Safety and Tolerability of an Antimalarial Herbal Remedy in Healthy Volunteers: An Open-Label, Single-Arm, Dose-Escalation Study on Maytenus senegalensis in Tanzania
Abstract
1. Introduction
2. Materials and Methods
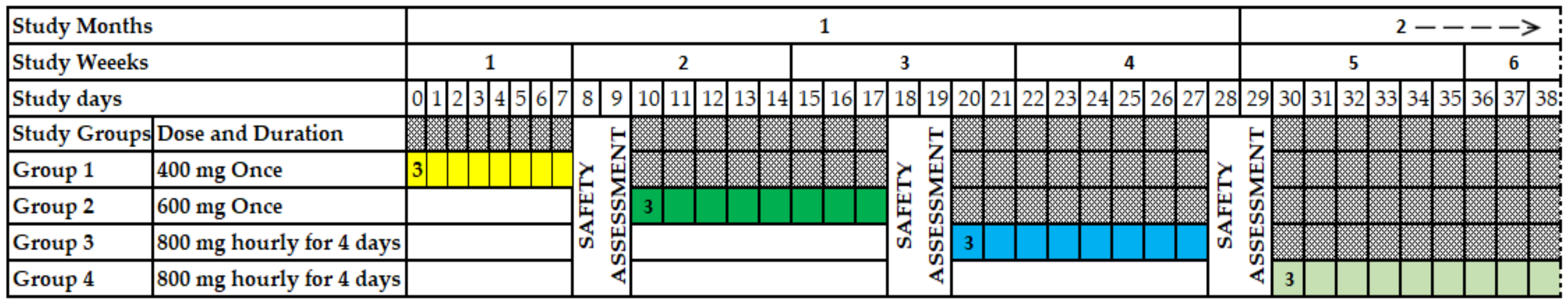
2.1. Study Design
2.2. Study Population
2.3. Sample Size
2.4. Preparation of M. senegalensis
2.5. Safety Assessment
2.6. Data Analysis
3. Results
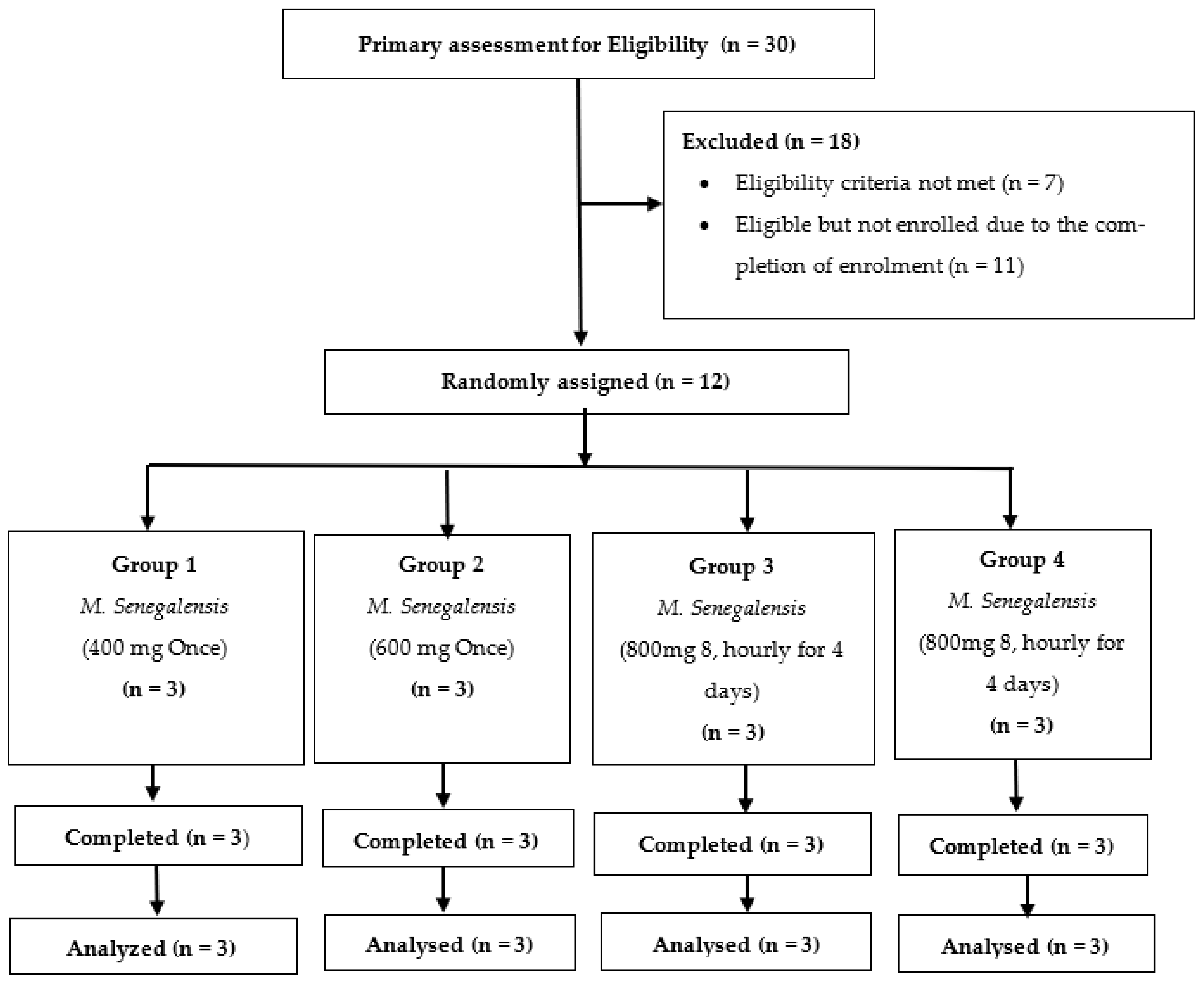
3.1. Study Volunteer Disposition
3.2. Demographic Characteristics
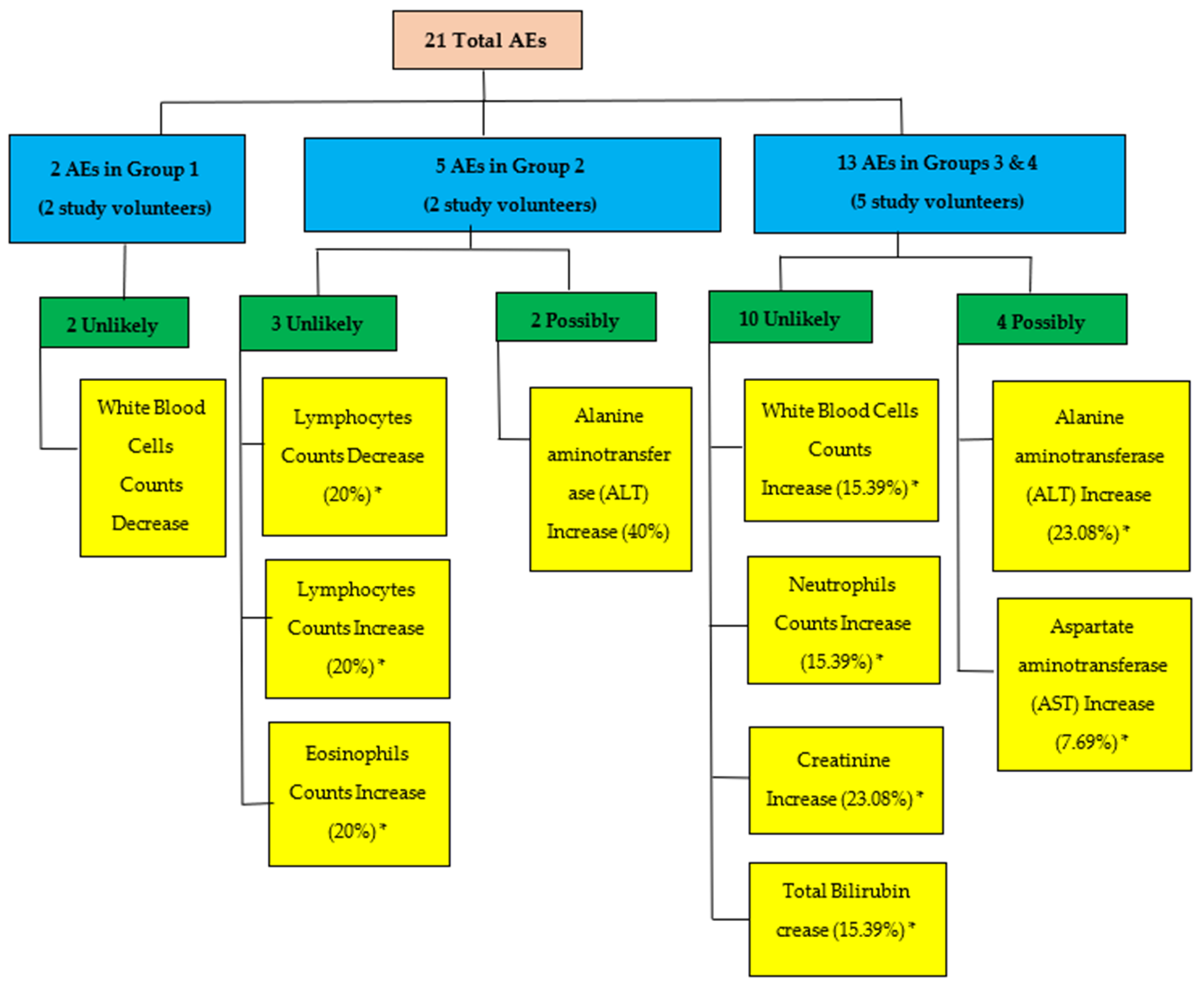
3.3. Adverse Events
3.4. Laboratory Test Results (Biochemistry and Hematology Responses)
3.5. Laboratory Test Results (Biochemistry and Hematology Responses)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Silva, G.; Taniça, M.; Rocha, J.; Serrano, R.; Gomes, E.; Sepodes, B.; Silva, O. In vivo anti-inflammatory effect and toxicological screening of Maytenus heterophylla and Maytenus senegalensis extracts. Hum. Exp. Toxicol. 2011, 30, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.P.V.; Rastrelli, L.; Romussi, G.; Oliveira, A.B.; Vilegas, J.H.Y.; Vilegas, W.; Pizza, C. Isolation and HPLC quantitative analysis of flavonoid glycosides from Brazilian beverages (Maytenus ilicifolia and M. aquifolium). J. Agric. Food Chem. 2001, 49, 3796–3801. [Google Scholar] [CrossRef] [PubMed]
- Sosa, S.; Morelli, C.; Tubaro, A.; Cairoli, P.; Speranza, G.; Manitto, P. Anti-inflammatory activity of Maytenus senegalensis root extracts and of maytenoic acid. Phytomedicine 2007, 14, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Malebo, H.M.; Wiketye, V.; Katani, S.J.; A Kitufe, N.; A Nyigo, V.; Imeda, C.P.; Ogondiek, J.W.; Sunguruma, R.; Mhame, P.P.; Massaga, J.J.; et al. In vivo antiplasmodial and toxicological effect of Maytenus senegalensis traditionally used in the treatment of malaria in Tanzania. Malar. J. 2015, 14, 79. [Google Scholar] [CrossRef]
- Umar, S.I.; Ndako, M.; Jigam, A.A.; Adefolalu, S.F.; Ibikunle, G.F.; Lawal, B. Anti-plasmodial, Anti-inflammatory, anti-nociceptive and safety profile of Maytenus senegalensis root bark extract on hepato-renal integrity in experimental animals. Comp. Clin. Pathol. 2019, 28, 1571–1579. [Google Scholar] [CrossRef]
- Kpoyizoun, P.K.; Metowogo, K.; Kantati, Y.T.; Missebukpo, A.; Dare, T.; Lawson-Evi, P.; Eklu-Gadegbeku, K.; A Aklikokou, K.; Egu, E.U. Antiinflammatory and antioxidant evaluation of Maytenus senegalensis hydroalcoholic roots extract fractions in allergic asthma. J. Phytopharm. 2020, 9, 252–257. [Google Scholar] [CrossRef]
