Implementing a Substance-Use Screening and Intervention Program for People Living with Rifampicin-Resistant Tuberculosis: Pragmatic Experience from Khayelitsha, South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Setting
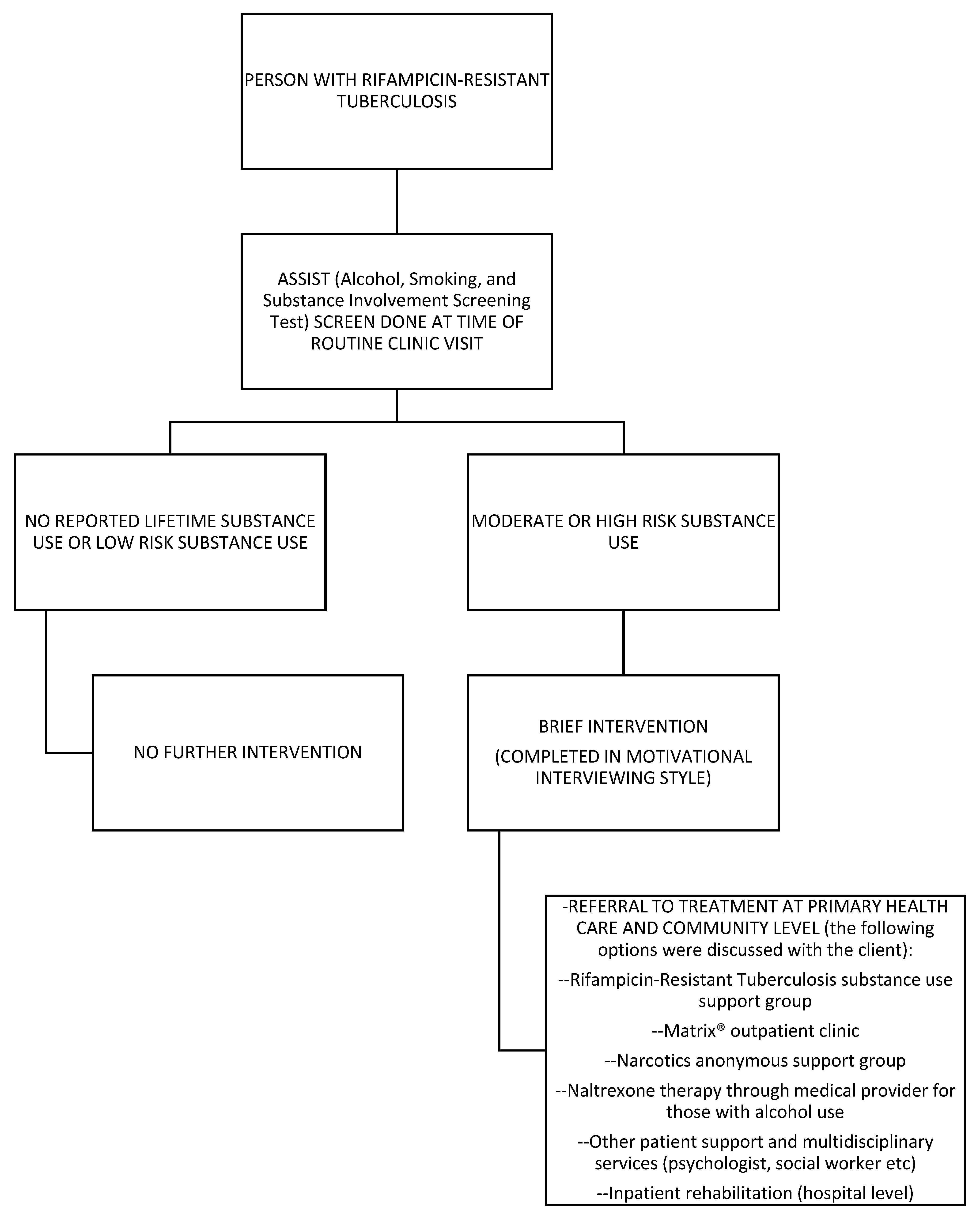
2.3. Screening, Brief Intervention, and Referral to Treatment (SBIRT) Description
2.3.1. Screening
2.3.2. Brief Intervention
2.3.3. Referral/Treatment
2.4. Follow Up Care
2.5. Outcome Measures and Definitions
2.6. Data Collection and Analysis
2.7. Ethics
3. Results
3.1. Clinical and Demographic Characteristics Based on Substance-Use (SU) Screening
