Arbovirus Seroprevalence Study in Bangphae District, Ratchaburi Province, Thailand: Comparison between ELISA and a Multiplex Rapid Diagnostic Test (Chembio DPP® ZCD IgG)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Serum Samples
2.2. In-House DENV IgG ELISA
2.3. In-House ZIKV NS1 IgG ELISA
2.4. CHIKV IgG ELISA
2.5. Chembio DPP® ZCD IgM/IgG System
2.6. Criteria for Seropositivity
2.7. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Arbovirus Seroprevalences
3.3. Co-Seropositivity for DENV, ZIKV, and CHIKV
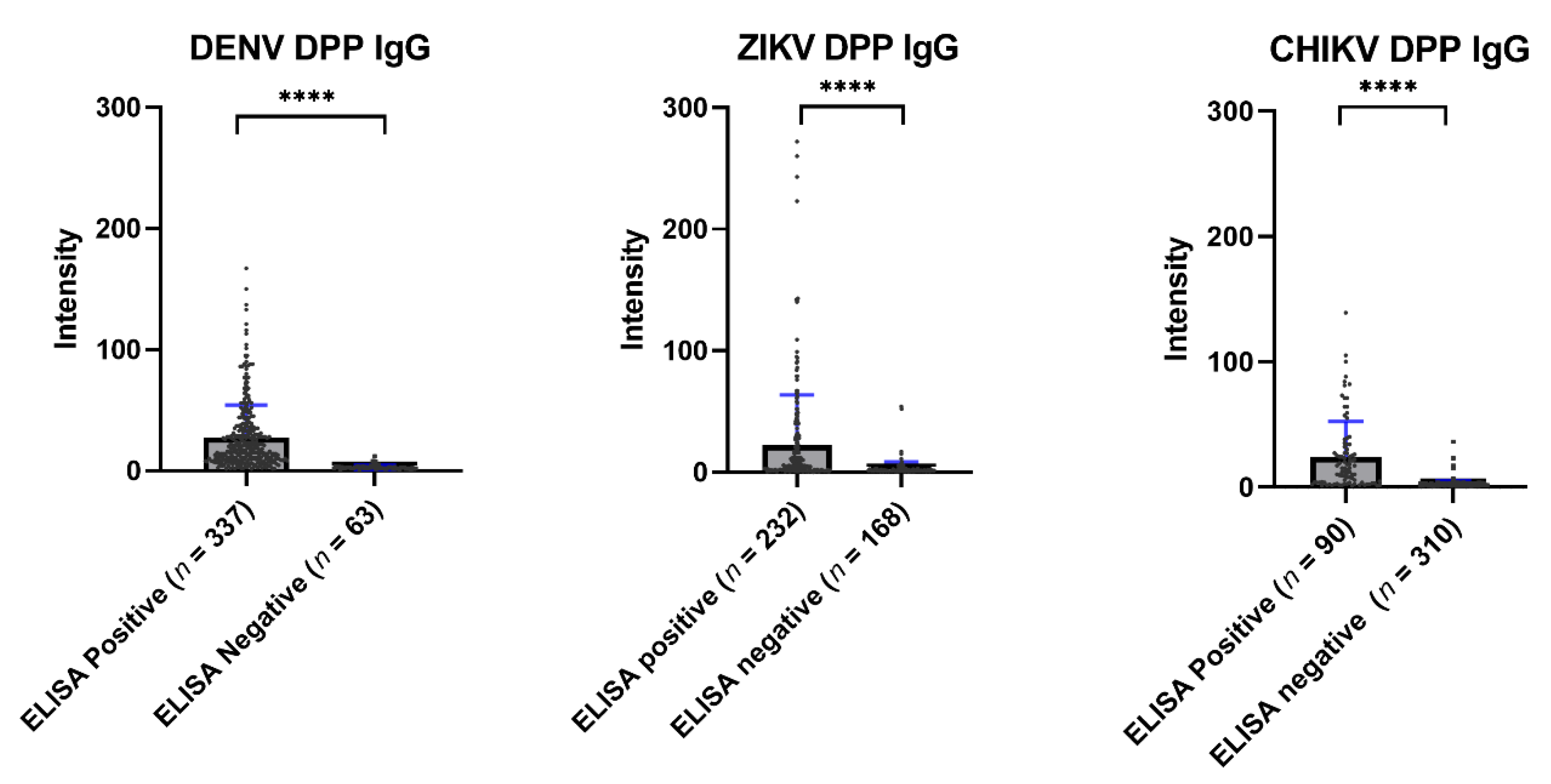
3.4. Comparison of ELISA and Chembio DPP® ZCD IgG
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bassett, M. The Emergence and History of Arboviruses, Microbiology Society. Available online: https://microbiologysociety.org/blog/the-emergence-and-history-of-arboviruses.html (accessed on 10 January 2022).
