Abstract
This study evaluated the in vitro and in vivo antiplasmodial efficacy and toxicity of aqueous and ethanolic extracts from traditional recipes used in Thailand. The aqueous and ethanolic extracts of ten traditional recipes were tested for in vitro antiplasmodial activity (parasite lactate dehydrogenase assay), cytotoxicity (MTT assay), and hemolysis). Oxidant levels were measured using cell-permeable probe 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate fluorescent dye-based assays. The best candidate was chosen for testing in mouse models using 4-day suppressive and acute toxicity assays. An in vitro study showed that ethanolic extracts and three aqueous extracts exhibited antiplasmodial activity, with an IC50 in the range of 2.8–15.5 µg/mL. All extracts showed high CC50 values, except for ethanolic extracts from Benjakul, Benjalotiga, and Trikatuk in HepG2 and Benjalotiga and aqueous extract from Chan-tang-ha in a Vero cell. Based on the results of the in vitro antiplasmodial activity, an aqueous extract of Triphala was chosen for testing in mouse models. The aqueous extract of Triphala exhibited good antiplasmodial activity, was safe at an oral dose of 2 g/kg, and is a potential candidate as a new source for the development of antimalarial drugs.
1. Introduction
The malaria burden has an impact around the world and is the heaviest, according to the World Health Organization (WHO). The African region accounted for approximately 95% of all cases and 96% of all deaths in 2020 [1]. There are five Plasmodium species that cause devastation to human life—P. falciparum, P. vivax, P. malariae, P. ovale, and P. knowlesi. A key feature of these infections is their ability to invade erythrocytes [2]. Sequestered parasites can lead to impaired blood flow and induce negative effects on critical organs [3]. However, effective tools for malaria control and elimination rely on potent antimalarial agents. The WHO recommends six artemisinin combinations (ACTs) as first-line treatment for uncomplicated falciparum malaria: artemether-lumefantrine, artesunate-amodiaquine, artesunate-mefloquine, artesunate-sulfadoxine-pyrimethamine, dihydroartemisinin-piperaquine, and artesunate-pyronaridine [4]. Blood-stage malaria plays a crucial role in the development of symptoms and clinical complications and can be terminated by blood schizonticides. Unfortunately, artemisinin and partner drugs slow parasite clearance along the Thai-Cambodian and Thai-Myanmar borders and spread to other areas [5]. The emergence of resistance makes malaria control and elimination challenging, highlighting the need for novel drug strategies. Traditional herbal medicine comprises pharmaceutical agents and is still commonly used to reduce the risk of disease in rural areas around the world [6]. The reasons for the use of herbal medicines are their safety, effectiveness, cultural preferences, inexpensiveness, and easy availability [7]. Today, traditional healers are still active, play a role in healing health in Thai society, and can be found in all parts of Thailand [6,8]. The concept of traditional therapeutic herbal strategies is based on two principles that involve single and polyherbal (herbal recipe) methods [9]. Even though individual herbs confer some benefits, herbal recipes evidently provide extra therapeutic effects from positive synergistic interaction [9].
In Ayurveda literature, one of the traditional medicinal systems in India, plant combinations are chosen rather than individual plants because of their ability to produce a greater result of varying potency [9]. Triphala and Trikatuk are traditional herbal recipes widely used in Ayurvedic and Thai traditional medicine. Triphala is commercially available and is recommended to treat several health ailments, such as fever, cough, asthma, jaundice, anemia, inflammation, cardiovascular disorders, and liver dysfunction [10,11]. Trikatuk has been used to treat a wide range of illnesses owing to its anti-allergic, anti-inflammatory, anticholinesterase, and antioxidant effects [12,13]. Trisamo has analgesic, antipyretic, antibacterial, and antioxidant properties; promotes general health; and is used as an antipyretic in Thai traditional medicine [14]. Jatu-phala-tiga possesses strong free-radical-scavenging properties [15]. Benjakul is a Thai herbal recipe on the Thailand National List of Essential Medicines. This recipe possesses anti-allergic, anti-inflammatory, and anticancer activities [16]. In Thai traditional medicine, Benjalotiga, Gaysorn-tang-ha, Benjathian, Benjagot, and Chan-tang-ha recipes are widely used in primary health care and associated with potential health benefits such as antipyretic, cardio-tonic, and hematic tonic prescriptions.
Interestingly, the multiple health-promoting properties of herbal medicines have inspired us to discover the elements as key antimalarial agents, and ten existing traditional medicines used in Thailand have not yet been investigated for their antimalarial activities. Therefore, the aim of this study was to evaluate the antiplasmodial activities of ten traditional recipes against P. falciparum infection in in vitro cultures and assess the antimalarial activity and acute toxicity of a good candidate in mouse models.
2. Materials and Methods
2.1. Herbal Material and Traditional Recipe
The 32 plants shown in Table 1 were authorized by a botanist after being obtained from a traditional Thai drug store in Muang District, Nakhon Si Thammarat Province, southern Thailand. The plant identification was performed using morphological characteristics and also confirmed by comparison with the herbarium specimens. The authorization for plant materials complied with the relevant guidelines and regulations of the Plant Varieties Protection, Department of Agriculture, Ministry of Agriculture and Cooperatives, Thailand. Voucher specimens were identified and deposited at the Department of Medical Sciences, School of Medicine, Walailak University, Thailand. The plants were washed with tap water and then dried in a hot air oven (Memmert, Model; SFE600, Schwabach, Germany). The plant parts were ground into a fine powder using a grinder (Taizhou Jincheng Pharmaceutical Machinery Co., Ltd., Model; SF, Jiangsu, China). The particle size of the powdered plant was 2.36 mm. Ten traditional herbal recipes were prepared by blending equal portions of the ingredients [17,18]. The final weight was made at 60 g to achieve an adequate quantity of crude extract.

Table 1.
List of traditional recipes.
2.2. Preparation of Crude Extract
The extraction process was performed as previously described [19]. The maceration method was performed to prepare ethanolic extracts. Sixty grams of the powdered recipe was extracted for 72 h in 600 mL of 80% ethanol at 25 °C. The decoction method was used to make aqueous extracts. Sixty grams of each herb powder was extracted three times by mixing with 600 mL of distilled water and allowed to boil for 30 min. Subsequently, the liquid portion was separated from the residue using filter paper (Whatman, Buckinghamshire, England). The marcs of ethanolic and aqueous extract were re-extracted twice, with 600 mL of solvents each time. The filtrates were combined and concentrated using a rotary evaporator (Rotavapor, Buchi, China) at 45 rpm and 45 °C. Further drying was performed in a freeze-drying machine at −89 °C (Martin Christ, Germany). The crude extracts were stored in a refrigerator until further use.
2.3. Phytochemical Analysis
Twenty different extracts were tested for phytochemicals according to standard procedures, as previously described [20,21]. Qualitative phytochemical screening was performed for flavonoids, terpenoids, alkaloids, tannins, anthraquinone, cardiac glycosides, saponins, and coumarins.
2.4. In Vitro Culture of Plasmodium Parasites
K1 chloroquine-resistant P. falciparum strain was used in this study. The cryopreserved parasite-infected blood was thawed using a 12% and 1.6% NaCl concentration gradient. The culture of the parasite was slightly modified from the original methods developed by Trager and Jensen [22]. It was cultured in a T-75 flask containing 2% human O+ erythrocytes, base media of RPMI-1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 2 mg/mL NaHCO3, 4.8 mg/mL HEPES (Himedia, Mumbai, India), 10 μg/mL hypoxanthine (Sigma-Aldrich, New Delhi, India), 2.5 μg/mL gentamicin (Sigma-Aldrich, New Delhi, India), and 0.5% albumax II (Gibco MA, USA) in a saturated atmosphere of 5% CO2 at 37 °C. In order to assess parasitemia and developmental stages, a thin blood smear was stained with Giemsa dye, and intracellular parasites were visualized under an oil immersion lens (100×) using a light microscope (Olympus CX31, Model CX31RBSFA, Tokyo, Japan).
2.5. Cell Culture and Maintenance
The HepG2 cells obtained from the ATCC cell bank (HB-8065™) and Vero cells (Elabscience, Wuhan, Hubei, China) were individually cultured in DMEM media (Gibco, Carlsbad, CA, USA) supplemented with 10% FBS (Sigma-Aldrich, New Delhi, India), 1% (v/v) penicillin/streptomycin (Sigma-Aldrich, St Louis, MO, USA) at 37 °C in an atmosphere of 5% CO2. The cell confluence and morphology were observed using a phase-contrast inverted microscope (Olympus, Model CK X31, Hicksville, NY, USA). Once the cells reached approximately 80% confluence, 2.5% trypsin-EDTA (Gibco, Carlsbad, CA, USA) was used to detach cells before subculturing.
