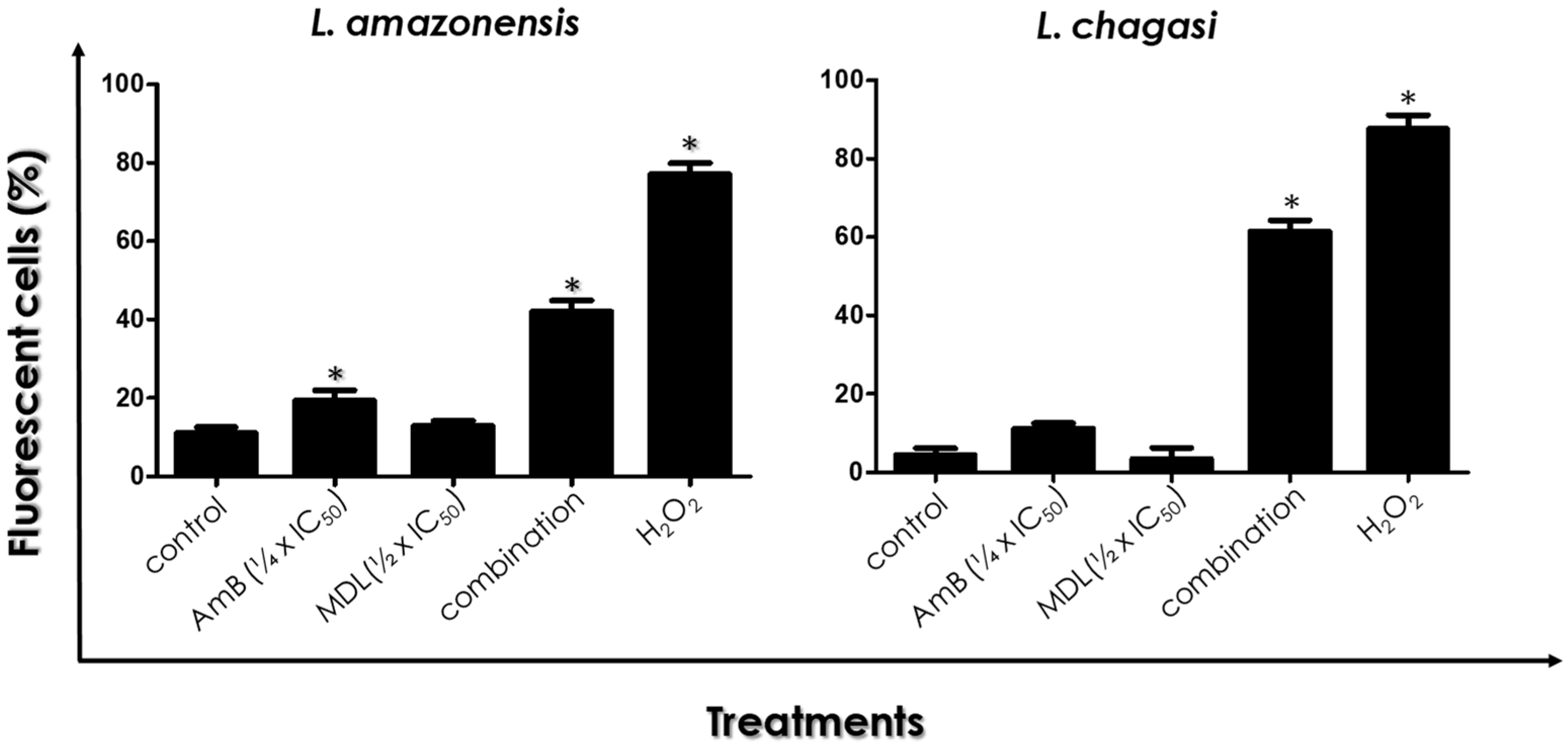
2.8. ROS Production
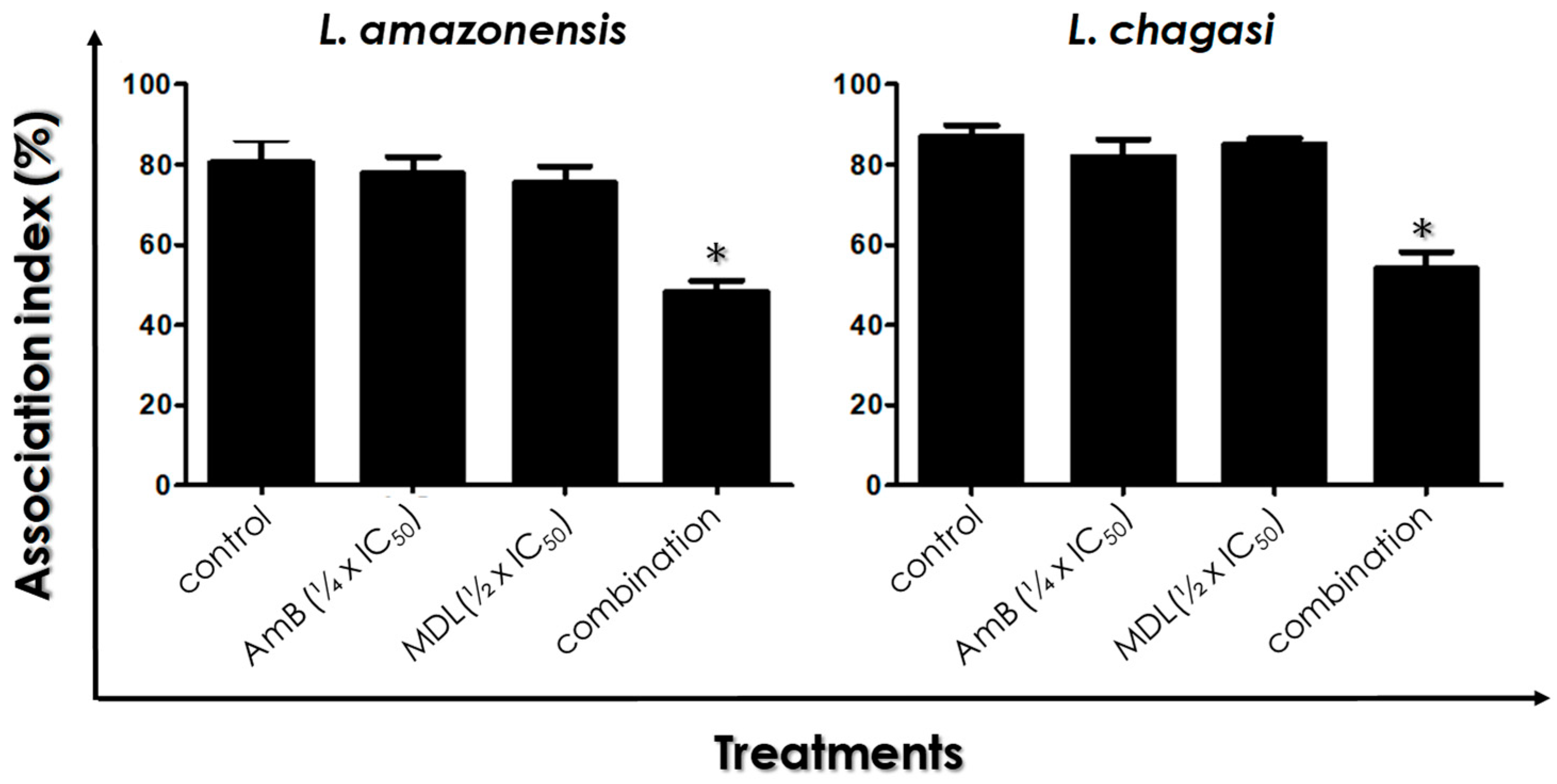
2.9. Leishmania–Macrophage Interaction
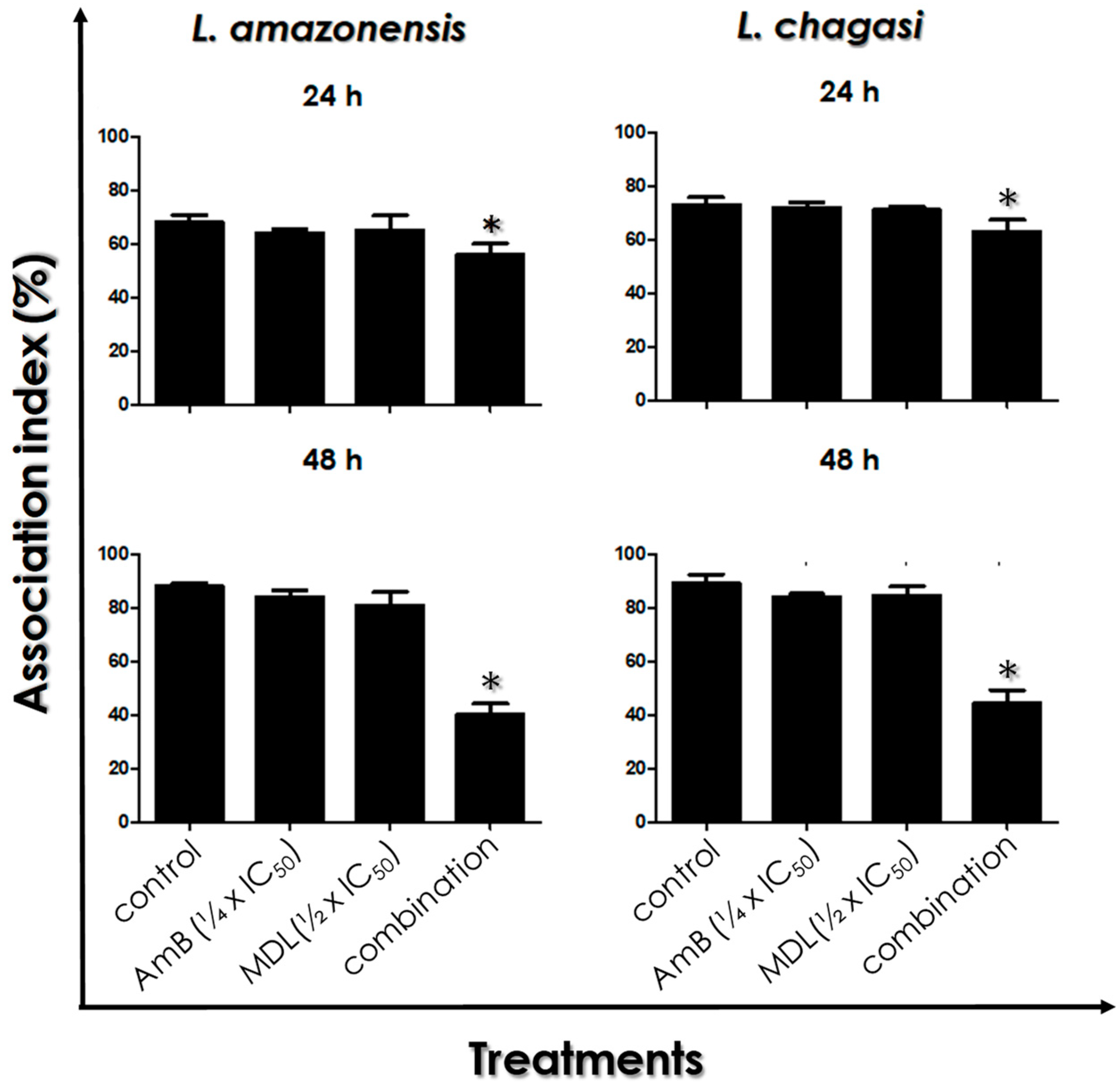
2.10. Intracellular Amastigotes Viability
2.11. Statistical Analysis
3. Results
3.1. Combination Therapy against Leishmania: MDL28170 and AmB
3.2. Promastigotes Morphology
3.3. Plasma Membrane Integrity
3.4. ΔΨ m Measurements
3.5. ROS Production
3.6. Leishmania–Macrophage Interaction: Pre-Treatment
3.7. Leishmania–Macrophage Interaction: Post-Treatment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- World Health Organization. Leishmaniasis. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab_1 (accessed on 15 January 2022).