- Girard, M.; Nelson, C.B.; Picot, V.; Gubler, D.J. Arboviruses: A global public health threat. Vaccine 2020, 38, 3989–3994. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Lee, H.; Nishiura, H. Analysis of international traveler mobility patterns in Tokyo to identify geographic foci of dengue fever risk. Theor. Biol. Med. Model. 2021, 18, 17. [Google Scholar] [CrossRef]
- Endy, T.P.; Chunsuttiwat, S.; Nisalak, A.; Libraty, D.H.; Green, S.; Rothman, A.L.; Vaughn, D.W.; Ennis, F.A. Epidemiology of inapparent and symptomatic acute dengue virus infection: A prospective study of primary school children in Kamphaeng Phet, Thailand. Am. J. Epidemiol. 2002, 156, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Fuentes, O.; Martinez, E.; Perez, A.B. Dengue. In International Encyclopedia of Public Health, 2nd ed.; Quah, S.R., Ed.; Academic Press: Oxford, UK, 2017; pp. 233–257. [Google Scholar]
- Gubler, D.J. Dengue/dengue haemorrhagic fever: History and current status. Novartis Found. Symp. 2006, 277, 3–16; discussion 16–22, 71–73, 251–253. [Google Scholar] [CrossRef]
- Singhi, S.; Kissoon, N.; Bansal, A. Dengue and dengue hemorrhagic fever: Management issues in an intensive care unit. J. Pediatr. 2007, 83, S22–S35. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy for Dengue Prevention and Control 2012–2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/75303/9789241504034_eng.pdf (accessed on 10 January 2022).
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Macnamara, F.N. Zika virus: A report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1954, 48, 139–145. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef]
- Hennessey, M.; Fischer, M.; Staples, J.E. Zika Virus Spreads to New Areas—Region of the Americas, May 2015–January 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 55–58. [Google Scholar] [CrossRef]
- Schuler-Faccini, L.; Ribeiro, E.M.; Feitosa, I.M.; Horovitz, D.D.; Cavalcanti, D.P.; Pessoa, A.; Doriqui, M.J.; Neri, J.I.; Neto, J.M.; Wanderley, H.Y.; et al. Possible Association Between Zika Virus Infection and Microcephaly—Brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 59–62. [Google Scholar] [CrossRef]
- Pillinger, M. WHO Declared a Public Health Emergency about Zika’s Effects. Here Are Three Takeaways. Available online: https://www.washingtonpost.com/news/monkey-cage/wp/2016/02/02/who-declared-a-public-health-emergency-about-zikas-effects-here-are-three-takeaways/ (accessed on 10 January 2022).