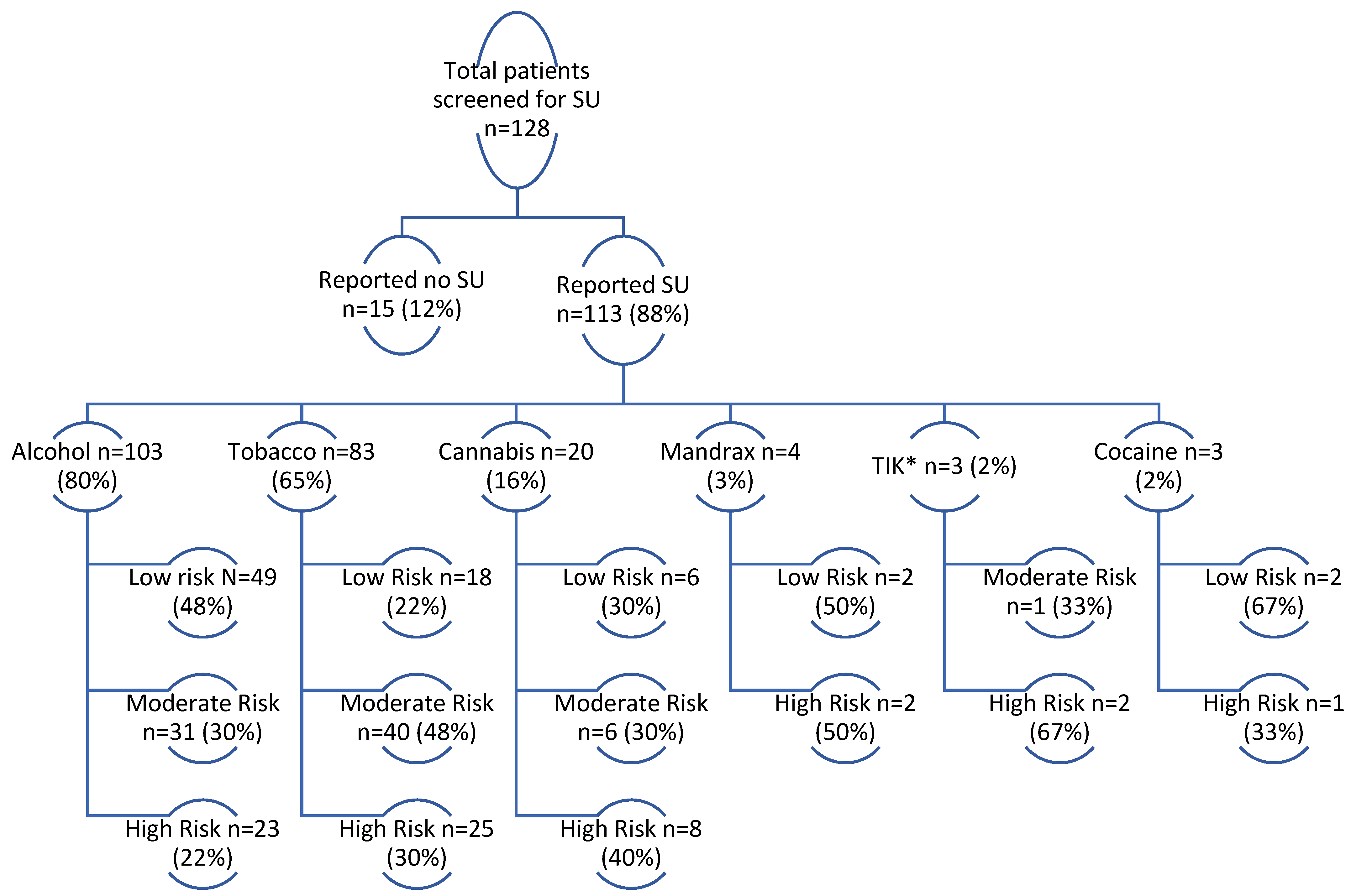
3.2. Substance Use Screening: Substances of Use and Risk Classification
3.3. Clinical and Demographic Characteristics Based on Highest Risk Classification
3.4. Rifampicin-Resistant Tuberculosis (RR-TB) Treatment Outcomes among Patients Screened for Substance Use Disorder within 2 Months of RR-TB Treatment Initiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Global Tuberculosis Report 2021. Available online: https://www.who.int/publications/i/item/9789240037021 (accessed on 1 November 2021).