2.6. Detection of Parasite Lactate Dehydrogenase (pLDH) Activity
A stock solution (20 mg/mL) of the aqueous extracts was prepared by dissolving the extract in phosphate-buffered saline (PBS), whereas the ethanolic extracts were dissolved in DMSO (dimethyl sulfoxide). A two-fold serial dilution was prepared using PBS (aqueous extracts) or DMSO (ethanolic extracts), covering a range of final concentration from 1.56 to 100 µg/mL. The antiplasmodial activity was determined by the concentration of the extracts that inhibited 50 percent of the parasite growth (IC50) by the measurement of pLDH activity, as previously described [23]. The crude extract (1µL) was incubated with 1% parasitized red blood cells (pRBCs) and 2% hematocrit (199 µL) in a 96-well cell culture plate (SPL Life Sciences, Pocheon-si, Gyeonggi-do, Korea) in an atmosphere of 5% CO2 at 37 °C for 72 h. The test was performed in triplicate for each concentration. Artesunate (Sigma, St Louis, MO USA) was used as the positive control (final concentration ranging from 1.56 to 100 ng/mL). PBS and DMSO were used as negative controls. Non-infected red blood cells served as blank controls. The suspension was frozen at −80 °C and thawed at 37 °C to lyse red cell pellets. The pLDH enzyme was detected by the reaction between 20 µL of their contents from the released red cells, 100 µL of malstate reagent, and 20 µL of nitroblue tetrazolium/phenazine ethosulfate solution (Calbiochem, Sigma-Aldrich, New Delhi, India) in a new 96-well plate flat bottom. The absorbance was measured at 650 nm (Biotek Eon, Winooski, VT, USA) after the reaction was incubated in the dark for an hour. The percent inhibition was calculated compared to the negative control after subtraction of the background using the following formula:
% inhibition = 100 × [(OD negative well − OD sample well)/OD negative well]
IC50 was determined for each sample by plotting % growth as a function of concentration and estimating the concentration which caused 50% growth inhibition.
2.7. Cytotoxicity Assessment by 3-[4,5-Dimethylthiazol-2-yl]-2,5 diphenyl Tetrazolium Bromide (MTT) Assay
Crude extracts were evaluated for toxicity at concentrations ranging from 12.5 to 800 µg/mL. Serial dilutions were prepared using a 160 mg/mL stock solution. Cell cultures at a density of 104 cells/well in 200 μL culture medium were seeded in a 96-well cell culture plate and then incubated at 37 °C for 24 h in an atmosphere of 5% CO2 until they reached confluence. HepG2 and Vero cell lines (199 µL) were treated with different concentrations of the extract (1 µL), with doxorubicin (final concentration ranging from 0.3 to 20 µg/mL) (Sigma-Aldrich, New Delhi, India) as a positive control. DMSO and PBS served as negative controls for ethanolic and aqueous extracts. Treated cells were incubated in an incubator at 37 °C for 48 h. The assays were performed in triplicates. MTT reagent (5 mg/mL) was added to each well at the end of the exposure period and then incubated at 37 °C for 3 h. Subsequently, the reagent was removed and replaced with 100 µL of DMSO. The absorbance was read at a wavelength of 590 nm using a microplate reader. The percent cytotoxicity was calculated to determine the cytotoxicity as follows:
% cytotoxicity = 100 − [100 × (OD sample well/OD negative well)]
The data in this assay are presented as the 50% cytotoxicity concentration (CC50), as determined by regression analysis using GraphPad Prism 6.
2.8. Hemolysis Measurement
The toxicity of crude extracts in human erythrocytes was evaluated by monitoring the hemolysis of the red cell suspension. Venous blood was drawn from healthy donors into EDTA-blood collection tubes, and the red cell pellet was washed three times with PBS. The plasma and buffy coat were discarded after centrifugation at 3000 rpm for 5 min. Cell suspension at 2% hematocrit was incubated with 50 µg/mL of the extracts in a final volume of 0.2 mL in an incubator at 37 °C for 72 h. Triton X-100 (Sigma-Aldrich, New Delhi, India) was used as a positive control. DMSO and PBS were used as negative controls. The plate was centrifuged at 3000 rpm for 5 min, and the supernatant was transferred to a new 96-well plate. The release of hemoglobin was measured at 570 nm, and the percentage of hemolysis was calculated as follows:
% hemolysis = [100 × (OD sample well − OD negative well)/(OD positive well − OD negative well)]
2.9. Measurement of Intracellular Oxidant in pRBCs
Oxidant generation was measured using the cell-permeable probe 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) (Invitrogen, Carlsbad, CA, USA). In this study, parasite culture at 1% parasitemia and 2% hematocrit was incubated with the presence of proximity IC50 concentration of drug or crude extracts in a 96-well plate for 72 h. Artesunate was used as the positive control. Negative controls were obtained using 0.5% DMSO and PBS. pRBCs were labeled with a fluorescence probe after disposal of the medium. The pellet was incubated in the dark with 100 µL CM-H2DCFDA solution at a final concentration of 10 mM for 30 min. Labeled cells were imaged using a Leica TCS SP5 confocal microscope (Leica, Mannheim, Germany) at an excitation/emission wavelength of 488/520 nm. In order to measure oxidant levels, five fields of each sample were acquired, and two hundred and twenty-five cells per sample were used for analysis. The integrated density, cell area, and background fluorescence were measured using the free software ImageJ Fiji. Corrected total cell fluorescence (CTCF), in terms of normalized values, was calculated as follows:
CTCF = integrated density − (area of selected cell × mean fluorescence of background readings).
The relative change was obtained by the division of CTCF from negative controls.
2.10. In Vivo Antimalarial Activity Model
The antimalarial efficacy of the crude extract against early malarial infection was evaluated using Peters’ 4-day suppressive test [24]. Male ICR mice (n = 25) aged 6–8 weeks were purchased from Nomura Siam International Co., Ltd., Bangkok, Thailand. They were randomly divided into five groups of five mice each. Group I was treated with PBS. Group II was treated with 25 mg/kg of chloroquine as the standard drug. Groups III, IV, and V received the aqueous extract of Triphala at doses of 200, 400, and 600 mg/kg, respectively. The animals were acclimatized for a week in the presence of food pellets and clean drinking water ad libitum before initiating the experiments. The Plasmodium berghei (P. berghei) ANKA strain (chloroquine-sensitive) was contributed by Thomas F. McCutchan and obtained from BEI Resources, NIAID, NIH. Mice infected with P. berghei were used as a donor, and then 25 mice were injected with 0.2 mL of 107 infected red cells per milliliter through the intraperitoneal route. Oral administration started at 3 h post-infection, followed by 24, 48, and 72 h daily. On day 4, blood samples from each mouse were collected to prepare a thin blood film and stained with Giemsa solution. The percentages of parasitemia and suppression were calculated using the following formulas:
% parasitemia = (number of infected red blood cells/number of total red blood cells) × 100
% suppression = [(mean parasitemianegative control − mean parasitemiaexperimental group)/mean parasitemianegative control] × 100
2.11. Acute Toxicity Test
The test was conducted according to the Organization for Economic Co-operation and Development (OECD) guidelines for testing chemicals, with a limit test at a dose of 2 g/kg (1). Ten male ICR mice were randomly divided into two groups: PBS solution and extract solution. A single oral dose was administered directly to the stomach through a feeding tube. Abnormalities were observed within the first 30 min and once daily for 14 days after administration. The parameters that were observed for signs of toxicity included general activity, body weight changes, tremors, convulsions, ataxia, diarrhea, urination, changes in skin fur, and death. On the last day, mice were anesthetized with 2% isoflurane and then euthanized by cardiac puncture after opening the thorax cavity. Blood samples were collected and placed into serum clot activator tubes for the analysis of liver and kidney enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), blood urea nitrogen (BUN), and creatinine. The analysis was performed using an AU480 chemistry analyzer (Beckman Coulter, USA). In addition, histological alterations of the liver and kidneys were performed using hematoxylin and eosin staining as previously described [25]. The relative weights of the liver and kidneys were calculated using the following formula:
Relative organ weight = (organ weight/body weight) × 100
2.12. The Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry (LC-QTOF-MS) Analysis
Product profiling of the crude extract was performed using an LC-QTOF-MS instrument (1290 Infinity II LC-6545 Quadrupole-TOF, Agilent Technologies, Santa Clara, CA, USA). Chromatographic separation was performed on a Zorbax Rapid Resolution HD Eclipse Plus C18 column (150 mm length × 2.1 mm inner-diameter, particle size 1.8 μm) from Agilent (Agilent, Waldbronn, Germany). The elution gradient was performed with 0.1% formic acid water (mobile phase A) and acetonitrile (mobile phase B) at a flow rate of 0.20 mL/min. The column was equilibrated (A: B; v/v) at 90:10 (1 min), and elution was carried out with the following steps; 80:20 (from 1 to 12 min), 75:25 (from 12 to 20 min), 70:30 (from 20 to 25 min), 65:35 (from 25 to 28 min), 60:40 (from 28 to 38 min), and 90:10 (from 38 to 45 min). The column temperature was set to 25 °C. The instrument was set to an MS range of 100–1200 m/z in both negative and positive modes. The injection volume was 2 μL. Data acquisition was controlled using the MassHunter WorkStation Qualitative Analysis Workflows V8 software (Agilent Technologies, Santa Clara, CA, USA). Compounds were identified by comparing retention times, mass data, and fragmentation patterns with a compound database in the library search of the Mass Hunter METLIN database (Agilent Technologies). The peak with similarity scores of 90% compared to the database was selected to confirm peak identification.
2.13. Statistical Analysis
The data are expressed as the mean ± standard error of the mean. The relative CTCF was tested for normality before statistically assessing differences with an independent t-test; p < 0.05 was considered significant using SPSS for Microsoft Windows (version 17.0; IBM, Armonk, NY, USA). Statistical analysis of in vivo studies was performed using one-way analysis of variance at p < 0.05 after the values showed a normal distribution using SPSS for Microsoft Windows version 17.0.
3. Results
3.1. Percentage Yield of Crude Plant Material
The percentage yield of each extract obtained from the dried crude extract divided by the initial amount of powdered plant part is presented in Table 2. The maximum extract yield (40.12%) was obtained from the aqueous extract of the Jatu-phala-tiga recipe, whereas the minimum yield (2.07%) was recorded for the ethanolic extract of the Benjatian recipe. The yield of aqueous extracts from Benjalotiga and Chan-tang-ha was lower than that of the ethanolic extract; however, other extracts exhibited a higher percentage yield than that of the ethanolic extract.

Table 2.