- Quan, T.M.; Phuong, H.T.; Vy, N.H.T.; Thanh, N.T.L.; Lien, N.T.N.; Hong, T.T.K.; Dung, P.N.; Chau, N.V.V.; Boni, M.F.; Clapham, H.E. Evidence of previous but not current transmission of chikungunya virus in southern and central Vietnam: Results from a systematic review and a seroprevalence study in four locations. PLOS Negl. Trop. Dis. 2018, 12, e0006246. [Google Scholar] [CrossRef] [PubMed]
- Soumahoro, M.-K.; Boelle, P.-Y.; Gaüzere, B.-A.; Atsou, K.; Pelat, C.; Lambert, B.; La Ruche, G.; Gastellu-Etchegorry, M.; Renault, P.; Sarazin, M.; et al. The Chikungunya Epidemic on La Réunion Island in 2005–2006: A Cost-of-Illness Study. PLOS Negl. Trop. Dis. 2011, 5, e1197. [Google Scholar] [CrossRef] [PubMed]
- Van Bortel, W.; Dorleans, F.; Rosine, J.; Blateau, A.; Rousset, D.; Matheus, S.; Leparc-Goffart, I.; Flusin, O.; Prat, C.; Cesaire, R.; et al. Chikungunya outbreak in the Caribbean region, December 2013 to March 2014, and the significance for Europe. Eur. Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2014, 19, 20759. [Google Scholar] [CrossRef] [PubMed]
- Hapuarachchi, H.C.; Bandara, K.B.; Sumanadasa, S.D.; Hapugoda, M.D.; Lai, Y.L.; Lee, K.S.; Tan, L.K.; Lin, R.T.; Ng, L.F.; Bucht, G.; et al. Re-emergence of Chikungunya virus in South-east Asia: Virological evidence from Sri Lanka and Singapore. J. Gen. Virol. 2010, 91, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Wiwanitkit, V. The current status of Zika virus in Southeast Asia. Epidemiol. Health 2016, 38, e2016026. [Google Scholar] [CrossRef]
- Forshey, B.M.; Guevara, C.; Laguna-Torres, V.A.; Cespedes, M.; Vargas, J.; Gianella, A.; Vallejo, E.; Madrid, C.; Aguayo, N.; Gotuzzo, E.; et al. Arboviral etiologies of acute febrile illnesses in Western South America, 2000–2007. PLoS Negl. Trop. Dis. 2010, 4, e787. [Google Scholar] [CrossRef]