- Ahmad, N.; Ahuja, S.D.; Akkerman, O.W.; Alffenaar, J.C.; Anderson, L.F.; Baghaei, P.; Bang, D.; Barry, P.M.; Bastos, M.L.; Behera, D.; et al. Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB treatment–2017. Lancet 2018, 392, 821–834. [Google Scholar] [CrossRef]
- Law, S.; Daftary, A.; O’Donnell, M.; Padayatchi, N.; Calzavara, L.; Menzies, D. Interventions to improve retention-in-care and treatment adherence among patients with drug-resistant tuberculosis: A systematic review. Eur. Respir. J. 2018, 53, 1801030. [Google Scholar] [CrossRef] [PubMed]
- Shean, K.; Streicher, E.; Pieterson, E.; Symons, G.; van Zyl-Smit, R.; Theron, G.; Lehloenya, R.J.; Padanilam, X.; Wilcox, P.; Victor, T.C.; et al. Drug-Associated Adverse Events and Their Relationship with Outcomes in Patients Receiving Treatment for Extensively Drug-Resistant Tuberculosis in South Africa. PLoS ONE 2013, 8, e63057. [Google Scholar] [CrossRef]
- Morris, M.D.; Quezada, L.; Bhat, P.; Moser, K.; Smith, J.; Perez, H.; Laniado-Laborin, R.; Estrada-Guzman, J.; Rodwell, T.C. Social, economic, and psychological impacts of MDR-TB treatment in Tijuana, Mexico: A patient’s perspective. Int. J. Tuberc. Lung Dis. 2013, 17, 954–960. [Google Scholar] [CrossRef]
- Isaakidis, P.; Rangan, S.; Pradhan, A.; Ladomirska, J.; Reid, T.; Kielmann, K. ‘I cry every day’: Experiences of patients co-infected with HIV and multidrug-resistant tuberculosis. Trop. Med. Int. Health 2013, 18, 1128–1133. [Google Scholar] [CrossRef]
- Gelmanova, I.; Keshavjee, S.; Golubchikova, V.; Berezina, V.; Strelis, A.; Yanova, G.; Atwood, S.; Murray, M. Barriers to successful tuberculosis treatment in Tomsk, Russian Federation: Non-adherence, default and the acquisition of multidrug resistance. Bull. World Health Organ. 2007, 85, 703–711. [Google Scholar] [CrossRef]
- Holtz, T.H.; Lancaster, J.; Laserson, K.F.; Wells, C.D.; Thorpe, L.; Weyer, K. Risk factors associated with default from multidrug-resistant tuberculosis treatment, South Africa, 1999–2001. Int. J. Tuberc. Lung Dis. 2006, 10, 649–655. [Google Scholar]
- Baddeley, A. A Systematic Literature Review to Assess the Impact of Alcoholism on Tuberculosis Control and Strategies to Encourage Compliance Amongst Alcoholic TB Patients. Master’s Thesis, University of London, London, UK, 2008. [Google Scholar]
- Kendall, E.A.; Theron, D.; Franke, M.F.; Van Helden, P.; Victor, T.C.; Murray, M.B.; Warren, R.M.; Jacobson, K.R. Alcohol, Hospital Discharge, and Socioeconomic Risk Factors for Default from Multidrug Resistant Tuberculosis Treatment in Rural South Africa: A Retrospective Cohort Study. PLoS ONE 2013, 8, e83480. [Google Scholar] [CrossRef]
- Ngouleun, W.; Nya, P.C.B.; Pieme, A.C.; Telefo, P.B.; Williams, N.; Prosper, B.N.; Anatole, P.C.; Bruno, T.P. Risk assessment of hepatotoxicity among tuberculosis and human immunodeficiency virus/AIDS-coinfected patients under tuberculosis treatment. Int. J. Mycobacteriol. 2016, 5, 482–488. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Brief Intervention: The ASSIST-Linked Brief Intervention for Hazardous and Harmful Substance Use: Manual for Use in Primary Care. Available online: https://www.who.int/publications/i/item/the-assist-linked-brief-intervention-for-hazardous-and-harmful-substance-use (accessed on 1 November 2021).
- World Health Organization (WHO). Management of Substance Dependence Review Series: A Systematic Review of Opioid Antagonists for Alcohol Dependence. Available online: https://www.who.int/substance_abuse/publications/en/opioid.pdf (accessed on 1 November 2021).