Percentage extraction yields of aqueous and ethanolic extracts.
3.2. Phytochemical Profile
Qualitative analysis of the ten traditional recipes (aqueous/ethanolic extracts) revealed different phytocomponents, which are displayed in Table 3 and Figure 1. All extracts, except the ethanolic extracts from Trikatuk, Benjalotiga, Benjakul, and Chan-tang-ha, contained tannins. A majority of terpenoids were found in all extracts, excluding the aqueous extract from Benjagot. Anthraquinones and cardiac glycosides were not detected. Saponins were mainly found in the aqueous extracts.

Table 3.
Phytochemical results of the ten traditional recipes (aqueous/ethanolic extracts).
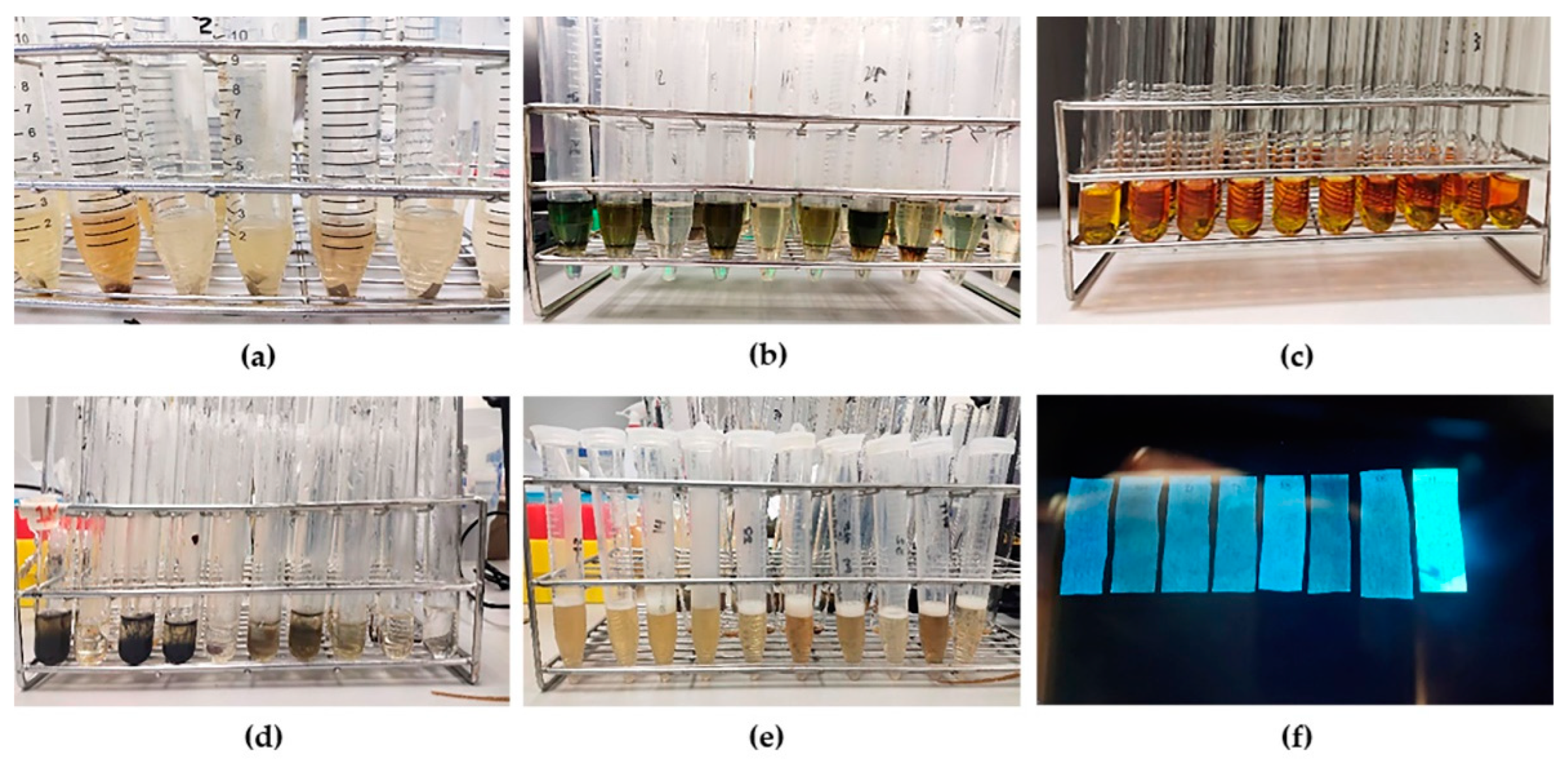
Figure 1.
Qualitative phytochemical screening (a) screening for flavonoid, (b) screening for terpenoid, (c) screening for alkaloid, (d) screening for tannin, (e) screening for saponin, (f) screening for coumarin.
3.3. In Vitro Antiplasmodial Activity
The antiplasmodial activities of the ten traditional recipes are shown in Table 4. According to these criteria of in vitro antiplasmodial assessment of products derived from plants [26], extracts with IC50 < 5 µg/mL are considered as exhibiting potent antiplasmodial activity, IC50 between 5–15 µg/mL and 15–50 µg/mL as good and moderate activity, respectively, and IC50 > 100 µg/mL as inactive. Crude ethanolic extracts from Gaysorn-tang-ha, Trikatuk, and Triphala exhibited potent activity against the P. falciparum K1 strain with IC50 values of 2.81, 4.37, and 4.39 µg/mL, respectively. The ethanolic extract of Benjatian revealed moderate antiplasmodial activity (IC50 = 15.5 µg/mL), and all other extracts showed good activity with IC50 ranging from 6.8–8.5 µg/mL. On the other hand, most of the aqueous extracts showed no activity (IC50 > 5 µg/mL). Interestingly, aqueous extract from Jatu-phala-tiga had a potent activity with an IC50 of 5.0 µg/mL, and extracts from Triphala and Trisamo expressed good activity with IC50 of 5.7 and 6.1 µg/mL, respectively.

Table 4.
IC50 values for antiplasmodial activity, CC50 value against Hep G2 and Vero cell lines and selectivity index (SI) of ten traditional recipes.
3.4. In Vitro Cytotoxicity on HepG2 and Vero Cells
The cytotoxic effects of the ten traditional recipes against HepG2 and Vero cells are shown in Table 4. A benchmark of toxicity levels based on the National Cancer Institute criteria was used. Recipes with CC50 values <30 µg/mL are considered cytotoxic after 48–72 h of exposure [27]. The results of PBS and 0.5% DMSO treated cells were considered 100% of living cells for aqueous and ethanolic extracts. The cytotoxic effects on HepG2 cells revealed that most of the extracts had CC50 values greater than the specified criteria. Only three extracts showed cytotoxicity with a CC50 of <30 μg/mL, indicating mild cytotoxicity [28]. The ethanolic extracts from Benjakul, Benjalotiga, and Trikatuk exhibited CC50 values of 10.9, 20.1, and 26.5 μg/mL, respectively. A comparison between the effects of aqueous and ethanolic extracts on HepG2 cells showed that the ethanolic extract had a greater toxic effect. The cytotoxic effects on Vero cells demonstrated that ethanolic extract from Chan-tang-ha is more toxic compared to that of the others, which produced CC50 value <12.5 μg/mL, and Benjalotiga had toxic effects with CC50 of 15.3 μg/mL for aqueous and 20.5 μg/mL for ethanolic extracts.
3.5. Interpretation of Selectivity Index (SI)
The SI was used to calculate risk-benefit assessment and identify progressible extracts that measure the ratio between 50% toxic concentration (CC50) and 50% antiplasmodial concentration (IC50) [29]. An extract with values <1 could be toxic and not applicable; however, a high value will give active extracts without undue risk. As shown in Table 4, both aqueous and ethanolic extracts of Triphala, Trisamo, and Jatu-phala-tiga showed high values of >27 in both HepG2 and Vero cells. The ethanolic extracts of Gaysorn-tang-ha exhibited high SI values above 20 in HepG2 cells, whereas the ethanolic extracts of Benjathian, Benjagot, and Chan-tang-ha showed high SI values in Vero cells. In addition, the ethanolic extract from other extracts exhibited values greater than that of the aqueous extract in both cell lines.