- Labeaud, A.D.; Bashir, F.; King, C.H. Measuring the burden of arboviral diseases: The spectrum of morbidity and mortality from four prevalent infections. Popul. Health Metr. 2011, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Tongthainan, D.; Mongkol, N.; Jiamsomboon, K.; Suthisawat, S.; Sanyathitiseree, P.; Sukmak, M.; Wajjwalku, W.; Poovorawan, Y.; Ieamsaard, G.; Sangkharak, B.; et al. Seroprevalence of Dengue, Zika, and Chikungunya Viruses in Wild Monkeys in Thailand. Am. J. Trop. Med. Hyg. 2020, 103, 1228–1233. [Google Scholar] [CrossRef]
- Limkittikul, K.; Chanthavanich, P.; Lee, K.S.; Lee, J.S.; Chatchen, S.; Lim, S.K.; Arunsodsai, W.; Yoon, I.K.; Lim, J.K. Dengue virus seroprevalence study in Bangphae district, Ratchaburi, Thailand: A cohort study in 2012–2015. PLoS Negl. Trop. Dis. 2022, 16, e0010021. [Google Scholar] [CrossRef] [PubMed]
- Sirivichayakul, C.; Limkittikul, K.; Chanthavanich, P.; Yoksan, S.; Ratchatatat, A.; Lim, J.K.; Arunsodsai, W.; Sabchareon, A. Monoclonal antibody-based capture ELISA in the diagnosis of previous dengue infection. Virol. J. 2019, 16, 125. [Google Scholar] [CrossRef]
- Sriburin, P.; Sittikul, P.; Kosoltanapiwat, N.; Sirinam, S.; Arunsodsai, W.; Sirivichayakul, C.; Limkittikul, K.; Chatchen, S. Incidence of Zika Virus Infection from a Dengue Epidemiological Study of Children in Ratchaburi Province, Thailand. Viruses 2021, 13, 1802. [Google Scholar] [CrossRef] [PubMed]
- Sittikul, P.; Sriburin, P.; Rattanamahaphoom, J.; Limkittikul, K.; Sirivichayakul, C.; Chatchen, S. Combining Immunoassays to Identify Zika Virus Infection in Dengue-Endemic Areas. Trop. Med. Infect. Dis. 2022, 7, 254. [Google Scholar] [CrossRef] [PubMed]
- Phatihattakorn, C.; Wongsa, A.; Pongpan, K.; Anuwuthinawin, S.; Mungmanthong, S.; Wongprasert, M.; Tassaneetrithep, B. Seroprevalence of Zika virus in pregnant women from central Thailand. PLoS ONE 2021, 16, e0257205. [Google Scholar] [CrossRef] [PubMed]