- El Tahir, A.; Satti, G.M.; A Khalid, S. Antiplasmodial activity of selected Sudanese medicinal plants with emphasis on Maytenus senegalensis (Lam.) Exell. J. Ethnopharmacol. 1999, 64, 227–233. [Google Scholar] [CrossRef]
- Lindsey, K.; Budesinsky, M.; Kohout, L.; van Staden, J. Antibacterial activity of maytenonic acid isolated from the root-bark of Maytenus senegalensis. S. Afr. J. Bot. 2006, 72, 473–477. [Google Scholar] [CrossRef]
- Jain, N.; Light, M.; Van Staden, J. Antibacterial activity of hairy-root cultures of Maytenus senegalensis. South Afr. J. Bot. 2008, 74, 163–166. [Google Scholar] [CrossRef]
- Makgatho, M.; Nxumalo, W.; Raphoko, L. Anti-mycobacterial, -oxidative, -proliferative and -inflammatory activities of dichloromethane leaf extracts of Gymnosporia senegalensis (Lam.) Loes. S. Afr. J. Bot. 2018, 114, 217–222. [Google Scholar] [CrossRef]
- Zangueu, C.B.; Olounlade, A.P.; Ossokomack, M.; Djouatsa, Y.N.N.; Alowanou, G.G.; Azebaze, A.G.B.; Llorent-Martínez, E.J.; De Córdova, M.L.F.; Dongmo, A.B.; Hounzangbe-Adote, M.S. In vitro effects of aqueous extract from Maytenus senegalensis (Lam.) Exell stem bark on egg hatching, larval migration and adult worms of Haemonchus contortus. BMC Vet. Res. 2018, 14, 147. [Google Scholar] [CrossRef]
- Gessler, M.C.; Tanner, M.; Chollet, J.; Nkunya, M.H.H.; Heinrich, M. Tanzanian medicinal plants used traditionally for the treatment of malaria: In vivo antimalarial and in vitro cytotoxic activities. Phytother. Res. 1995, 9, 504–508. [Google Scholar] [CrossRef]
- Mueller, M.S.; Mechler, E. Medicinal Plants in Tropical Countries. Traditional Use-Experience-Facts. Medicinal Plants in Tropical Countries. Traditional Use-Experience-Facts. George Thieme Verlag: New York, NY, USA; Stuttgart, Germany. 2005, pp. 101–106. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=lah&AN=20053197835&site=ehost-live (accessed on 21 March 2022).
- Kamanzi Atindehou, K.; Schmid, C.; Brun, R.; Koné, M.W.; Traore, D. Antitrypanosomal and antiplasmodial activity of medicinal plants from Côte d’Ivoire. J. Ethnopharmacol. 2004, 90, 221–227. [Google Scholar] [CrossRef]
- Haule, E.E.; Moshi, M.J.; Nondo, R.S.O.; Mwangomo, D.T.; Mahunnah, R.L.A. A study of antimicrobial activity, acute toxicity and cytoprotective effect of a polyherbal extract in a rat ethanol-HCl gastric ulcer model. BMC Res. Notes 2012, 5, 546. [Google Scholar] [CrossRef]
- Fong, H.H.S. Integration of herbal medicine into modern medical practices: Issues and prospects. Integr. Cancer 2002, 1, 287–293, discussion 293. [Google Scholar] [CrossRef]