- Ghorbani, M.; Farhoudi, R. Leishmaniasis in humans: Drug or vaccine therapy? Drug Des Devel Ther. 2017, 12, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Uliana, S.R.B.; Trinconi, C.T.; Coelho, A.C. Chemotherapy of leishmaniasis: Present challenges. Parasitology 2018, 145, 464–480. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Singh, B. Emerging therapeutic targets for treatment of leishmaniasis. Expert Opin. Ther. Targets 2018, 22, 467–486. [Google Scholar] [CrossRef] [PubMed]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef] [PubMed]
- Roatt, B.M.; de Oliveira Cardoso, J.M.; De Brito, R.C.F.; Coura-Vital, W.; de Oliveira Aguiar-Soares, R.D.; Reis, A.B. Recent advances and new strategies on leishmaniasis treatment. Appl. Microbiol. Biotechnol. 2020, 104, 8965–8977. [Google Scholar] [CrossRef]
- d’Avila-Levy, C.M.; Marinho, F.A.; Santos, L.O.; Martins, J.L.; Santos, A.L.; Branquinha, M.H. Antileishmanial activity of MDL 28170, a potent calpain inhibitor. Int. J. Antimicrob. Agents 2006, 28, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Marinho, F.A.; Gonçalves, K.C.; Oliveira, S.S.; Gonçalves, D.S.; Matteoli, F.P.; Seabra, S.H.; Oliveira, A.C.; Bellio, M.; Oliveira, S.S.; Souto-Padrón, T.; et al. The calpain inhibitor MDL28170 induces the expression of apoptotic markers in Leishmania amazonensis promastigotes. PLoS ONE 2014, 9, e87659. [Google Scholar] [CrossRef]
- Marinho, F.A.; Sangenito, L.S.; Oliveira, S.; De Arruda, L.B.; D’Ávila-Levy, C.M.; Santos, A.; Branquinha, M.H. The potent cell permeable calpain inhibitor MDL28170 affects the interaction of Leishmania amazonensis with macrophages and shows anti-amastigote activity. Parasitol. Int. 2017, 66, 579–583. [Google Scholar] [CrossRef]
- de Sousa Araújo, P.S.; de Oliveira, S.; d’Avila-Levy, C.M.; Dos Santos, A.; Branquinha, M.H. Susceptibility of promastigotes and intracellular amastigotes from distinct Leishmania species to the calpain inhibitor MDL28170. Parasitol. Res. 2018, 117, 2085–2094. [Google Scholar] [CrossRef]
- Ersfeld, K.; Barraclough, H.; Gull, K. Evolutionary relationships and protein domain architecture in an expanded calpain superfamily in kinetoplastid parasites. J. Mol. Evol. 2005, 61, 742–757. [Google Scholar] [CrossRef] [PubMed]
- Branquinha, M.H.; Marinho, F.A.; Sangenito, L.S.; Oliveira, S.S.; Goncalves, K.C.; Ennes-Vidal, V.; d’Avila-Levy, C.M.; Santos, A.L. Calpains: Potential targets for alternative chemotherapeutic intervention against human pathogenic trypanosomatids. Curr. Med. Chem. 2013, 20, 3174–3185. [Google Scholar] [CrossRef] [PubMed]
- Ennes-Vidal, V.; Vitório, B.D.S.; Menna-Barreto, R.F.S.; Pitaluga, A.N.; Gonçalves-da-Silva, S.A.; Branquinha, M.H.; Santos, A.L.S.; d’Avila-Levy, C.M. Calpains of Leishmania braziliensis: Genome analysis, differential expression, and functional analysis. Memórias Inst. Oswaldo Cruz 2019, 23, e190147. [Google Scholar] [CrossRef] [PubMed]