3.6. Hemolysis Results
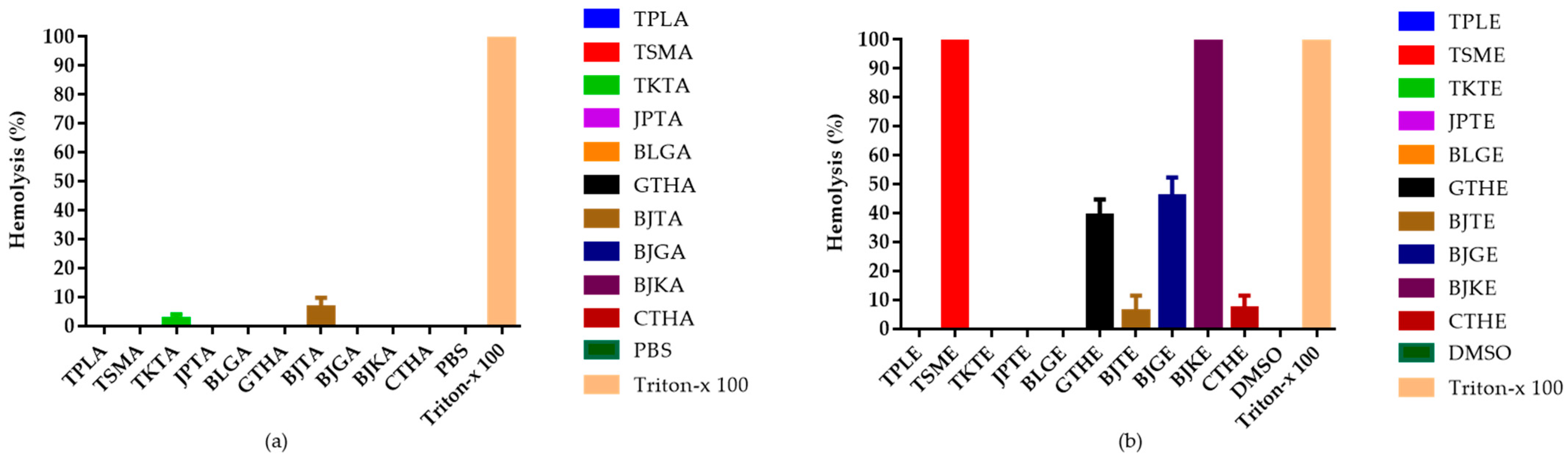
The hemolytic effects of the ten recipes were determined to indicate hematotoxicity in human erythrocytes, and the results are shown in Figure 2. Triton X-100 (0.5% v/v) was used for complete hemolysis of blood cells, which was then compared with the samples. Cell lysis was measured as the amount of free hemoglobin in the red cell suspension. Aqueous extracts at a fixed dose of 50 μg/mL did not show lytic effects on erythrocytes, except for Benjathian (6.53%) and Trikatuk (2.46%), whereas some ethanolic extracts showed hemolytic effects. The effect of Trisamo and Benjakul recipes reached 100% hemolysis after incubation for 72 h. Benjagot, Gaysorn-tang-ha, Chan-tang-ha, and Benjathian exhibited hemolysis percentages of 45.59, 39.18, 6.09, and 5.79, respectively.
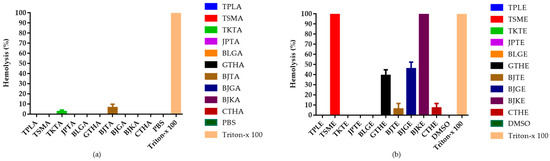
Figure 2.
Percentage of hemolysis from in vitro hemolysis assay against human erythrocyte. (a) Hemolytic effects of aqueous extracts from ten recipes at 50 µg/mL; (b) Hemolytic effects of ethanolic extracts at 50 µg/mL concentration. TPLA, aqueous extracts from Triphala; TSMA, aqueous extracts from Trisamo; TKTA, aqueous extracts from Trikatuk; JPTA, aqueous extracts from Jatu-phala-tiga; BLGA, aqueous extracts from Benjalotiga; GTHA, aqueous extracts from Gaysorn-tang-ha; BJTA, aqueous extracts from Benjathian; BJGA, aqueous extracts from Benjagot; BJKA, aqueous extracts from Benjakul; CTHA, aqueous extracts from Chan-tang-ha; TPLE, ethanolic extracts from Triphala; TSME, ethanolic extracts from Trisamo; TKTE, ethanolic extracts from Trikatuk; JPTE, ethanolic extracts from Jatu-phala-tiga; BLGE, ethanolic extracts from Benjalotiga; GTHE, ethanolic extracts from Gaysorn-tang-ha; BJTE, ethanolic extracts from Benjathian; BJGE, ethanolic extracts from Benjagot; BJKE, ethanolic extracts from Benjakul; CTHE, ethanolic extracts from Chan-tang-ha; PBS, phosphate-buffered saline; and DMSO, dimethyl sulfoxide.
3.7. Estimation of Intracellular Reactive Oxygen Species Production
CM-H2DCFDA was used to detect free radical production. The reaction of an oxidant and a CM-H2DCFDA non-fluorescent dye generates a DCF fluorescent component. Oxidant production is indicated by green fluorescence, as shown in Figure 3. Cells treated with DMSO or PBS were considered to have basal levels of oxidant generation. The positive control exhibited the highest oxidant production. The average CTCF values from positive control; aqueous extract of Trisamo; ethanolic extracts of Triphala, Trisamo, and Trikatuk recipes significantly increased ROS levels compared with the levels in negative groups at p < 0.05 by 17.06%, 15.36%, 6.26%, 9.20%, and 3.60%, respectively (Figure 4). Contrastingly, oxidant generation in the ethanolic extract group of the Benjagot recipe was significantly decreased by 0.50%.
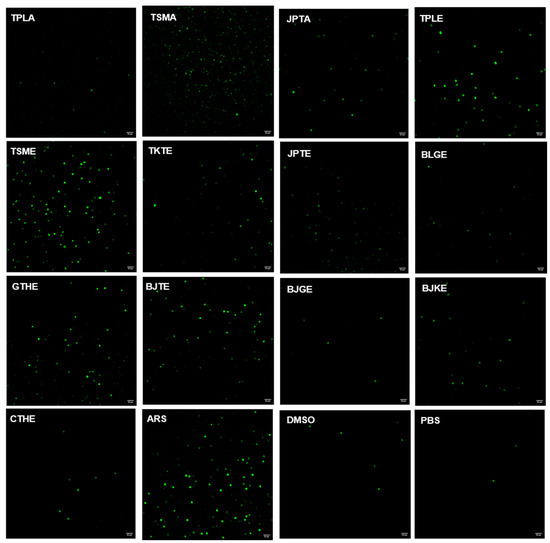
Figure 3.
Evaluation of intracellular oxidant generation in P. falciparum-infected red blood cells using CM-H2DCFDA staining; Scale bar = 20 µm., TPLA, aqueous extracts from Triphala; TSMA, aqueous extracts from Trisamo; JPTA, aqueous extracts from Jatu-phala-tiga; TPLE, ethanolic extracts from Triphala; TSME, ethanolic ex-tracts from Trisamo; TKTE, ethanolic extracts from Trikatuk; JPTE, ethanolic extracts from Ja-tu-phala-tiga; BLGE, ethanolic extracts from Benjalotiga; GTHE, ethanolic extracts from Gaysorn-tang-ha; BJTE, ethanolic extracts from Benjathian; BJGE, ethanolic extracts from Benjagot; BJKE, ethanolic extracts from Benjakul; CTHE, ethanolic extracts from Chan-tang-ha; ARS, artesunate; PBS, phosphate-buffered saline; DMSO, dimethyl sulfoxide.
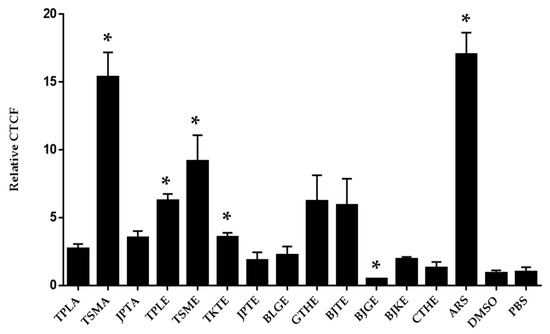
Figure 4.
Detection of oxidant levels by confocal fluorescence microscopy. Data were analyzed by independent t-test. * p < 0.05 versus the negative control groups; TPLA, aqueous extracts from Triphala; TSMA, aqueous extracts from Trisamo; JPTA, aqueous extracts from Jatu-phala-tiga; TPLE, ethanolic extracts from Triphala; TSME, ethanolic extracts from Trisamo; TKTE, ethanolic extracts from Trikatuk; JPTE, ethanolic extracts from Jatu-phala-tiga; BLGE, ethanolic extracts from Benjalotiga; GTHE, ethanolic extracts from Gaysorn-tang-ha; BJTE, ethanolic extracts from Benjathian; BJGE, ethanolic extracts from Benjagot; BJKE, ethanolic extracts from Benjakul; CTHE, ethanolic extracts from Chan-tang-ha; ARS, artesunate; PBS, phosphate-buffered saline; DMSO, dimethyl sulfoxide.
3.8. Selection of Crude Extract as a Candidate in a Mouse Model
Table 4 shows the SI values of the 20 crude extracts on the HepG2 and Vero cell lines. Nine SI values that were not determined included the values of aqueous extracts of Gaysorn-tang-ha, Benjagot, and Benjakul on both HepG2 and Vero cells, and aqueous extracts of Trikatuk, Benjatian, and Chan-tang-ha on Vero cells, because SI values cannot be calculated. The aqueous extract of Triphala showed the highest values in HepG2 cells, whereas the ethanolic extract showed a high constant value of 176.13 in Vero cells. It is imperative to review the formula used to calculate SI indices. The values of IC50 produced the same antiplasmodial activity, which was 5.7 ± 0.2 for aqueous and 4.4 ± 1.3 μg/mL for ethanolic extracts, whereas the values of cytotoxicity aqueous extract were greater than ethanolic in both HepG2 and Vero cell lines. Therefore, an aqueous extract of Triphala was selected for testing in mouse models.