- Cui, L.; Wang, Z.; Miao, J.; Miao, M.; Chandra, R.; Jiang, H.; Su, X.-Z.; Cui, L. Mechanisms of in vitro resistance to dihydroartemisinin in Plasmodium falciparum. Mol. Microbiol. 2012, 86, 111–128. [Google Scholar] [CrossRef]
- Dondorp, A.M.; Nosten, F.; Yi, P.; Das, D.; Phyo, A.P.; Tarning, J.; Lwin, K.M.; Ariey, F.; Hanpithakpong, W.; Lee, S.J.; et al. Artemisinin Resistance in Plasmodium falciparum Malaria. N. Engl. J. Med. 2009, 361, 455–467. [Google Scholar] [CrossRef]
- Ajayi, N.A.; Ukwaja, K.N. Possible artemisinin-based combination therapy-resistant malaria in Nigeria: A report of three cases. Rev. Soc. Bras. Med. Trop. 2013, 46, 525–527. [Google Scholar] [CrossRef]
- Saxena, A.; Rubens, M.; Ramamoorthy, V.; Zhang, Z.; Ahmed, A.; McGranaghan, P.; Das, S.; Veledar, E. A Brief Overview of Adaptive Designs for Phase I Cancer Trials. Cancers 2022, 14, 1566. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry Establishing Bioequivalence Statistical Approaches. Analysis 2001, 8. [Google Scholar]
- Teschke, R.; Eickhoff, A.; Wolff, A.; Frenzel, C.; Schulze, J. Herbal hepatotoxicity and WHO global introspection method. Ann. Hepatol. 2013, 12, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Saadh, M.J.; Haddad, M.; Dababneh, M.F.; Bayan, M.F.; Al-Jaidi, B.A. A guide for estimating the maximum safe starting dose and conversion it between animals and humans. Syst. Rev. Pharm. 2020, 11, 98–101. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory, 3rd ed.; Clinical and Laboratory Standatd Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Zeh Clement, E.; Collins Odhiambo, O.; Lisa, M.A. Laboratory Reference Intervals in Africa. In Blood Cell-An Overview of Studies in Hematology; Odhiambo, C.O., Ed.; IntechOpen: London, UK, 2012; pp. 306–307, Ch. 15. [Google Scholar] [CrossRef][Green Version]
- Blumenreich, M.S. The White Blood Cell and Differential Count. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Berinyuy, E.; Lawal, B.; Olalekan, L.; Olalekan, I.; Yusuf, A.; Sakpe, S.; Ossai, P. Hematological Status and Organs/Body-weight Parameters in Wister Rats during Chronic Administration of Cassia occidentalis. Int. Blood Res. Rev. 2015, 4, 1–7. [Google Scholar] [CrossRef]
- Bhoopalan, S.V.; Huang, L.J.-S.; Weiss, M.J. Erythropoietin regulation of red blood cell production: From bench to bedside and back. F1000Research 2020, 9, 1153. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. Essential Oil Safety: A Guide for Health Care Professionals; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013; Available online: https://books.google.co.tz/books?id=DbEKAQAAQBAJ (accessed on 30 March 2022).