- Legros, V.; Jeannin, P.; Burlaud-Gaillard, J.; Chaze, T.; Gianetto, Q.G.; Butler-Browne, G.; Mouly, V.; Zoladek, J.; Afonso, P.V.; Gonzàlez, M.N.; et al. Differentiation-dependent susceptibility of human muscle cells to Zika virus infection. PLoS Negl. Trop. Dis. 2020, 14, e0008282. [Google Scholar] [CrossRef] [PubMed]
- Coelho, F.C.; Durovni, B.; Saraceni, V.; Lemos, C.; Codeco, C.T.; Camargo, S.; de Carvalho, L.M.; Bastos, L.; Arduini, D.; Villela, D.A.; et al. Higher incidence of Zika in adult women than adult men in Rio de Janeiro suggests a significant contribution of sexual transmission from men to women. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2016, 51, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S.; Kamaraj, P.; Khan, S.A.; Allam, R.R.; Barde, P.V.; Dwibedi, B.; Kanungo, S.; Mohan, U.; Mohanty, S.S.; Roy, S.; et al. Seroprevalence of chikungunya virus infection in India, 2017: A cross-sectional population-based serosurvey. Lancet Microbe 2021, 2, e41–e47. [Google Scholar] [CrossRef]
- Ruchusatsawat, K.; Wongjaroen, P.; Posanacharoen, A.; Rodriguez-Barraquer, I.; Sangkitporn, S.; Cummings, D.A.T.; Salje, H. Long-term circulation of Zika virus in Thailand: An observational study. Lancet Infect. Dis. 2019, 19, 439–446. [Google Scholar] [CrossRef]
- Sirinam, S.; Chatchen, S.; Arunsodsai, W.; Guharat, S.; Limkittikul, K. Seroprevalence of Zika Virus in Amphawa District, Thailand, after the 2016 Pandemic. Viruses 2022, 14, 476. [Google Scholar] [CrossRef]
- Khongwichit, S.; Chansaenroj, J.; Thongmee, T.; Benjamanukul, S.; Wanlapakorn, N.; Chirathaworn, C.; Poovorawan, Y. Large-scale outbreak of Chikungunya virus infection in Thailand, 2018–2019. PLoS ONE 2021, 16, e0247314. [Google Scholar] [CrossRef]