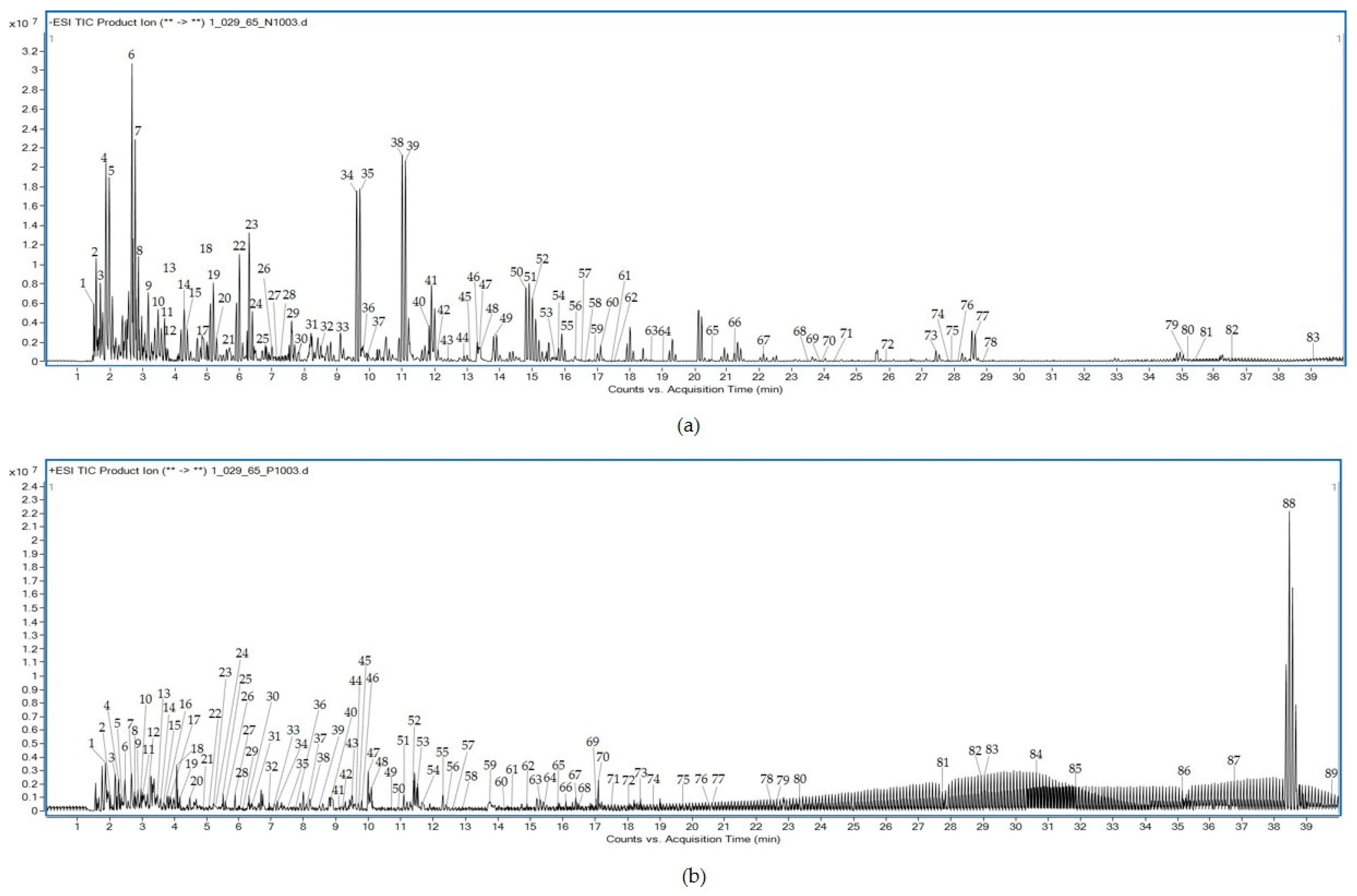
3.9. Chemical Profiling of Aqueous Extract of Triphala
A total of 83 metabolites in the negative ion mode and 89 metabolites in the positive ion mode were identified from the aqueous extract of Triphala using LC-QTOF-MS. Table 5 shows several compounds identified from the Mass Hunter METLIN database library by matching their accurate masses. The peak chromatograms are presented in Figure 5. Figure 6 and Figure 7 showed proposed fragmentation patterns in negative and positive modes, respectively.

Table 5.
Identification of the chemical constituents from aqueous extract from Triphala by LC-QTOF-MS.
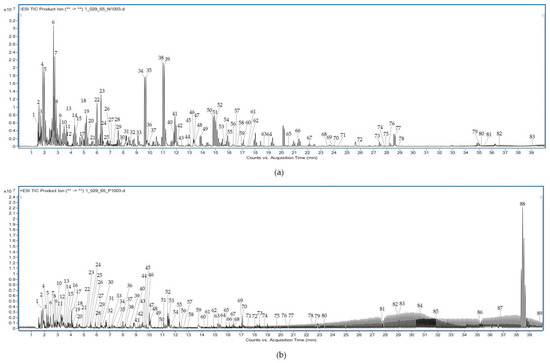
Figure 5.
LC-QTOF-MS full-scan chromatogram of aqueous extract from Triphala in negative (a) and positive (b) modes.
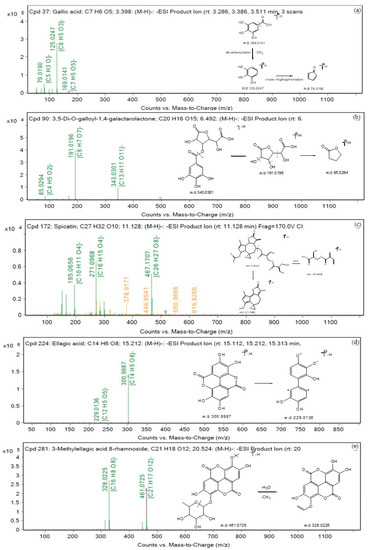
Figure 6.
Proposed fragmentation patterns in negative mode (a) gallic acid, (b) 3,5-Di-O-galloyl-1,4-galactarolactone, (c) spicatin, (d) ellagic acid, and (e) 3-Methylellagic acid 8-rhamnoside.
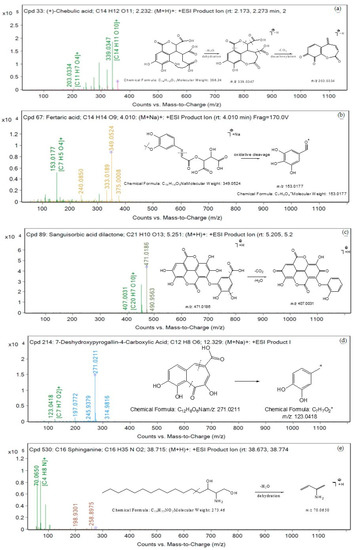
Figure 7.
Proposed fragmentation patterns in positive mode (a) chebulic acid, (b) fertaric acid, (c) sanguisorbic acid dilactone, (d) 7-deshydroxypyrogallin-4-carboxylic acid, and (e) C16 Sphinganine.
3.10. Effects of Aqueous Extract of Triphala on 4-Day Suppressive Test
Table 6 shows the percentage of parasitemia and suppression in mice infected with P. berghei. Although the most effective suppression was exhibited in the chloroquine group, which produced 100% suppression, the administration of the extract at all doses revealed a significant (p < 0.05) increase in percent suppression when compared with that of the negative control. Different doses of the extract reduced the percentage of parasitemia in a dose-dependent manner. The extract at 600 mg/kg showed the best suppressive effect (75.47%), followed by 44.13% at the dose of 400 mg/kg and 42.72% at the dose of 200 mg/kg.

Table 6.
In vivo antimalarial activity of aqueous extract from Triphala.
3.11. Acute Toxicity Test
The mice that received the aqueous extract of Triphala at 2 g/kg did not experience any death or abnormalities of the eyes, fur, or skin and did not show behavioral changes such as general activity, tremors, convulsions, ataxia, or diarrhea, indicating that the LD50 value of the extract was greater than 2 g/kg. Both groups of mice showed a percent increase in body weight, and no significant difference (p < 0.05) was observed between the two groups (Table 7). The relative organ weights of the liver and kidney in mice that received the extract showed no significant difference (p < 0.05) compared to the control group (Table 8). In addition, the extract did not induce significant (p < 0.05) changes in the liver and kidney enzymes, including BUN, creatinine, AST, ALT, and ALP (Table 9).

Table 7.
Effect of aqueous extract from Triphala on body weight changes in acute toxicity test.

Table 8.
Relative weight of liver and kidney in acute toxicity test of aqueous extract from Triphala.

Table 9.
Effect of aqueous extract from Triphala on liver and kidney functions.
Figure 8 shows the histological examination of the control and treatment groups, which showed normal structures of the liver and kidney in both groups. The liver sections did not show dilation of the central vein, cytoplasmic vacuolization, inflammatory cell infiltration, or congestion in the hepatic sinusoids. Kidney histological changes in extract-treated mice exhibited no obvious damage compared to the control group. Figure 8b,d show normal renal tubules and glomeruli in the glomerular basement membrane.
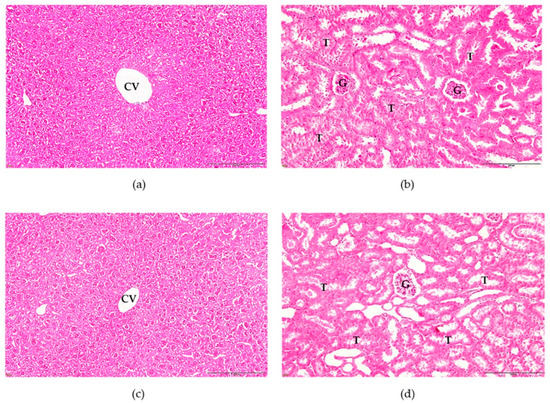
Figure 8.
Histopathology of the liver and kidney from ICR mice that received aqueous extract from Triphala in acute toxicity test; (a) liver histology in control mice, (b) kidney histology in control mice, (c) liver histology in extract-treated mice and (d) kidney histology in extract-treated mice. All images were acquired at 20X magnification. Bar = 20 μm. CV, central vein; T, renal tubule; G, glomerulus.