- Ndako, M.; Jigam, A.A.; Kabiru, A.Y.; Umar, S.I.; Lawal, B. Polar extracts from Gymnosporia senegalensis (syn. Maytenus senegalensis) root bark, its effects on nociception, edema, and malarial infection. Phytomedicine Plus 2021, 1, 100113. [Google Scholar] [CrossRef]
- Gounden, V.; Bhatt, H.; Jialal, I. Renal Function Tests. In Statpearls [Internet]; Statpearls Publishing: Treasure Island, FL, USA, 2022; [Updated 18 July 2022]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507821/ (accessed on 3 August 2022).
- Pimenta, E.; Jensen, M.; Jung, D.; Schaumann, F.; Boxnick, S.; Truebel, H. Effect of Diet on Serum Creatinine in Healthy Subjects During a Phase I Study. J. Clin. Med. Res. 2016, 8, 836–839. [Google Scholar] [CrossRef]
- Kalakonda, A.; Jenkins, B.A.; John, S. Physiology, Bilirubin. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022; [Updated 12 September 2022]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470290/ (accessed on 25 September 2022).
- Alolga, R.N.; Fan, Y.; Zhang, G.; Li, J.; Zhao, Y.-J.; Kakila, J.L.; Chen, Y.; Li, P.; Qi, L.-W. Pharmacokinetics of a multicomponent herbal preparation in healthy Chinese and African volunteers. Sci. Rep. 2015, 5, 12961. [Google Scholar] [CrossRef]
- Yang, T.; Liu, S.; Wang, C.H.; Tao, Y.Y.; Zhou, H.; Liu, C.H. Comparative pharmacokinetic and tissue distribution profiles of four major bioactive components in normal and hepatic fibrosis rats after oral administration of Fuzheng Huayu recipe. J. Pharm. Biomed. Anal. 2015, 114, 152–158. [Google Scholar] [CrossRef]

| Product name: M. senegalensis Batch number: MS2020-0002 Description: 200 mg Capsule | ||||
| 01 Product description | Specification | Test method | ||
| Plant part used | Root bark | Visual | ||
| Botanical name | M. senegalensis | Macroscopic | ||
| Plant: % of Extract yield | 11.2% | By weight | ||
| 02 Physical data | Specification | Result | Test method | |
| Appearance | - | Capsule | Visual | |
| Aroma | Neutral Odor | Odor of Ingredients | Organoleptic test | |
| Particle Size | - | 900 µm | Sieve analysis | |
| Average Moisture % | - | 1.87 | Loss on drying method | |
| 03 Chemical data | Specification | Result | Test method | |
| Alkaloids | - | Detected | Precipitation test | |
| Anthraquinones | - | Not detected | Color test | |
| Flavonoids | - | Not detected | Color test | |
| Saponins | - | Detected | Foam test | |
| Pesticide residues | - | Not detected | “GC-MS/MS” | |
| Lead | 10 ppm | <0.001 ppm | ICP-OES | |
| Arsenic | 2 ppm | <0.005 ppm | ICP-OES | |
| Cadmium | 0.3 ppm | <0.001 ppm | ICP-OES | |
| Chromium | 2 ppm | <0.001 ppm | ICP-OES | |
| 04 Microbiological data | Specification | Result | Test method | |
| Aerobic bacteria | Max 105 (cfu/g) | 3.4 × 102 (cfu/g) | Spread technique | |
| Clostridia | Absent | 0 per gram | Spread technique | |
| Total Coliform counts (MPN/g) | Max 103 (MPN/g) | <3 (MPN/g) | MPN technique | |
| E. coli count | Absent | <3 (MPN/g) | MPN technique | |
| Salmonella spp. | Absent | Absent | Conventional technique | |
| Yeast and Mould Identification: Yeasts | Max 104 (cfu/g) | 0 (cfu/g) | Spread technique | |
| Aflatoxin G1, μg/Kg | - | Not detected | FCL/SOP-TM/13-02 | |