- Srisurapanont, M.; Jarusuraisin, N. Opioid antagonists for alcohol dependence. Cochrane Database Syst. Rev. 2005, 1, CD001867. [Google Scholar] [CrossRef]
- Cape Town City Health. City of Cape Town–2011 Census–Khayelitsha Health District. Available online: https://resource.capetown.gov.za/documentcentre/Documents/Maps%20and%20statistics/Khayelitsha%20Health%20District.pdf (accessed on 1 November 2021).
- Cox, H.; Hughes, J.; Daniels, J.; Azevedo, V.; McDermid, C.; Poolman, M.; Boulle, A.; Goemaere, E.; Van Cutsem, G. Community-based treatment of drug-resistant tuberculosis in Khayelitsha, South Africa. Int. J. Tuberc. Lung Dis. 2014, 18, 441–448. [Google Scholar] [CrossRef] [PubMed]
- University of Cape Town. A Mixed-Methods Study of the Nature and Extent of the Alcohol Trade in Khayelitsha—And Community Perceptions. Available online: https://movendi.ngo/wp-content/uploads/2020/04/khayelitsha_-_alcohol_trade_and_community_perceptions_report.pdf (accessed on 1 November 2021).
- Peltzer, K.; Phaswana-Mafuya, N. Drug use among youth and adults in a population-based survey in South Africa. S. Afr. J. Psychiatry 2018, 24, 1139. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, J.D. Evidence about the use of naltrexone and for different ways of using it in the treatment of alcoholism. Alcohol Alcohol. 2001, 36, 2–10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Health Organization (WHO). Definitions and Reporting Framework for Tuberculosis–2013 Revision. Available online: http://apps.who.int/iris/handle/10665/79199 (accessed on 1 November 2021).
- REDCap. Research Electronic Data Capture: REDCap. Available online: https://projectredcap.org/software/ (accessed on 1 November 2021).
- Jonas, D.E.; Garbutt, J.C.; Brown, J.M.; Amick, H.R.; Brownley, K.A.; Council, C.L.; Viera, A.J.; Wilkins, T.M.; Schwartz, C.; Richmond, E.M.; et al. Screening, Behavioral Counseling, and Referral in Primary Care to Reduce Alcohol Misuse. Comp. Eff. Rev. 2012, 64, 1–87. [Google Scholar]
- van der Westhuizen, C.; Malan, M.; Naledi, T.; Roelofse, M.; Myers, B.; Stein, D.J.; Lahri, S.; Sorsdahl, K. Patient outcomes and experience of a task-shared screening and brief intervention service for problem substance use in South African emergency centres: A mixed methods study. Addict. Sci. Clin. Pract. 2021, 16, 31. [Google Scholar] [CrossRef]
- Madhombiro, M.; Kidd, M.; Dube, B.; Dube, M.; Mutsvuke, W.; Muronzie, T.; Zhou, D.T.; Derveeuw, S.; Chibanda, D.; Chingono, A.; et al. Effectiveness of a psychological intervention delivered by general nurses for alcohol use disorders in people living with HIV in Zimbabwe: A cluster randomized controlled trial. J. Int. AIDS Soc. 2020, 23, e25641. [Google Scholar] [CrossRef]
- Calligaro, G.L.; de Wit, Z.; Cirota, J.; Orrell, C.; Myers, B.; Decker, S.; Stein, D.J.; Sorsdahl, K.; Dawson, R. Brief psychotherapy administered by non-specialised health workers to address risky substance use in patients with multidrug-resistant tuberculosis: A feasibility and acceptability study. Pilot Feasibility Stud. 2021, 7, 28. [Google Scholar] [CrossRef]
- Wennerstrom, A.; Hargrove, L.; Minor, S.; Kirkland, A.L.; Shelton, S.R. Integrating Community Health Workers Into Primary Care to Support Behavioral Health Service Delivery. J. Ambul. Care Manag. 2015, 38, 263–272. [Google Scholar] [CrossRef]