- Boeras, D.; Diagne, C.T.; Pelegrino, J.L.; Grandadam, M.; Duong, V.; Dussart, P.; Brey, P.; Ruiz, D.; Adati, M.; Wilder-Smith, A.; et al. Evaluation of Zika rapid tests as aids for clinical diagnosis and epidemic preparedness. EClinicalMedicine 2022, 49, 101478. [Google Scholar] [CrossRef]
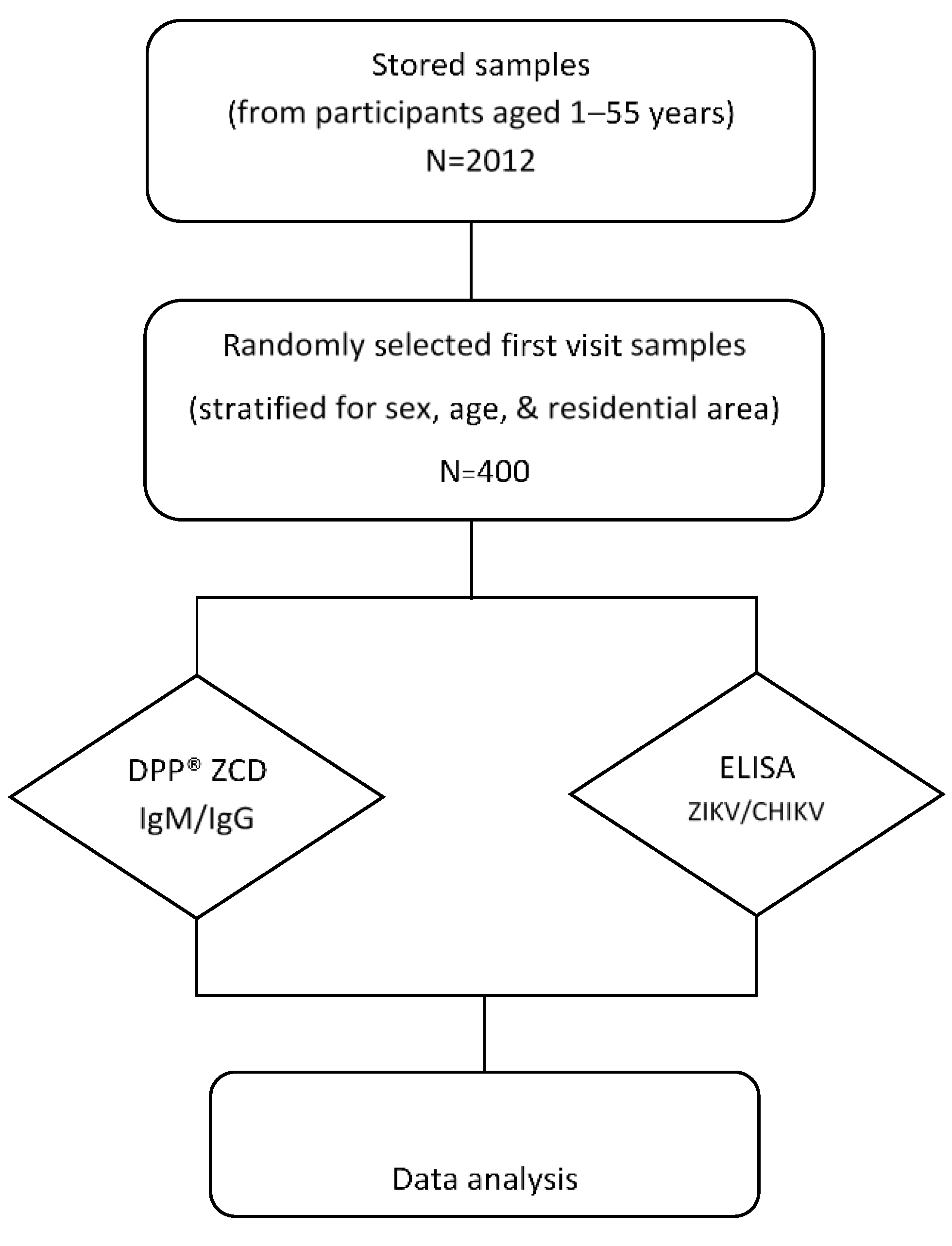

| Visit 1 | Selected | |
|---|---|---|
| number/samples | 2012 | 400 |
| Male:Female | 903:1109 | 200:200 |
| 0.81:1 | 1:1 | |
| Sub-district | ||
| Wang-Yen | 348 (17.30) | 57 |
| Bang-Phae | 301 (15.00) | 58 |
| Wat-Kaew | 250 (12.40) | 57 |
| Hau-Pho | 254 (12.60) | 57 |
| Don-Kha | 152 (7.60) | 57 |
| Don-Yai | 203 (10.00) | 57 |
| Pho-Hak | 504 (25.00) | 57 |
| Age group | ||
| 1–10 | 695 (34.54) | 80 |
| 11–20 | 733 (36.43) | 80 |
| 21–30 | 180 (8.95) | 80 |
| 31–40 | 170 (8.45) | 80 |
| 41–55 | 234 (11.63) | 80 |
| DENV | ZIKV | CHIKV | |
|---|---|---|---|
| number/samples | 337 (84.25) | 232 (58.00) | 90 (22.50) |
| Male:Female | 163:174 | 117:115 | 39:51 |
| 0.94:1 | 1.02:1 | 0.76:1 | |
| Sub-district | |||
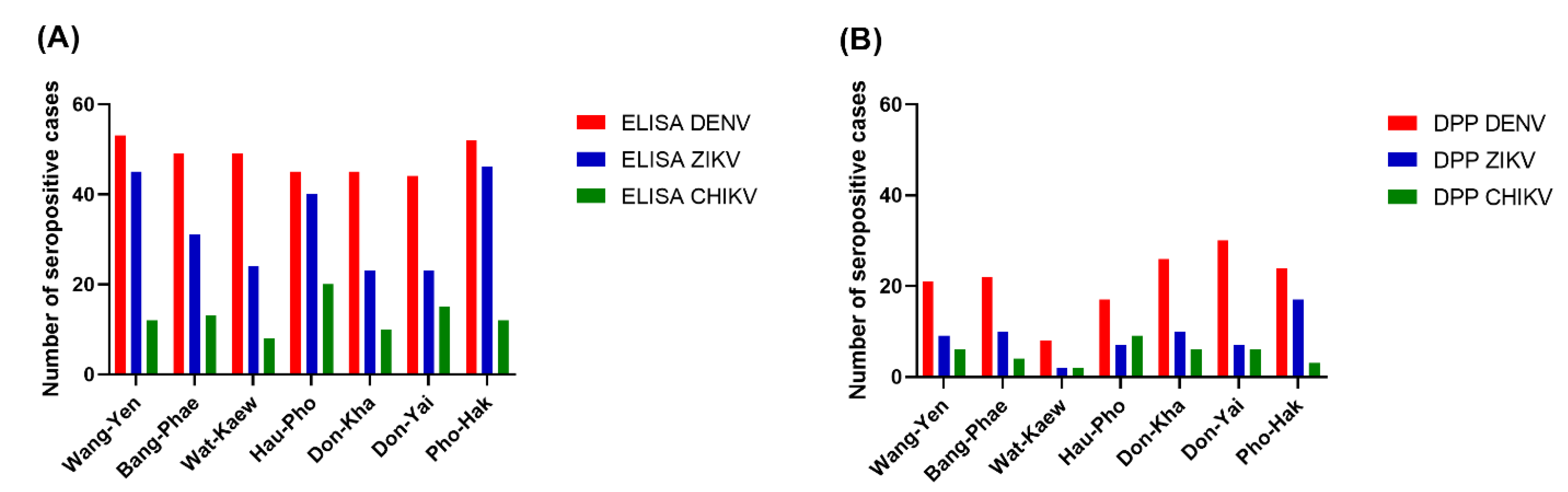
| Wang-Yen | 53 (92.98) | 45 (78.95) | 12 (21.05) |
| Bang-Phae | 49 (84.48) | 31 (53.45) | 13 (22.41) |
| Wat-Kaew | 49 (85.96) | 24 (42.11) | 8 (14.04) |
| Hau-Pho | 45 (78.95) | 40 (70.18) | 20 (35.09) |
| Don-Kha | 45 (78.95) | 23 (40.35) | 10 (17.54) |
| Don-Yai | 44 (77.19) | 23 (40.35) | 15 (26.32) |
| Pho-Hak | 52 (91.23) | 46 (80.70) | 12 (21.05) |
| Age group | |||
| 1–10 | 37 (46.25) | 25 (31.25) | 4 (5.00) |
| 11–20 | 65 (81.25) | 51 (63.75) | 5 (6.25) |
| 21–30 | 76 (95.00) | 40 (50.00) | 6 (7.50) |
| 31–40 | 80 (100.00) | 57 (71.25) | 26 (32.50) |
| 41–55 | 79 (98.75) | 59 (73.75) | 49 (61.25) |
| DENV IgM | DENV IgG | ZIKV IgM | ZIKV IgG | CHIKV IgM | CHIKV IgG | |
|---|---|---|---|---|---|---|
| number/ samples | 28 (7.00) | 148 (37.00) | 5 (1.25) | 62 (15.50) | 14 (3.50) | 35 (8.75) |
| Male:Female | 17:11 | 72:76 | 3:2 | 30:32 | 6:8 | 14:21 |