| Aflatoxin G2, μg/Kg | - | Not detected | FCL/SOP-TM/13-02 | |
| Aflatoxin B2, μg/Kg | - | Not detected | FCL/SOP-TM/13-02 | |
| Aflatoxin B1, μg/Kg | - | Not detected | FCL/SOP-TM/13-02 | |
| Total Aflatoxin, μg/Kg | - | Not detected | FCL/SOP-TM/13-02 | |
| 05 Additional information | ||||
| Extraction method | Cold maceration | |||
| Packing | Hard-shelled capsule, then in blister bags containing 100 capsules | |||
| Storage | Temperature below 30 °C away from exposure to sunlight and moisture | |||
| Shelf life | 2 years | |||
| Country of origin/manufactured | Tanzania | |||
| Non-irradiation | This material has not been subjected to irradiation | |||
| Characteristics | Statistics | Group 1 (n = 3) | Group 2 (n = 3) | Group 3 (n = 3) | Group 4 (n = 3) | All (n = 12) |
|---|---|---|---|---|---|---|
| Age | Mean | 27.21 | 23.63 | 28.62 | 31.35 | 27.70 |
| SD | 2.62 | 4.88 | 4.95 | 11.14 | 6.41 | |
| Range | 4.96 | 9.76 | 9.43 | 19.49 | 25.48 | |
| Sex | ||||||
| Male | n (%) | 3 (25) | 3 (25) | 3 (25) | 3 (25) | 12 (100) |
| Race | ||||||
| African | n (%) | 25 | 25 | 25 | 25 | 100 |
| Height, Kg | Mean | 169.33 | 164.67 | 170.5 | 165.17 | 167.42 |
| SD | 4.16 | 3.06 | 3.91 | 7.75 | 5.06 | |
| Range | 4.16 | 6 | 7 | 15.5 | 16.5 | |
| Weight, Kg | Mean | 76.67 | 55.67 | 63.67 | 60 | 64 |
| SD | 6.11 | 6.66 | 2.89 | 8.54 | 9.84 | |
| Range | 12 | 13 | 5 | 17 | 32 | |
| BMI, Kg/m2 | Mean | 26.71 | 20.58 | 21.91 | 21.89 | 22.77 |
| SD | 1.166 | 2.99 | 1.03 | 1.06 | 2.87 | |
| Range | 2.24 | 5.42 | 1.8 | 2.09 | 9.05 |
| Characteristics | Group 1 (n = 3) | Group 2 (n = 3) | Group 3 (n = 3) | Group 4 (n = 3) |
|---|---|---|---|---|
| Nervous System | ||||
| Drowsiness | 0 | 0 | 0 | 0 |
| Nervousness | 0 | 0 | 0 | 0 |
| Insomnia | 0 | 0 | 0 | 0 |
| Nightmares | 0 | 0 | 0 | 0 |
| Shivering | 0 | 0 | 0 | 0 |
| Numbness | 0 | 0 | 0 | 0 |
| Ageusia | 0 | 0 | 0 | 0 |
| Tinnitus | 0 | 0 | 0 | 0 |
| Blurred vision | 0 | 0 | 0 | 0 |
| Unpleasant taste | 0 | 0 | 0 | 0 |
| Thirst | 0 | 0 | 0 | 0 |
| Cardiovascular System | ||||
| Fast heartbeat | 0 | 0 | 0 | 0 |
| Irregular heartbeat | 0 | 0 | 0 | 0 |
| Heartbeat awareness | 0 | 0 | 0 | 0 |
| Respiratory system | ||||
| Cough | 0 | 0 | 0 | 0 |
| Chest pain | 0 | 0 | 0 | 0 |
| Stuffy nose | 0 | 0 | 0 | 0 |
| Difficulty in breathing | 0 | 0 | 0 | 0 |
| Gastrointestinal System | ||||
| Heartburn | 0 | 0 | 0 | 0 |
| Abdominal pain | 0 | 0 | 0 | 0 |
| Diarrhea | 0 | 0 | 0 | 0 |
| Nausea and vomiting | 0 | 0 | 0 | 0 |
| Constipation | 0 | 0 | 0 | 0 |
| Intestinal wind | 0 | 0 | 0 | 0 |
| Abnormal stool color | 0 | 0 | 0 | 0 |
| Genito-urinary System | ||||
| Dysuria | 0 | 0 | 0 | 0 |
| Polyuria | 0 | 0 | 0 | 0 |
| Nocturia | 0 | 0 | 0 | 0 |
| Dark urine | 0 | 0 | 0 | 0 |
| Change in sexual ability/desire | 0 | 0 | 0 | 0 |
| Muco-cutaneous System | ||||
| Skin rash | 0 | 0 | 0 | 0 |
| Pruritus | 0 | 0 | 0 | 0 |
| Dry mouth | 0 | 0 | 0 | 0 |
| Others | ||||
| Jaundice | 0 | 0 | 0 | 0 |
| Fever | 0 | 0 | 0 | 0 |
| Headache | 0 | 0 | 0 | 0 |
| Hematology Parameters Reference Range (Unit) | Visit Code | G1 | G2 | G3 and 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | Median (Range) | * p Value | n | Mean ± SD | Median (Range) | * p Value | n | Mean ± SD | Median (Range) | p Value | ||