- Necho, M.; Tsehay, M.; Seid, M.; Zenebe, Y.; Belete, A.; Gelaye, H.; Muche, A. Prevalence and associated factors for alcohol use disorder among tuberculosis patients: A systematic review and meta-analysis study. Subst. Abus. Treat. Prev. Policy 2021, 16, 2. [Google Scholar] [CrossRef]
- Mohr-Holland, E.; Reuter, A.; Hughes, J.; Daniels, J.; Beko, B.; Makhanda, G.; De Avezedo, V.; Kock, Y.; Cox, H.; Furin, J.; et al. Correspondence regarding “Delamanid for rifampicin-resistant tuberculosis: A retrospective study from South Africa”. Eur. Respir. J. 2020, 56, 2000837. [Google Scholar] [CrossRef]
- Shin, S.; Livchits, V.; Connery, H.S.; Shields, A.; Yanov, S.; Yanova, G.; Fitzmaurice, G.M.; Nelson, A.K.; Greenfield, S.F. Tomsk Tuberculosis Alcohol Working Group Effectiveness of alcohol treatment interventions integrated into routine tuberculosis care in Tomsk, Russia. Addiction 2013, 108, 1387–1396. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Total n = 333 | Not Screened n = 205 | Screened n = 128 | p-Value | |
|---|---|---|---|---|
| Male | 195 (58.6) | 117 (57.1) | 78 (60.9) | 0.49 |
| Median Age, years | 34 (28–42) | 34 (28–42) | 35 (29–43) | 0.58 |
| Age Category, years | ||||
| <20 | 19 (5.7) | 10 (4.9) | 9 (7.0) | |
| 20–29 | 87 (26.1) | 58 (28.3) | 29 (22.7) | |
| 30–39 | 119 (35.7) | 72 (35.1) | 47 (36.7) | |
| 40–49 | 66 (19.8) | 38 (18.5) | 28 (21.9) | |
| ≥50 | 42 (12.6) | 27 (13.2) | 15 (11.7) | 0.69 |
| Disease classification | ||||
| Xpert MTB/RIF unconfirmed | 34 (10.2) | 25 (12.2) | 9 (7.0) | |
| Rifampicin-mono resistance | 77 (23.1) | 53 (25.9) | 24 (18.8) | |
| MDR including injectable resistance | 191 (57.4) | 113 (55.1) | 78 (60.9) | |
| MDR plus fluroquinolone resistance | 31 (9.3) | 14 (6.8) | 17 (13.3) | 0.051 |
| Previous TB treatment history None | 143 (42.9) | 90 (43.9) | 53 (41.4) | |
| Previous 1st line TB treatment | 162 (48.7) | 96 (46.8) | 66 (51.6) | |
| Previous 2nd line TB treatment | 28 (8.4) | 19 (9.3) | 9 (7.0) | 0.64 |
| Disease Site | ||||
| Pulmonary TB | 314 (94.3) | 194 (94.6) | 120 (93.8) | |
| Extra-Pulmonary TB | 19 (5.7) | 11 (5.4) | 8 (6.3) | 0.74 |
| Site of Treatment initiation | 288 (86.5) | 167 (81.5) | 121 (94.5) | |
| Primary Health Care Facility Hospital | 45 (13.5) | 38 (18.5) | 7 (5.5) | 0.001 * |
| HIV Positive | 226 (67.9) | 138 (67.3) | 88 (68.8) | 0.79 |
| Median CD4 count | 79 (28–239) ^ | 73 (24–217) ^ | 102 (35–247) ^ | 0.30 |
| On Antiretroviral Therapy | 218 (96.5) | 132 (95.7) | 86 (97.7) | 0.38 |
| Total Screened n = 128 | No-/Low-Risk SU n = 45 | Moderate-/High-Risk SU n = 83 | p-Value | |
|---|---|---|---|---|
| Male | 78 (60.9) | 14 (31.1) | 64 (77.1) | <0.001 * |
| Median Age, years | 35 (29–43) | 34 (25–47) | 35 (30–42) | 0.64 |
| Age Category, years | ||||
| <20 20–29 30–39 40–49 | 9 (7.0) 29 (22.7) 47 (36.7) 28 (21.9) | 5 (11.1) 13 (28.9) 11 (24.4) 6 (13.3) | 4 (4.8) 16 (19.3) 36 (43.4) 22 (26.5) | |
| >=50 | 15 (11.7) | 10 (22.2) | 5 (6.0) | 0.006 * |
| Time to SU screening | ||||
| <=2 months | 77 (60.2) | 32 (71.1) | 45 (54.2) | |
| >2 months | 51 (39.8) | 13 (28.9) | 38 (45.8) | 0.062 |
| Disease classification | ||||
| Xpert MTB/RIF unconfirmed Rifampicin-mono resistance MDR including injectable resistance | 9 (7.0) 24 (18.8) 78 (60.9) | 3 (6.7) 8 (17.8) 25 (55.5) | 6 (7.2) 16 (19.3) 53 (63.9) | |