4. Discussion
Over the years, drug resistance has become one of the biggest problems in infectious diseases, including malaria. Partial resistance to artemisinin has emerged and spread, leading to delayed parasite clearance after treatment with ACT [30]. The development of antimalarial agents has been urgently needed; hence, phytomedicine became an interesting idea [31]. This study investigated the antiplasmodial activity of ten traditional recipes that provided scientific justification for malaria treatment. Solvent choices for extraction were selected according to the traditionally used phytomedicine in Thailand, which has otherwise been used as a polar and slightly nonpolar solvent for the study of biological activities. An aqueous solution is considered the best choice because of its low cost, nontoxicity, and health safety, and ethanol can be added to increase the solubilization of polar substances [32]. In addition, different solvent polarities affect the extract yields. The Benjalotiga and Chan-tang-ha recipes produced higher ethanolic yields than that of aqueous solutions. This result is in agreement with previous studies, where they found that the compounds present in trees can be extracted using organic solvents [33]. This finding suggests that alcohol can improve the solubilization of nonpolar molecules from wood [34].
Antiplasmodial properties of herbal recipes revealed that high activity against P. falciparum was mostly present in ethanolic extracts with an IC50 value ranging from 2.8–8.5 μg/mL. In comparison, the Gaysorn-tang-ha recipe showed the highest activity, followed by Trikatuk, Triphala, Benjalotiga, Benjagot, Chan-tang-ha, Jatu-phala-tiga, Benjakul, and Benjathian recipes. This suggests that the solubility of potentially active substances in semi-polar solutes may be greater than that in polar solvents, which is consistent with previous research. Potent antiplasmodial compounds are concentrated in the medium-polar solvent [35]. In addition, single ethanolic extracts from Dracaena loureiri Gagnep, Myristica fragrans Houtt, and Piper chaba Hunt were reported to possess antiplasmodial activity with IC50 of 10.5, 8.9, and 5.3 μg/mL, respectively [19,36]. In contrast, only three aqueous extracts from Triphala, Trisamo, and Jatu-phala-tiga exhibited good activity with an IC50 of 5.7 ± 0.2, 6.1 ± 0.7, and 5.0 ± 0.3 μg/mL, respectively, which is in agreement with previous evidence. The ingredients in water extracts of Triphala, Trisamo, and Jatu-phala-tiga recipes such as Terminalia bellerica (Gaertn) Roxb., Phyllanthus emblica L., and Terminalia chebula Retz were reported antiplasmodial activities with an IC50 of 14.3, 14.4, and 15.4 μg/mL, respectively [37]. Therefore, our findings imply that the antiplasmodial activity of these recipes is caused by the combined effect or synergism of the ingredients.
Historically, plants have been considered to have pharmacological properties that respond to the presence of phytocompounds [38]. This study investigated eight phytoconstituents based on the main components present in medicinal plants [39]. Flavonoids, a group of natural substances with aromatic organic structures, are potential sources of antimalarial compounds [40]. Its mechanism of action is believed to be its interference with functional biomolecules, such as protein, enzymes, DNA, etc., under cellular oxidative stress and inhibition of fatty acid biosynthesis during the intraerythrocytic cycle [40]. Terpenoids play a key role in the eradication of malarial parasites. Artemisinin, a sesquiterpene lactone compound, is currently the most effective antimalarial drug derived from the medicinal plant [41]. They create radical ions that can damage various proteins, including sarco-endoplasmic reticulum Ca2+-ATPase, and inhibit PfATP6, leading to the breakage of mitochondrial and parasitic membranes [42]. Alkaloids are a broad class of biological compounds with antiplasmodial properties. Quinoline alkaloids are well-known compounds in malaria research, such as quinine and quinidine. For quinine, the structure has been modified to improve efficacy and reduce toxicity. Its mechanism is related to hemoglobin breakdown pathways. [43,44]. Tannins have a positive effect on antiplasmodial prophylaxis [45,46]. Saponins and coumarins have been reported as attractive compounds against malaria [47,48]. Therefore, plant secondary metabolites are strongly correlated with antiplasmodial activities, and it is suggested that the good activity of the traditional recipes in this study may be owing to the action of one individual or synergistic effects of phytocompounds. Traditional herbal medicines not only provide benefits but also generate potentially harmful effects or side effects from the plants [49]. This encouraged us to investigate the toxic effects of these medicinal plants.
An in vitro cell-based approach was used as the model for toxicity screening. The liver is the primary site for drug-induced toxicity, whereas the kidney is the primary organ involved in drug clearance for oral drug delivery [50]. Consequently, we investigated the cytotoxic effects of ten traditional recipes on both Hep-G2 and Vero cells and found that all extracts showed CC50 against Hep-G2 at concentrations greater than 30 μg/mL, except for the ethanolic extracts from Benjakul, Benjalotiga, and Trikatuk, whereas Benjalotiga and the aqueous extract of Chan-tang-ha exhibited CC50 against Vero cells at concentrations below 30 μg/mL. These results suggest that the extracts with a CC50 below 30 μg/mL exhibited cytotoxicity. This finding is consistent with those of previous studies [51,52]. The ethanolic extract of Benjakul possesses anticarcinogenic activity, which is a response of at least three cytotoxic components of plumbagin, piperine, and 6-gingerol. Likewise, the toxic effects of Trikatuk may be caused by its components. For the Benjalotiga recipe, Santalol, the major constituent of Santalum album L., was reported to have antitumor properties on human hepatocellular carcinoma cell lines, and Silvestrol and episilvestrol were announced as potential anticancer agents against human oral epidermoid carcinoma [53,54]. Taccalonolides isolated from plants of the genus Tacca, such as Tacca chantrieri, are a new class of microtubule-stabilizing anticancer agents [55]. This finding suggests that the cytotoxicity of the recipes might be caused by toxic compounds that are deposited in plants. However, the toxic effects of these recipes on Vero cells exhibited CC50 greater than those on HepG2 cells, except for Benjalotiga. It was implied that ethanolic extracts from Trikatuk and Benjakul displayed selective toxicity towards cancer cell lines owing to the contribution of antitumor or anticancer compounds.
Furthermore, drug-induced hemolysis is a serious toxicity liability, particularly in malaria. The hemolytic toxicity of the recipes was quantified to forecast the direct harmful effects. Aqueous extracts except for Benjathian and Trikatuk at a fixed dose of 50 μg/mL did not promote the breakdown of red blood cells, and only three aqueous extracts from Triphala, Trisamo, and Jatu-phala-tiga exhibited high SI values. These results suggest that aqueous extracts from Triphala, Trisamo, and Jatu-phala-tiga are good candidates for further evaluation in animal models. In contrast, some ethanolic extracts exhibited hemolytic effects. Trisamo and Benjakul recipes produced 100% hemolysis after incubation for 72 h. Benjagot, Gaysorn-tang-ha, Chan-tang-ha, and Benjathian exhibited percent hemolysis at 45.59 ± 6.81, 39.18 ± 9.62, 6.09 ± 9.14, and 5.79 ± 9.66, respectively. The extracts may be attributed to the presence of toxic substances that affect hematopoietic cells. In addition, this finding may imply that the antiplasmodial activity of ethanolic extracts of Gaysorn-tang-ha, Benjagot, Chan-tang-ha, Trisamo, Benjakul and Benjatian with IC50 of 2.8, 6.8, 7.2, 7.7, 8.5 and 15.5 μg/mL, respectively could be due to hemolytic activity at 50 µg/mL.
Based on these findings, we propose that extracts not inducing hemolysis must be considered for use in medicine, whereas extracts that show hemolysis effects of more than 10% should be identified as toxic components or used with caution.
In previous findings, the antiparasitic effect of artesunate has been linked with DNA damage by increasing ROS production [56]. In our study, we observed oxidant generation of the extracts that had the ability to kill the parasite. As shown in Figure 3, our results illustrated that aqueous extracts of Trisamo and ethanolic extracts of Triphala, Trikatuk, and Trisamo induced significantly higher oxidant levels, suggesting that parasite death might be related to enhancing of oxidant levels and that increasing the oxidant can be targeted to oxidative damage to intracellular proteins, lipids and nucleic acids [57]. This finding is in accordance with previous research. Phenolic compounds in Terminalia species could potentially behave as either antioxidants or prooxidants, depending on their concentration, redox state, and the ratio between compounds [58]. The antioxidant effect is due to acting with a variety of free radicals, whereas prooxidant properties are related to the presence of transition metal ions such as copper or iron in the extract. The ability to reduce the metal ions may exert a redox cycling mechanism resulting in the formation of prooxidants [58].
In order to investigate the antimalarial activity and toxicity in an animal model, an aqueous extract of Triphala was selected for the in vivo evaluation. However, additional candidates such as Trisamo and Jatu-phala-tiga can be chosen as candidates in further studies because extracts with SI values of ≥10 can be assumed as potential samples for further investigation [59]. ICR mice were used because they are susceptible to infection by P. berghei ANKA [60]. The aqueous extract of Triphala reduced the parasite load up to 75.47% at a concentration of 600 mg/kg. Although the standard drug eliminated the infection, the extract at all doses significantly suppressed parasitemia (p < 0.05) compared to that of the infected control. This finding implies that the extract possesses good antimalarial activity against P. berghei ANKA. This activity may have been derived from the active compounds deposited in the extract. Gallic acid increases ROS production in macrophages, which may enhance phagocytic activity, and ellagic acid has antimalarial activity [61,62,63]. In addition, previous studies found gallic acid, ellagic acid, and chebulinic acid to be the major constituents of the Triphala recipe [11]. There was consistency with our results by LC-QTOF-MS analysis. Furthermore, Triphala has been used to maintain appropriate homeostasis in the body. Thus, the reduction in parasites might be owing to indirect effects. Triphala possesses antioxidant, free-radical scavenging, and immunomodulatory activities [63,64]. Therefore, the extract may activate the mechanism of cell-mediated immunity or humoral-mediated immunity because it is responsible for the stimulation of the immune system [63].