| 1.55:1 | 0.95:1 | 1.50:1 | 0.94:1 | 0.75:1 | 0.67:1 | |
| Sub-district | ||||||
| Wang-Yen | 4 (7.02) | 21 (36.84) | 1 (1.75) | 9 (15.79) | 2 (3.51) | 6 (12.53) |
| Bang-Phae | 2 (3.45) | 22 (37.93) | 0 (0.00) | 10 (17.24) | 2 (3.45) | 4 (6.90) |
| Wat-Kaew | 1 (1.75) | 8 (14.04) | 1 (1.75) | 2 (3.51) | 3 (5.26) | 2 (3.51) |
| Hau-Pho | 1 (1.75) | 17 (29.82) | 1 (1.75) | 7 (12.28) | 2 (3.51) | 9 (15.79) |
| Don-Kha | 3 (5.26) | 26 (45.61) | 1 (1.75) | 10 (17.54) | 1 (1.75) | 5 (8.77) |
| Don-Yai | 11 (19.30) | 30 (52.63) | 1 (1.75) | 7 (12.28) | 2 (3.51) | 6 (10.53) |
| Pho-Hak | 6 (10.53) | 24 (42.11) | 0 (0.00) | 7 (12.28) | 2 (3.51) | 3 (5.26) |
| Age group | ||||||
| 1–10 | 3 (3.75) | 13 (16.25) | 1 (1.25) | 2 (2.50) | 4 (5.00) | 0 (0.00) |
| 11–20 | 6 (7.50) | 30 (37.50) | 2 (2.50) | 14 (17.50) | 2 (2.50) | 0 (0.00) |
| 21–30 | 8 (10.00) | 33 (41.25) | 1 (1.25) | 11 (13.75) | 3 (3.75) | 1 (1.25) |
| 31–40 | 5 (6.25) | 33 (41.25) | 1 (1.25) | 18 (22.50) | 2 (2.50) | 10 (12.50) |
| 41–55 | 6 (7.50) | 39 (48.75) | 0 (0.00) | 17 (21.25) | 3 (3.75) | 24 (30.00) |
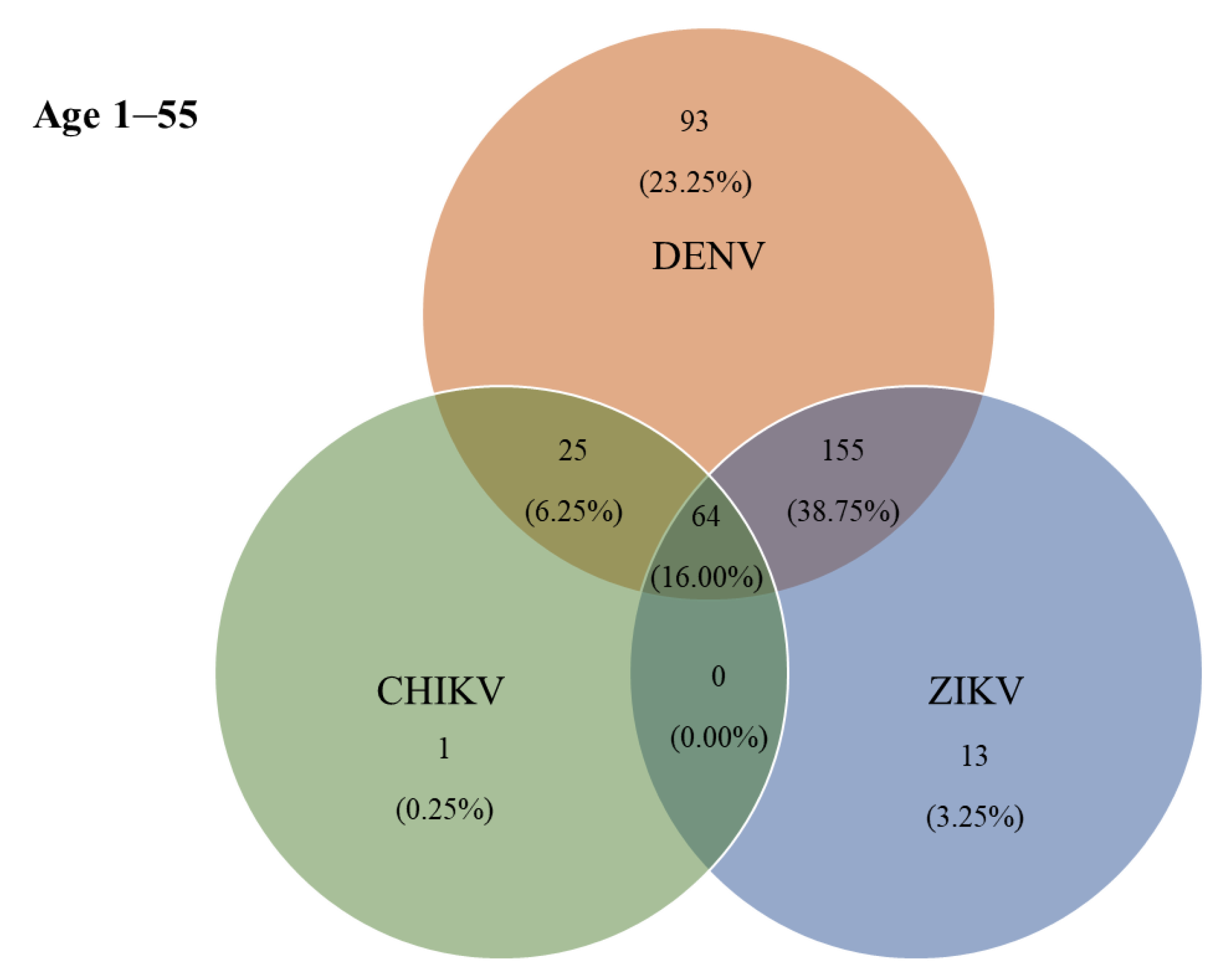
| DENV | ZIKV | CHIKV | D + Z | D + C | Z + C | D + Z + C | |
|---|---|---|---|---|---|---|---|
| number/ samples | 93 (23.25) | 13 (3.25) | 1 (0.25) | 155 (38.75) | 25 (6.25) | 0 (0.00) | 64 (16.00) |
| Age group | |||||||
| 1–10 | 17 (21.25) | 6 (7.50) | 1 (1.25) | 17 (21.25) | 1 (1.25) | 0 (0.00) | 2 (2.50) |
| 11–20 | 18 (22.50) | 6 (7.50) | 0 (0.00) | 42 (52.50) | 2 (2.50) | 0 (0.00) | 3 (3.75) |
| 21–30 | 33 (41.25) | 0 (0.00) | 0 (0.00) | 37 (46.25) | 3 (3.75) | 0 (0.00) | 3 (3.75) |
| 31–40 | 16 (20.00) | 0 (0.00) | 0 (0.00) | 38 (47.50) | 7 (8.75) | 0 (0.00) | 19 (23.75) |