- Ennes-Vidal, V.; Branquinha, M.H.; Dos Santos, A.L.S.; d’Avila-Levy, C.M. The Diverse Calpain Family in Trypanosomatidae: Functional Proteins Devoid of Proteolytic Activity? Cells 2021, 10, 299. [Google Scholar] [CrossRef] [PubMed]
- Ennes-Vidal, V.; Menna-Barreto, R.F.; Branquinha, M.H.; Dos Santos, A.L.; D’Avila-Levy, C.M. Why calpain inhibitors are interesting leading compounds to search for new therapeutic options to treat leishmaniasis? Parasitology 2017, 144, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Portes, J.; Netto, C.D.; da Silva, A.J.; Costa, P.R.; DaMatta, R.A.; dos Santos, T.A.; De Souza, W.; Seabra, S.H. A new type of pterocarpanquinone that affects Toxoplasma gondii tachyzoites in vitro. Vet. Parasitol. 2012, 186, 261–269. [Google Scholar] [CrossRef]
- de Macedo-Silva, S.T.; de Oliveira Silva, T.L.; Urbina, J.A.; de Souza, W.; Rodrigues, J.C. Antiproliferative, Ultrastructural, and Physiological Effects of Amiodarone on Promastigote and Amastigote Forms of Leishmania amazonensis. Mol. Biol. Int. 2011, 2011, 876021. [Google Scholar] [CrossRef]
- Curtin, J.F.; Donovan, M.; Cotter, T.G. Regulation and measurement of oxidative stress in apoptosis. J. Immunol. Methods 2002, 265, 49–72. [Google Scholar] [CrossRef]
- Santos, L.O.; Marinho, F.A.; Altoé, E.F.; Vitório, B.S.; Alves, C.R.; Britto, C.; Motta, M.C.; Branquinha, M.H.; Santos, A.L.; d’Avila-Levy, C.M. HIV aspartyl peptidase inhibitors interfere with cellular proliferation, ultrastructure and macrophage infection of Leishmania amazonensis. PLoS ONE 2009, 4, e4918. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J. An update on pharmacotherapy for leishmaniasis. Expert Opin. Pharmacother. 2015, 16, 237–252. [Google Scholar] [CrossRef]
- Seifert, K.; Croft, S.L. In vitro and in vivo interactions between miltefosine and other antileishmanial drugs. Antimicrob. Agents Chemother. 2006, 50, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Olliaro, P.L. Drug combinations for visceral leishmaniasis. Curr. Opin. Infect. Dis. 2010, 23, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Purkait, B.; Kumar, A.; Nandi, N.; Sardar, A.H.; Das, S.; Kumar, S.; Pandey, K.; Ravidas, V.; Kumar, M.; De, T.; et al. Mechanism of amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob. Agents Chemother. 2012, 56, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Tonin, F.S.; Steimbach, L.M.; Borba, H.H.; Sanches, A.C.; Wiens, A.; Pontarolo, R.; Fernandez-Llimos, F. Efficacy and safety of amphotericin B formulations: A network meta-analysis and a multicriteria decision analysis. J. Pharm. Pharmacol. 2017, 69, 1672–1683. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.S.C.; Branquinha, M.H.; Pires e Cruz, M.S.; Santos, A.L.S.; Sangenito, L.S. Trendings of amphotericin B-loaded nanoparticles as valuable chemotherapeutic approaches against leishmaniasis. In Applications of Nanobiotechnology for Neglected Tropical Diseases; Elsevier: Amsterdam, The Netherlands, 2021; pp. 291–327. [Google Scholar] [CrossRef]