The harmful effects of the aqueous extract of Triphala showed that the extract did not cause acute toxicity in ICR mice. The indications for safety were normal behavior and absence of alteration in body weight, organ weight, liver-kidney function level, and histology compared with that of the control mice. Changes in body and organ weights are important parameters for assessing toxicity. Weight loss may indicate that a substance is causing damage to the body. Organ weight is a sensitive indicator of chemical or drug-induced organ damage [65]. Thus, the results of this study indicate that the extract is safe for the liver and kidneys. In addition, the results of liver-kidney functions and histological examination showed no significant changes compared to the control group. Therefore, we conclude that the aqueous extract of Triphala is safe.
5. Conclusions
This study confirmed that all ethanolic and aqueous extracts from Triphala, Trisamo, and Jatu-phala-tiga could kill P. falciparum. However, high SI values in both HepG2 and Vero cells were present in aqueous extracts from Triphala, Trisamo, and Jatu-phala-tiga recipes. The aqueous extract of Triphala exhibited good antimalarial activity in a mouse model, and a single oral dose of 2 g/kg was safe in acute toxicity tests.
Author Contributions
Conceptualization, A.P., P.C., S.P. and C.P.; methodology, A.P., P.C. and C.P.; formal analysis, A.P., P.C., W.P., A.C. and C.P.; investigation, A.P., P.C., W.P. and C.P.; resources, P.C. and C.P.; data curation, A.P., P.C., S.P., A.W.S., A.C. and C.P.; writing—original draft preparation, A.P.; writing—review and editing, P.C., S.P., A.W.S., A.C. and C.P.; visualization, A.P., P.C., W.P. and C.P.; supervision, P.C. and C.P.; project administration, C.P.; funding acquisition, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Walailak University Graduate Research Fund, Thailand (Contract No. CGS-RF-2022/06).
Institutional Review Board Statement
The study protocol was reviewed and approved by the Human Ethics Committee of Walailak University prior to the recruitment of any participant (approval number: WUEC-22-153-01) and followed the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants before data and blood sample collection to establish models of blood-stage malaria infection and hemolysis, and the study protocol was reviewed and approved by the Animal Ethics Committee of Walailak University, National Research Council of Thailand (NRCT) (protocol number: WU-ACUC-65049).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
The data associated with this study are included in this published article. Additional files are available from the corresponding authors upon request.
Acknowledgments
This work was supported by Walailak University Ph.D. Scholarships for High-Potential Candidates to Enroll in Doctoral Programs (Contract No. HP004/2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Malaria Report. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 15 July 2022).
- Weatherall, D.J.; Miller, L.H.; Baruch, D.I.; Marsh, K.; Doumbo, O.K.; Casals-Pascual, C.; Roberts, D.J. Malaria and the red cell. Hematology 2002, 2002, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-C.; Russell, B.; Rénia, L. Sticking for a cause: The falciparum malaria parasites cytoadherence paradigm. Front. Immunol. 2019, 10, 1444. [Google Scholar] [CrossRef] [PubMed]
- WHO. Report on Antimalarial Drug Efficacy, Resistance and Response: 10 Years of Surveillance; WHO: Geneva, Switzerland, 2020; p. 78.
- Thriemer, K.; Hong, N.V.; Rosanas-Urgell, A.; Phuc, B.Q.; Ha, D.M.; Pockele, E.; Guetens, P.; Van, N.V.; Duong, T.T.; Amambua-Ngwa, A.; et al. Delayed parasite clearance after treatment with dihydroartemisinin-piperaquine in Plasmodium falciparum malaria patients in central Vietnam. Antimicrob Agents Chemother 2014, 58, 7049–7055. [Google Scholar] [CrossRef]
- Junsongduang, A.; Kasemwan, W.; Lumjoomjung, S.; Sabprachai, W.; Tanming, W.; Balslev, H. Ethnomedicinal knowledge of traditional healers in Roi Et, Thailand. Plants 2020, 9, 1177. [Google Scholar] [CrossRef]
- Noronha, M.; Pawar, V.; Prajapati, A.; Subramanian, R.B. A literature review on traditional herbal medicines for malaria. S. Afr. J. Bot. 2020, 128, 292–303. [Google Scholar] [CrossRef]
- Peltzer, K.; Pengpid, S. The use of herbal medicines among chronic disease patients in Thailand: A cross-sectional survey. J. Multidiscip. Healthc. 2019, 12, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, S.; Thing, G.S.; Dhanaraj, S.A. Polyherbal formulation: Concept of ayurveda. Pharmacogn. Rev. 2014, 8, 73–80. [Google Scholar] [CrossRef]
- Rudrapal, M.; Celik, I.; Khan, J.; Ansari, M.A.; Alomary, M.N.; Alatawi, F.A.; Yadav, R.; Sharma, T.; Tallei, T.E.; Pasala, P.K.; et al. Identification of bioactive molecules from Triphala (Ayurvedic herbal formulation) as potential inhibitors of SARS-CoV-2 main protease (Mpro) through computational investigations. J. King Saud Univ. Sci. 2022, 34, 101826. [Google Scholar] [CrossRef]
- Peterson, C.T.; Denniston, K.; Chopra, D. Therapeutic uses of Triphala in Ayurvedic medicine. J. Altern. Complement. Med. 2017, 23, 607–614. [Google Scholar] [CrossRef]
- Tappayuthpijarn, P.; Sattaponpan, C.; Sakpakdeecharoen, I.; Ittharat, A. Cholinesterase inhibitory and antioxidant activities of Thai traditional remedies potentially used for Alzheimer’s disease. Eur. J. East Asian Stud. 2012, 17, 18–25. [Google Scholar] [CrossRef]
- Pattanacharoenchai, N.; Itharat, A. Anti-allergic activity of Trikatuk Tripha and Trisarn remedies. TMJ 2017, 17, 548–556. [Google Scholar]
- Suksaeree, J.; Monton, C. Evaluation of the interaction of phenolic compounds contained in the Trisamo recipe using simplex lattice design. J. Sci. Technol. 2021, 11, 100–113. [Google Scholar]
- Wetchakul, P.; Goon, J.A.; Adekoya, A.E.; Olatunji, O.J.; Ruangchuay, S.; Jaisamut, P.; Issuriya, A.; Kunworarath, N.; Limsuwan, S.; Chusri, S. Traditional tonifying polyherbal infusion, Jatu-Phala-Tiga, exerts antioxidant activities and extends lifespan of Caenorhabditis elegans. BMC Complement. Altern. Med. 2019, 19, 209. [Google Scholar] [CrossRef] [PubMed]
- Makchuchit, S.; Rattarom, R.; Itharat, A. The anti-allergic and anti-inflammatory effects of Benjakul extract (a Thai traditional medicine), its constituent plants and its some pure constituents using in vitro experiments. Biomed. Pharmacother. 2017, 89, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Loetthammasak, P. Formulas of Thai Traditional Medicine; Pimdee: Samut Sakhon, Thailand, 2014. [Google Scholar]
- Sinsupan, N. Thai traditional medicine theory Part 3 Thai Pharmacy. J. Acad. Serv. Cent. 2002, 10. [Google Scholar]
- Chaniad, P.; Phuwajaroanpong, A.; Techarang, T.; Horata, N.; Chukaew, A.; Punsawad, C. Evaluation of the antimalarial activity and toxicity of Mahanil-Tang-Thong formulation and its plant ingredients. BMC Complement. Med. Ther. 2022, 22, 51. [Google Scholar] [CrossRef]
- Ngbolua, K.-T.-N. Phytochemical screening of some medicinal plants traditionally used by African women in Kinshasa city (DR Congo) for their intimate hygiene and evaluation of the pH of derived recipes. J. Mod. Drug Discov. Drug Deliv. Res. 2014, 1, 1–7. [Google Scholar]
- Malar, G.; Chinnachamy, C. Phytochemical screening, total flavonoid, total terpenoid and anti-inflammatory activity of aqueous stem extract of Salacia oblonga. J. Chem. Pharm. 2017, 10, 550. [Google Scholar]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. 1976. J. Parasitol. 2005, 91, 484–486. [Google Scholar] [CrossRef]
- Makler, M.T.; Hinrichs, D.J. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am. J. Trop. Med. Hyg. 1993, 48, 205–210. [Google Scholar] [CrossRef]
- Peters, W. The chemotherapy of rodent malaria, XXII. The value of drug-resistant strains of P. berghei in screening for blood schizontocidal activity. Ann. Trop. Med. Parasitol. 1975, 69, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Phuwajaroanpong, A.; Chaniad, P.; Horata, N.; Muangchanburee, S.; Kaewdana, K.; Punsawad, C. In vitro and in vivo antimalarial activities and toxicological assessment of Pogostemon Cablin (Blanco) Benth. J. Evid.-Based Integr. Med. 2020, 25, 2515690X20978387. [Google Scholar] [CrossRef] [PubMed]
- Lusakibanza, M.; Mesia, G.; Tona, G.; Karemere, S.; Lukuka, A.; Tits, M.; Angenot, L.; Frédérich, M. In vitro and in vivo antimalarial and cytotoxic activity of five plants used in congolese traditional medicine. J. Ethnopharmacol. 2010, 129, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Odira, H.O.; Mitema, S.O.; Mapenay, I.M.; Moriasi, G.A. Anti-inflammatory, analgesic, and cytotoxic effects of the phytexponent: A polyherbal formulation. J. Evid.-Based Integr. Med. 2022, 27, 2515690X221082986. [Google Scholar] [CrossRef]