| 41–55 | 9 (11.25) | 1 (1.25) | 0 (0.00) | 21 (26.25) | 12 (15.00) | 0 (0.00) | 37 (46.25) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakma, R.; Sriburin, P.; Sittikul, P.; Rattanamahaphoom, J.; Nuprasert, W.; Thammasonthijarern, N.; Maneekan, P.; Thaipadungpanit, J.; Arunsodsai, W.; Sirivichayakul, C.; et al. Arbovirus Seroprevalence Study in Bangphae District, Ratchaburi Province, Thailand: Comparison between ELISA and a Multiplex Rapid Diagnostic Test (Chembio DPP® ZCD IgG). Trop. Med. Infect. Dis. 2022, 7, 378. https://doi.org/10.3390/tropicalmed7110378
Chakma R, Sriburin P, Sittikul P, Rattanamahaphoom J, Nuprasert W, Thammasonthijarern N, Maneekan P, Thaipadungpanit J, Arunsodsai W, Sirivichayakul C, et al. Arbovirus Seroprevalence Study in Bangphae District, Ratchaburi Province, Thailand: Comparison between ELISA and a Multiplex Rapid Diagnostic Test (Chembio DPP® ZCD IgG). Tropical Medicine and Infectious Disease. 2022; 7(11):378. https://doi.org/10.3390/tropicalmed7110378
Chicago/Turabian StyleChakma, Ruba, Pimolpachr Sriburin, Pichamon Sittikul, Jittraporn Rattanamahaphoom, Warisa Nuprasert, Nipa Thammasonthijarern, Pannamas Maneekan, Janjira Thaipadungpanit, Watcharee Arunsodsai, Chukiat Sirivichayakul, and et al. 2022. "Arbovirus Seroprevalence Study in Bangphae District, Ratchaburi Province, Thailand: Comparison between ELISA and a Multiplex Rapid Diagnostic Test (Chembio DPP® ZCD IgG)" Tropical Medicine and Infectious Disease 7, no. 11: 378. https://doi.org/10.3390/tropicalmed7110378
APA StyleChakma, R., Sriburin, P., Sittikul, P., Rattanamahaphoom, J., Nuprasert, W., Thammasonthijarern, N., Maneekan, P., Thaipadungpanit, J., Arunsodsai, W., Sirivichayakul, C., Limkittikul, K., & Chatchen, S. (2022). Arbovirus Seroprevalence Study in Bangphae District, Ratchaburi Province, Thailand: Comparison between ELISA and a Multiplex Rapid Diagnostic Test (Chembio DPP® ZCD IgG). Tropical Medicine and Infectious Disease, 7(11), 378. https://doi.org/10.3390/tropicalmed7110378