- Ennes-Vidal, V.; Menna-Barreto, R.F.; Santos, A.L.; Branquinha, M.H.; d’Avila-Levy, C.M. Effects of the calpain inhibitor MDL28170 on the clinically relevant forms of Trypanosoma cruzi in vitro. J. Antimicrob. Chemother. 2010, 65, 1395–1398. [Google Scholar] [CrossRef]
- Ennes-Vidal, V.; Menna-Barreto, R.F.; Santos, A.L.; Branquinha, M.H.; d’Avila-Levy, C.M. MDL28170, a calpain inhibitor, affects Trypanosoma cruzi metacyclogenesis, ultrastructure and attachment to Rhodnius prolixus midgut. PLoS ONE 2011, 6, e18371. [Google Scholar] [CrossRef]
- Oliveira, S.S.; Gonçalves, D.S.; Garcia-Gomes, A.D.; Gonçalves, I.C.; Seabra, S.H.; Menna-Barreto, R.F.; Lopes, A.H.; D’Avila-Levy, C.M.; Santos, A.L.; Branquinha, M.H. Susceptibility of Phytomonas serpens to calpain inhibitors in vitro: Interference on the proliferation, ultrastructure, cysteine peptidase expression and interaction with the invertebrate host. Memórias Inst. Oswaldo Cruz 2017, 112, 31–43. [Google Scholar] [CrossRef][Green Version]
- Zukfiqar, B.; Shelper, T.B.; Avery, V.M. Leishmaniasis drug discovery: Recent progress and challengesin assay development. Drug Discov. Today. 2017, 22, 1516–1531. [Google Scholar] [CrossRef]
- Donkor, I.O. An updated patent review of calpain inhibitors (2012–2014). Expert Opin. Ther. Pat. 2015, 25, 17–31. [Google Scholar] [CrossRef]
- García-Hernández, R.; Manzano, J.I.; Castanys, S.; Gamarro, F. Leishmania donovani develops resistance to drug combinations. PLoS Negl. Trop. Dis. 2012, 6, e1974. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branquinha, M.H.; Araújo, P.S.S.; Oliveira, S.S.C.; Sangenito, L.S.; Gonçalves, D.S.; Seabra, S.H.; d’Avila-Levy, C.M.; Santos, A.L.S. Antileishmanial Efficacy of the Calpain Inhibitor MDL28170 in Combination with Amphotericin B. Trop. Med. Infect. Dis. 2022, 7, 29. https://doi.org/10.3390/tropicalmed7020029
Branquinha MH, Araújo PSS, Oliveira SSC, Sangenito LS, Gonçalves DS, Seabra SH, d’Avila-Levy CM, Santos ALS. Antileishmanial Efficacy of the Calpain Inhibitor MDL28170 in Combination with Amphotericin B. Tropical Medicine and Infectious Disease. 2022; 7(2):29. https://doi.org/10.3390/tropicalmed7020029
Chicago/Turabian StyleBranquinha, Marta H., Pedro S. S. Araújo, Simone S. C. Oliveira, Leandro S. Sangenito, Diego S. Gonçalves, Sérgio H. Seabra, Claudia M. d’Avila-Levy, and André L. S. Santos. 2022. "Antileishmanial Efficacy of the Calpain Inhibitor MDL28170 in Combination with Amphotericin B" Tropical Medicine and Infectious Disease 7, no. 2: 29. https://doi.org/10.3390/tropicalmed7020029
APA StyleBranquinha, M. H., Araújo, P. S. S., Oliveira, S. S. C., Sangenito, L. S., Gonçalves, D. S., Seabra, S. H., d’Avila-Levy, C. M., & Santos, A. L. S. (2022). Antileishmanial Efficacy of the Calpain Inhibitor MDL28170 in Combination with Amphotericin B. Tropical Medicine and Infectious Disease, 7(2), 29. https://doi.org/10.3390/tropicalmed7020029