| White Blood Cells (WBC) 3.48–9.11 (103/µL) | Day 1 | 3 | 5.76 ± 2.39 | 5.28 (3.65, 8.36) | 0.13 * | 3 | 4.097 ± 0.90 | 3.68 (3.48, 5.13) | 0.05 * | 6 | 5.13 ± 1.57 | 4.99 (3.2, 7.76) | 0.003 * |
| Day 3 | 3 | 4.723 ± 0.55 | 4.93 (4.1, 5.14) | 3 | 5.347 ± 1.26 | 5.45 (4.04, 6.55) | 6 | 7.465 ± 2.62 | 7.09 (4.78, 11.46) | ||||
| Day 7 | 3 | 4.87 ± 0.77 | 4.56 (4.33, 5.77) | 3 | 5.863 ± 2.18 | 6.17 (3.55, 7.87) | 6 | 7.66 ± 2.30 | 6.7 (5.85, 11.66) | ||||
| Day 14 | 3 | 4.53 ± 0.78 | 4.17 (4, 5.42) | 3 | 4.313 ± 1.74 | 4.16 (2.66, 6.12) | 6 | 5.68 ± 1.54 | 4.91 (3.97, 8.23) | ||||
| Day 28 | 3 | 3.883 ± 0.95 | 3.41 (3.26, 4.98) | 3 | 4.297 ± 1.30 | 4.95 (2.8, 5.14) | 6 | 5.27 ± 0.94 | 5.38 (3.68, 6.45) | ||||
| Red Blood Cells (RBC) 4.39–6.5 (106/µL) | Day 1 | 3 | 5.63 ± 0.37 | 5.59 (5.28, 6.02) | 3 | 5.28 ± 0.32 | 5.43 (4.91, 5.5) | 0.16 * | 6 | 5.44 ± 0.46 | 5.26 (5.07, 6.21) | 0.07 * | |
| Day 3 | 3 | 5.36 ± 0.37 | 5.35 (5, 5.74) | 3 | 5.54 ± 0.55 | 5.78 (4.91, 5.92) | 6 | 5.27 ± 0.46 | 5.16 (4.82, 6.04) | ||||
| Day 7 | 3 | 5.40 ± 0.40 | 5.45 (4.97, 5.77) | 0.07 * | 3 | 5.61 ± 0.37 | 5.64 (5.23, 5.96) | 6 | 5.34 ± 0.55 | 5.32 (4.67, 6.1) | |||
| Day 14 | 3 | 5.46 ± 0.39 | 5.43 (5.09, 5.86) | 3 | 5.34 ± 0.30 | 5.33 (5.05, 5.64) | 6 | 5.28 ± 0.52 | 5.26 (4.75, 6.01) | ||||
| Day 28 | 3 | 5.36 ± 0.37 | 5.26 (5.04, 5.77) | 3 | 5.6 ± 0.42 | 5.47 (5.26, 6.07) | 6 | 5.30 ± 0.42 | 5.34 (4.82, 5.97) | ||||
| Hemoglobin (HGB) 12.6–17.3 (g/dL) | Day 1 | 3 | 15.13 ± 1.66 | 15.3 (13.4, 16.7) | 0.08 * | 3 | 13.93 ± 0.46 | 14.2 (13.4, 14.2) | 0.08 * | 6 | 15.33 ± 1.29 | 15.55 (13.2, 16.8) | 0.31 * |
| Day 3 | 3 | 14.37 ± 1.70 | 14.5 (12.6, 16) | 3 | 14.73 ± 0.43 | 14.6 (14.4, 15.2) | 6 | 15.03 ± 1.15 | 15.15 (13, 16.2) | ||||
| Day 7 | 3 | 14.53 ± 1.90 | 14.6 (12.6, 16.4) | 3 | 14.8 ± 0.2 | 14.8 (14.6, 15) | 6 | 15.13 ± 1.53 | 15.55 (12.3, 16.7) | ||||
| Day 14 | 3 | 14.67 ± 1.65 | 14.7 (13, 16.3) | 3 | 14.1 ± 0.78 | 14.5 (13.2, 14.6) | 6 | 14.98 ± 1.37 | 15.35 (12.6, 16.4) | ||||
| Day 28 | 3 | 14.43 ± 1.52 | 14.7 (12.8, 15.8) | 3 | 14.77 ± 0.95 | 15.1 (13.7, 15.5) | 6 | 15.1 ± 1.28 | 15.5 (12.6, 16.1) | ||||
| Platelets (PLT) 107–396.2 (103/µL) | Day 1 | 3 | 231.67 ± 42.55 | 234 (188, 273) | 0.05 * | 3 | 246 ± 77.31 | 215 (189, 334) | 0.48 * | 6 | 249 ± 44.21 | 265.5 (175, 291) | 0.82 * |
| Day 3 | 3 | 213.67 ± 57.01 | 215 (156, 270) | 3 | 241.33 ± 62.78 | 220 (192, 312) | 6 | 247.83 ± 60.79 | 257 (169, 319) | ||||
| Day 7 | 3 | 210.33 ± 62.07 | 220 (144, 267) | 3 | 219.66 ± 55.08 | 193 (183, 283) | 6 | 249.33 ± 61.05 | 248.5 (168, 319) | ||||
| Day 14 | 3 | 236.66 ± 44.74 | 246 (188, 276) | 3 | 232.66 ± 68.12 | 228 (167, 303) | 6 | 246.33 ± 50.23 | 245 (184, 324) | ||||
| Day 28 | 3 | 206.67 ± 33.56 | 209 (172, 239) | 3 | 221 ± 55.76 | 201 (178, 284) | 6 | 238.5 ± 43.04 | 247 (186, 300) | ||||
| Neutrophils counts (NEUT_N) 1.18–5.46 (103/µL) | Day 1 | 3 | 3.11 ± 2.46 | 1.97 (1.42, 5.93) | 0.07 * | 3 | 1.48 ± 0.32 | 1.61 (1.12, 1.72) | 0.06 * | 6 | 2.77 ± 1.49 | 2.47 (1.14, 5.46) | 0.02 * |
| Day 3 | 3 | 2.15 ± 0.45 | 1.93 (1.85, 2.66) | 3 | 2.39 ± 0.56 | 2.29 (1.88, 2.99) | 6 | 4.23 ± 2.19 | 3.93 (1.97, 7.54) | ||||