- Ngemenya, M.N.; Djeukem, G.G.R.; Nyongbela, K.D.; Bate, P.N.N.; Babiaka, S.B.; Monya, E.; Kanso, R.K. Microbial, phytochemical, toxicity analyses and antibacterial activity against multidrug resistant bacteria of some traditional remedies sold in Buea Southwest Cameroon. BMC Complement. Altern. Med. 2019, 19, 150. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Chapter six-validation of in-vitro bioassay methods: Application in herbal drug research. In Profiles of Drug Substances, Excipients and Related Methodology; Al-Majed, A.A., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 46, pp. 273–307. [Google Scholar]
- Arya, A.; Kojom Foko, L.P.; Chaudhry, S.; Sharma, A.; Singh, V. Artemisinin-based combination therapy (ACT) and drug resistance molecular markers: A systematic review of clinical studies from two malaria endemic regions-India and sub-Saharan Africa. Int. J. Parasitol. Drugs Drug Resist. 2021, 15, 43–56. [Google Scholar] [CrossRef]
- Sarkar, B.; Chakraborty, S.; Pal, C. Phytomedicine Against Infectious Diseases; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 161–172. [Google Scholar]
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Tixier, A.-S.F. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef]
- Moore, R.K.; Smaglick, J.; Leitch, E.A.-R.M.; Mann, D. The effect of polarity of extractives on the durability of wood. In Proceedings of the 18th ISWFPC (International Symposium on Wood, Fiber, and Pulping), Vienna, Austria, 7–11 September 2015. [Google Scholar]
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Mojarrab, M.; Naderi, R.; Heshmati Afshar, F. Screening of different extracts from artemisia species for their potential antimalarial activity. Iran. J. Pharm. Res. 2015, 14, 603–608. [Google Scholar]
- Thiengsusuk, A.; Chaijaroenkul, W.; Na-Bangchang, K. Antimalarial activities of medicinal plants and herbal formulations used in Thai traditional medicine. Parasitol. Res. 2013, 112, 1475–1481. [Google Scholar] [CrossRef]
- Pinmai, K.; Hiriote, W.; Soonthornchareonnon, N.; Jongsakul, K.; Sireeratawong, S.; Tor-Udom, S. In vitro and in vivo antiplasmodial activity and cytotoxicity of water extracts of Phyllanthus emblica, Terminalia chebula, and Terminalia bellerica. J. Med. Assoc. Thai. 2010, 93 (Suppl. 7), S120–S126. [Google Scholar] [PubMed]
- Aye, M.M.; Aung, H.T.; Sein, M.M.; Armijos, C. A review on the phytochemistry, medicinal properties and pharmacological activities of 15 selected myanmar medicinal plants. Molecules 2019, 24, 293. [Google Scholar] [CrossRef] [PubMed]
- Agidew, M.G. Phytochemical analysis of some selected traditional medicinal plants in Ethiopia. Bull. Natl. Res. Cent. 2022, 46, 87. [Google Scholar] [CrossRef]
- Rudrapal, M.; Chetia, D.D. Plant flavonoids as potential source of future antimalarial leads. Syst. Rev. Pharm. 2016, 8, 13–18. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in pharmacological activities of Terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Jahangeer, M.; Fatima, R.; Ashiq, M.; Basharat, A.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Therapeutic and biomedical potentialities of terpenoids—A review. J. Pure Appl. Microbiol. 2021, 15, 471–483. [Google Scholar] [CrossRef]
- Murugan, K.; Panneerselvam, C.; Subramaniam, J.; Paulpandi, M.; Rajaganesh, R.; Vasanthakumaran, M.; Madhavan, J.; Shafi, S.S.; Roni, M.; Portilla-Pulido, J.S.; et al. Synthesis of new series of quinoline derivatives with insecticidal effects on larval vectors of malaria and dengue diseases. Sci. Rep. 2022, 12, 4765. [Google Scholar] [CrossRef]
- Uzor, P.F. Alkaloids from plants with antimalarial activity: A review of recent studies. Evid.-Based Complement. Altern. Med. 2020, 2020, 8749083. [Google Scholar] [CrossRef]
- Maugeri, A.; Lombardo, G.E.; Cirmi, S.; Süntar, I.; Barreca, D.; Laganà, G.; Navarra, M. Pharmacology and toxicology of tannins. Arch. Toxicol. 2022, 96, 1257–1277. [Google Scholar] [CrossRef]
- Lutgen, P. Tannins in Artemisia: The hidden treasure of prophylaxis. Pharm. Pharmacol. Int. 2018, 6, 176–181. [Google Scholar] [CrossRef][Green Version]
- Mikhail, N.; Adewuyi, A.; Abdulsalam, T.; Ashafa, T. Antimalarial activity and biochemical effects of saponin-rich extract of Dianthus basuticus Burtt Davy in Plasmodium berghei-infected mice. Adv. Tradit. Med. 2021, 22, 519–529. [Google Scholar]
- Hu, X.L.; Gao, C.; Xu, Z.; Liu, M.L.; Feng, L.S.; Zhang, G.D. Recent development of coumarin derivatives as potential antiplasmodial and antimalarial agents. Curr. Top. Med. Chem. 2018, 18, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, J.; Patil, M. A review on phytotoxins and qualitative tests for their detection. Pharmacogn. Phytochem. 2020, 9, 1502–1507. [Google Scholar]
- Sung, J.H. Multi-organ-on-a-chip for pharmacokinetics and toxicokinetic study of drugs. Expert Opin. Drug Metab. Toxicol. 2021, 17, 969–986. [Google Scholar] [CrossRef] [PubMed]
- Mahavorasirikul, W.; Viyanant, V.; Chaijaroenkul, W.; Itharat, A.; Na-Bangchang, K. Cytotoxic activity of Thai medicinal plants against human cholangiocarcinoma, laryngeal and hepatocarcinoma cells in vitro. BMC Complement. Altern. Med. 2010, 10, 55. [Google Scholar] [CrossRef]
- Ruangnoo, S.; Itharat, A.; Sakpakdeejaroen, I.; Rattarom, R.; Tappayutpijam, P.; Pawa, K.K. In vitro cytotoxic activity of Benjakul herbal preparation and its active compounds against human lung, cervical and liver cancer cells. J. Med. Assoc. Thai. 2012, 95 (Suppl. 1), S127–S134. [Google Scholar] [PubMed]
- Saraswati, S.; Kanaujia, P.; Sunder, S. OP-03 α-Santalol demonstrates antitumor and antiantiangiogenic activities in models of hepatocellular carcinoma in vitro and in vivo. Dig. Liver Dis. 2013, 45, S249–S250. [Google Scholar] [CrossRef]
- Hwang, B.Y.; Su, B.-N.; Chai, H.; Mi, Q.; Kardono, L.B.S.; Afriastini, J.J.; Riswan, S.; Santarsiero, B.D.; Mesecar, A.D.; Wild, R.; et al. Silvestrol and episilvestrol, potential anticancer rocaglate derivatives from Aglaia silvestris. J. Org. Chem. 2004, 69, 3350–3358. [Google Scholar] [CrossRef]
- Chen, X.; Winstead, A.; Yu, H.; Peng, J. Taccalonolides: A novel class of microtubule-stabilizing anticancer agents. Cancers 2021, 13, 920. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.M.; Kumar, N. Antimalarial action of artesunate involves DNA damage mediated by reactive oxygen species. Antimicrob. Agents Chemother. 2015, 59, 317–325. [Google Scholar] [CrossRef]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef]
- Cock, I.E. The medicinal properties and phytochemistry of plants of the genus Terminalia (Combretaceae). Inflammopharmacology 2015, 23, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Peña-Morán, O.A.; Villarreal, M.L.; Álvarez-Berber, L.; Meneses-Acosta, A.; Rodríguez-López, V. Cytotoxicity, post-treatment recovery, and selectivity analysis of naturally occurring podophyllotoxins from Bursera fagaroides var. fagaroides on breast cancer cell lines. Molecules 2016, 21, 1013. [Google Scholar] [CrossRef] [PubMed]
- Basir, R.; Rahiman, S.F.; Hasballah, K.; Chong, W.; Talib, H.; Yam, M.; Jabbarzare, M.; Tie, T.; Othman, F.; Moklas, M.; et al. Plasmodium berghei ANKA infection in ICR mice as a model of cerebral malaria. Iran. J. Parasitol. 2012, 7, 62–74. [Google Scholar]
- Khasanah, U.; WidyaWaruyanti, A.; Hafid, A.F.; Tanjung, M. Antiplasmodial activity of isolated polyphenols from Alectryon serratus leaves against 3D7 Plasmodium falciparum. Pharmacogn. Res. 2017, 9, S57–S60. [Google Scholar]
- Soh, P.N.; Witkowski, B.; Olagnier, D.; Nicolau, M.L.; Garcia-Alvarez, M.C.; Berry, A.; Benoit-Vical, F. In vitro and in vivo properties of ellagic acid in malaria treatment. Antimicrob. Agents Chemother. 2009, 53, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Belapurkar, P.; Goyal, P.; Tiwari-Barua, P. Immunomodulatory effects of triphala and its individual constituents: A review. Indian J. Pharm. Sci. 2014, 76, 467–475. [Google Scholar]
- Baliga, M.S.; Meera, S.; Mathai, B.; Rai, M.P.; Pawar, V.; Palatty, P.L. Scientific validation of the ethnomedicinal properties of the Ayurvedic drug Triphala: A review. Chin. J. Integr. Med. 2012, 18, 946–954. [Google Scholar] [CrossRef]
- Lazic, S.E.; Semenova, E.; Williams, D.P. Determining organ weight toxicity with Bayesian causal models: Improving on the analysis of relative organ weights. Sci. Rep. 2020, 10, 6625. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).