| MDR plus fluroquinolone resistance | 17 (13.3) | 9 (20.0) | 8 (9.6) | 0.43 |
| Previous TB treatment history | ||||
| None Previous 1st line TB treatment | 53 (41.4) 66 (51.6) | 21 (46.7) 20 (44.4) | 32 (38.6) 46 (55.4) | |
| Previous 2nd line TB treatment | 9 (7.0) | 4 (8.9) | 5 (6.0) | 0.48 |
| Disease Site | ||||
| Pulmonary TB | 120 (93.8) | 42 (93.3) | 78 (94.0) | |
| Extra-pulmonary TB | 8 (6.3) | 3 (6.7) | 5 (6.0) | 0.89 |
| Site of Treatment initiation | ||||
| Primary Health Care Facility | 121 (94.5) | 41 (91.1) | 80 (96.4) | |
| Hospital | 7 (5.5) | 4 (8.9) | 3 (3.6) | 0.21 |
| HIV Positive | 88 (68.8) | 29 (64.4) | 59 (71.1) | 0.44 |
| Median CD4 count | 102 (35–247) ^ | 100 (35–238) | 107 (38–257) ^ | 0.95 |
| On Antiretroviral Therapy | 86 (97.7) | 27 (93.1) | 59 (100.0) | 0.041 * |
| Overall n = 77 n (%) | No-/Low-Risk SU n = 32 n (%) | Moderate/High Risk n = 45 n (%) | |
|---|---|---|---|
| Treatment Success | 53 (68.8) | 21 (65.6) | 32 (71.1) |
| Loss to Follow-up | 9 (11.7) | 2 (6.2) | 7 (15.6) |
| Died | 9 (11.7) | 5 (15.6) | 4 (8.9) |
| Failed by Treatment | 3 (3.9) | 3 (9.4) | 0 (0) |
| Not Evaluated | 3 (3.9) | 1 (3.1) | 2 (4.4) |
| Overall n = 33 n (%, Column) | Did not Receive Naltrexone n = 22 | Received Naltrexone n = 11 | |
|---|---|---|---|
| Treatment Success | 23 (69.7) | 16 (72.7) | 7 (63.6) |
| Loss to Follow-up | 6 (18.2) | 4 (18.2) | 2 (18.2) |
| Died | 2 (6.1) | 1 (4.5) | 1 (9.1) |
| Not Evaluated | 2 (6.1) | 1 (4.5) | 1 (9.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reuter, A.; Beko, B.; Memani, B.; Furin, J.; Daniels, J.; Rodriguez, E.; Reuter, H.; Weich, L.; Isaakidis, P.; von der Heyden, E.; et al. Implementing a Substance-Use Screening and Intervention Program for People Living with Rifampicin-Resistant Tuberculosis: Pragmatic Experience from Khayelitsha, South Africa. Trop. Med. Infect. Dis. 2022, 7, 21. https://doi.org/10.3390/tropicalmed7020021
Reuter A, Beko B, Memani B, Furin J, Daniels J, Rodriguez E, Reuter H, Weich L, Isaakidis P, von der Heyden E, et al. Implementing a Substance-Use Screening and Intervention Program for People Living with Rifampicin-Resistant Tuberculosis: Pragmatic Experience from Khayelitsha, South Africa. Tropical Medicine and Infectious Disease. 2022; 7(2):21. https://doi.org/10.3390/tropicalmed7020021
Chicago/Turabian StyleReuter, Anja, Buci Beko, Boniwe Memani, Jennifer Furin, Johnny Daniels, Erickmar Rodriguez, Hermann Reuter, Lize Weich, Petros Isaakidis, Erin von der Heyden, and et al. 2022. "Implementing a Substance-Use Screening and Intervention Program for People Living with Rifampicin-Resistant Tuberculosis: Pragmatic Experience from Khayelitsha, South Africa" Tropical Medicine and Infectious Disease 7, no. 2: 21. https://doi.org/10.3390/tropicalmed7020021
APA StyleReuter, A., Beko, B., Memani, B., Furin, J., Daniels, J., Rodriguez, E., Reuter, H., Weich, L., Isaakidis, P., von der Heyden, E., Kock, Y., & Mohr-Holland, E. (2022). Implementing a Substance-Use Screening and Intervention Program for People Living with Rifampicin-Resistant Tuberculosis: Pragmatic Experience from Khayelitsha, South Africa. Tropical Medicine and Infectious Disease, 7(2), 21. https://doi.org/10.3390/tropicalmed7020021