| Day 7 | 3 | 2.38 ± 0.20 | 2.34 (2.2, 2.59) | 3 | 2.2 ± 0.41 | 2.4 (1.73, 2.47) | 6 | 4.13 ± 1.96 | 3.52 (1.82, 7.26) | ||||
| Day 14 | 3 | 1.82 ± 0.13 | 1.89 (1.67, 1.9) | 3 | 1.44 ± 0.65 | 1.64 (0.71, 1.96) | 6 | 2.76 ± 1.16 | 2.43 (1.77, 4.88) | ||||
| Day 28 | 3 | 1.40 ± 0.30 | 1.28 (1.19, 1.74) | 3 | 1.56 ± 0.72 | 1.19 (1.1, 2.39) | 6 | 2.645 ± 0.76 | 2.81 (1.26, 3.41) | ||||
| Lymphocytes counts (LYMP_N) 1.19–3.4 (103/µL) | Day 1 | 3 | 1.95 ± 0.36 | 1.74 (1.74, 2.36) | 3 | 1.98 ± 0.35 | 1.81 (1.75, 2.38) | 0.86 * | 6 | 1.85 ± 0.10 | 1.83 (2.03) | 0.001 * | |
| Day 3 | 3 | 1.85 ± 0.34 | 1.72 (1.6, 2.23) | 3 | 2.01 ± 0.53 | 1.9 (1.55, 2.59) | 6 | 2.40 ± 0.37 | 2.30 (2.99) | ||||
| Day 7 | 3 | 1.85 ± 0.30 | 1.79 (1.59, 2.17) | 0.25 * | 3 | 2.53 ± 1.20 | 2.98 (1.17, 3.44) | 6 | 2.61 ± 0.48 | 2.60 (3.37) | |||
| Day 14 | 3 | 2.02 ± 0.43 | 1.92 (1.67, 2.48) | 3 | 1.93 ± 0.39 | 2.02 (1.51, 2.27) | 6 | 1.83 ± 0.27 | 1.73 (2.24) | ||||
| Day 28 | 3 | 1.40 ± 0.30 | 1.28 (1.19, 1.74) | 3 | 1.94 ± 0.66 | 2.08 (1.23, 2.52) | 6 | 1.94 ± 0.24 | 1.91 (2.24) | ||||
| Eosinophils counts (EOS_N) 0–0.78 (103/µL) | Day 1 | 3 | 0.31 ± 0.24 | 0.20 (0.15, 0.58) | 0.29 * | 3 | 0.28 ± 0.27 | 0.24 (0.03, 0.56) | 0.90 * | 6 | 0.12 ± 0.08 | 0.11 (0.03, 0.24) | 0.01 * |
| Day 3 | 3 | 0.27 ± 0.23 | 0.18 (0.11, 0.53) | 3 | 0.38 ± 0.39 | 0.25 (0.08, 0.82) | 6 | 0.21 ± 0.10 | 0.24 (0.09, 0.35) | ||||
| Day 7 | 3 | 0.26 ± 0.21 | 0.16 (0.12, 0.5) | 3 | 0.46 ± 0.45 | 0.25 (0.15, 0.98) | 6 | 0.19 ± 0.10 | 0.22 (0.05, 0.32) | ||||
| Day 14 | 3 | 0.27 ± 0.23 | 0.21 (0.08, 0.52) | 3 | 0.45 ± 0.57 | 0.18 (0.07, 1.1) | 6 | 0.18 ± 0.15 | 0.14 (0.03, 0.47) | ||||
| Day 28 | 3 | 0.23 ± 0.16 | 0.17 (0.12, 0.41) | 3 | 0.25 ± 0.19 | 0.22 (0.08, 0.45) | 6 | 0.15 ± 0.08 | 0.17 (0.03, 0.24) | ||||
| Biochemistry Parameters Reference Range (Unit) | Visit Code | G1 | G2 | G3 and 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | Median (Range) | * p Value | n | Mean ± SD | Median (Range) | * p Value | n | Mean ± SD | Median (Range) | p Value | ||
| Aspartate aminotransferase (AST) 12.3–74.7 (U/L) | Day 1 | 3 | 21.47 ± 6.45 | 22.3 (16.1, 29) | 0.07 * | 3 | 22.5 ± 5.55 | 22.4 (17, 28.1) | 0.14 * | 6 | 28.6 ± 4.67 | 27.25 (22.4, 36.1) | 0.62 * |
| Day 3 | 3 | 27.47 ± 13.92 | 24.6 (15.2, 42.6) | 3 | 31.33± 7.36 | 29.9 (24.8, 39.3) | 6 | 39.42 ± 23.31 | 34.85 (19.2, 84.1) | ||||
| Day 7 | 3 | 32.5 ± 10.11 | 29.4 (24.3, 43.8) | 3 | 34.8 ± 12.70 | 37.7 (20.9, 45.8) | 6 | 53.25 ± 47.17 | 36.3 (29.3, 149.3) | ||||
| Day 14 | 3 | 23.6 ± 5.40 | 23.8 (18.1, 28.9) | 3 | 23.1 ± 3.40 | 22.4 (20.1, 26.8) | 6 | 38.2 ± 14.55 | 35.65 (25.7, 65.6) | ||||
| Day 28 | 3 | 23.83 ± 4.76 | 23.4 (19.3, 28.8) | 3 | 52.83 ± 14.86 | 58.9 (35.9, 63.7) | 6 | 35.42 ± 5.27 | 35.7 (26.9, 40.8) | ||||
| Alanine aminotransferase (ALT) 3.5–46.8 (U/L) | Day 1 | 3 | 3 | 13.77± 5.25 | 12.9 (9, 19.4) | 0.03 | 6 | 23.25 ± 6.49 | 20.85 (17.3, 34 | 0.002 * | |||
| Day 3 | 3 | 3 | 28.97 ± 5.66 | 29.9 (22.9, 34.1) | 6 | 36.23 ± 24.01 | 27.45 (18.2, 81.8) | ||||||
| Day 7 | 3 | 3 | 45 ±21.81 | 50.4 (21, 63.6) | 6 | 68.62 ± 53.51 | 46.05 (33.8, 174.7) | ||||||
| Day 14 | 3 | 3 | 23.73 ± 0.51 | 27.8 (11.8, 31.6) | 6 | 48.35 ± 23.55 | 44.65 (25.3, 89.1) | ||||||
| Day 28 | 3 | 3 | 12.87 ± 2.82 | 13.8 (9.7, 15.1) | 6 | 26.87 ± 13.60 | 21.1 (16.1, 53.3) | ||||||
| Total Bilirubin 3.1–31.1 (μmol/L) | Day 1 | 3 | 11.07 ± 4.38 | 12.7 (6.1,14.4) | 0.23 * | 3 | 8.63± 0.68 | 8.4 (8.1, 9.4) | 0.41 | 6 | 15.65 ± 13.35 | 10.4 (7.7, 42.7) | 0.0004 * |
| Day 3 | 3 | 5.6 ± 2.98 | 6.1 (2.4, 8.3) | 3 | 7.43 ± 2.40 | 8.4 (4.7, 9.2) | 6 | 08.03 ± 6.91 | 5.2 (2.9, 21.6) | ||||
| Day 7 | 3 | 6.37 ± 2.88 | 6.8 (3.3, 9) | 3 | 8.93± 2.90 | 9 (6, 11.8) | 6 | 09.4 ± 6.39 | 6.3 (4.9, 21.7) | ||||
| Day 14 | 3 | 8.13 ± 1.83 | 7.5 (6.7,10.2) | 3 | 11.13 ± 4.85 | 8.9 (7.8, 16.7) | 6 | 15.27 ± 9.09 | 13.2 (7.2, 32.9) | ||||
| Day 28 | 3 | 8.67± 5.55 | 8.5 (3.2, 14.3) | 3 | 9.93 ± 1.86 | 9.7 (8.2, 11.9) | 6 | 13.37 ± 8.69 | 10.25 (7.2, 30.2) | ||||
| Creatinine 49–95.3 (μmol/L) | Day 1 | 3 | 83.33 ± 7.77 | 81 (77, 92) | 0.10 * | 3 | 77.67 ± 7.57 | 81 (69, 83) | 0.13 | 6 | 79.67 ± 8.45 | 82.5 (67, 88) | 0.03 * |
| Day 3 | 3 | 70± 3 | 70 (67, 73) | 3 | 76.67± 7.57 | 80 (68, 82) | 6 | 96.83 ± 9.70 | 99 (82, 108) | ||||
| Day 7 | 3 | 86 ± 5.57 | 87 (80, 91) | 3 | 82 ±10.54 | 83 (71, 92) | 6 | 80.27 ± 8.31 | 79.8 (7, 91) | ||||
| Day 14 | 3 | 87.33± 2.08 | 88 (85, 89) | 3 | 79.67 ± 8.02 | 79 (72, 88) | 6 | 83.13 ± 7.02 | 83 (75.3, 94) | ||||
| Day 28 | 3 | 89 ± 3.61 | 90 (85, 92) | 3 | 85.33 ± 7.37 | 88 (77, 91) | 6 | 88.03 ± 10.75 | 88.4 (70.8, 104.3) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassimu, K.; Milando, F.; Omolo, J.; Mdemu, A.; Nyaulingo, G.; Mbarak, H.; Mohamed, L.; Rashid, R.; Ahmed, S.; Rashid, M.; et al. Safety and Tolerability of an Antimalarial Herbal Remedy in Healthy Volunteers: An Open-Label, Single-Arm, Dose-Escalation Study on Maytenus senegalensis in Tanzania. Trop. Med. Infect. Dis. 2022, 7, 396. https://doi.org/10.3390/tropicalmed7120396
Kassimu K, Milando F, Omolo J, Mdemu A, Nyaulingo G, Mbarak H, Mohamed L, Rashid R, Ahmed S, Rashid M, et al. Safety and Tolerability of an Antimalarial Herbal Remedy in Healthy Volunteers: An Open-Label, Single-Arm, Dose-Escalation Study on Maytenus senegalensis in Tanzania. Tropical Medicine and Infectious Disease. 2022; 7(12):396. https://doi.org/10.3390/tropicalmed7120396
Chicago/Turabian StyleKassimu, Kamaka, Florence Milando, Justin Omolo, Abel Mdemu, Gloria Nyaulingo, Hussein Mbarak, Latipha Mohamed, Ramla Rashid, Saumu Ahmed, Mohammed Rashid, and et al. 2022. "Safety and Tolerability of an Antimalarial Herbal Remedy in Healthy Volunteers: An Open-Label, Single-Arm, Dose-Escalation Study on Maytenus senegalensis in Tanzania" Tropical Medicine and Infectious Disease 7, no. 12: 396. https://doi.org/10.3390/tropicalmed7120396
APA StyleKassimu, K., Milando, F., Omolo, J., Mdemu, A., Nyaulingo, G., Mbarak, H., Mohamed, L., Rashid, R., Ahmed, S., Rashid, M., Msami, H., Damiano, D., Simon, B., Mbaga, T., Issa, F., Lweno, O., Balige, N., Hassan, O., Mwalimu, B., ... Abdulla, S. (2022). Safety and Tolerability of an Antimalarial Herbal Remedy in Healthy Volunteers: An Open-Label, Single-Arm, Dose-Escalation Study on Maytenus senegalensis in Tanzania. Tropical Medicine and Infectious Disease, 7(12), 396. https://doi.org/10.3390/tropicalmed7120396






