Abstract
In spite of ongoing eradication programs, helminth infections are still a medical issue in Ghana. For follow-up assessments on the decline of regional helminth infections, historic baseline prevalence values obtained with standardized diagnostic procedures can be helpful. In this retrospective cross-sectional study, real-time PCR targeting the nematodes Ancylostoma spp. (ITS2), Ascaris lumbricoides (ITS1), Enterobius vermicularis (ITS1), Necator americanus (ITS2), Strongyloides stercoralis (18S rRNA) and Trichuris trichiura (18S rRNA), the trematodes Schistosoma spp. (ITS2) as well as the cestodes Hymenolepis nana (ITS1), Taenia saginata (ITS1) and Taenia solium (ITS1) was applied with 2046 DNA eluates from stool samples of Ghanaian children from the Ashanti region collected between 2007 and 2008 in order to retrospectively define prevalence values. The overall prevalence was low with 3.8% (n = 77) and only 0.1% (n = 2) double infections with helminths were recorded. The three most frequently detected enteric helminth species comprised 2% S. stercoralis (n = 41), 0.8% H. nana (n = 16), and 0.7% N. americanus (n = 14), while only sporadic infection events were recorded for other helminth species comprising 0.1% E. vermicularis (n = 2), 0.1% Schistosoma spp. (n = 2), 0.1% T. saginata (n = 1) and 0.1% T. trichiura (n = 1). A. lumbricoides, Ancylostoma spp. and T. solium were not detected at all. In conclusion, the retrospective assessment suggests a low prevalence of enteric helminth infections in Ghanaian children from the Ashanti Region within the assessment period between 2007 and 2008.
Keywords:
helminth; epidemiology; diarrhea; Ascaris; hookworm; Strongyloides; Trichuris; Taenia; Schistosoma; Hymenolepis; Enterobius; Ghana 1. Introduction
Intestinal helminth infections are common, particularly in resource-limited tropical settings [1,2] where access even to baseline hygiene precautions such as hand washing with soap is sometimes scarcely available [3]. In addition, meta-analyses have suggested the association of specific helminth infections with age, sex, co-infections, previous treatment, and lifestyle [4,5]. In the tropics, co-infections with different helminths as well as co-infections of helminths and other severe infections are quite frequently observed, making mutual supportive interactions likely [6,7,8,9,10]. In contrast, the worm burden declines in settings showing socio-economic development where systematic deworming programs are implemented [11].
From the public health perspective, deworming programs are useful because intestinal helminth infections have been reported to be associated with stunted growth, cognitive impairment [12,13], likely effects even on adult productivity [14] as well as with pregnancy and birth complications [15,16]. As a complication of hookworm infections in Ghana, resistance determinants against benzimidazoles are common [17], which might partially explain the varying effectiveness of benzimidazole-based treatment as observed in Ghanaian patients [18,19].
For West African Ghana, the prevalence of multiple helminth infections has been reported. In Ghanaian individuals, infection rates with intestinal helminths have been shown to be in the range of 2–22% [20,21,22,23] with declining prevalence over recent decades [24], urogenital schistosomiasis in the range of 2.5–12% [20,24], while intestinal schistosomiasis was regionally reported for more than 90% of assessed Ghanaian children [25] but in less than 2% for other Ghanaian patients [22]. Swimming in surface water is an independent risk factor for schistosomiasis in Ghana [26,27], and reinfection rates are high with up to 40% within six months in some areas [28]. In historic assessments covering the previous decades, very high infection rates >50% were quite common for schistosomiasis in Ghana [29,30,31,32,33]. In exposed individuals such as waste handlers, prevalence rates for soil-transmissible helminths of 5% have been reported [34], and prevalence rates >20% were observed in farmers [35]. In the case of Ghanaian farmers, wastewater irrigation increases the risk of intestinal helminth infections by factor 3 [36]. Cases of taeniasis have been detected in Ghana including cerebral affections [21,37] same as trematode infections with Fasciola gigantica [38] and Dicrocoelium dendriticum [39]. Even solitary egg findings suggesting infections with small liver flukes like Clonorchis spp. or Opisthorchis spp. have been reported from Ghana [40]. Frequently detected intestinal helminths in Ghana comprise Ascaris lumbricoides, hookworms with higher infection rates for Necator americanus than for Ancylostoma spp., Hymenolepis spp., Taenia spp., Strongyloides stercoralis, Schistosoma spp. and Trichuris trichiura [21,22,23,41,42,43]. In spite of declining overall helminth infection rates, intestinal helminths are yet among the top five outpatient morbidities in Ghana [44], making Ghana a suitable site to study inference between helminth infections and other diseases like, e.g., allergic diatheses [45]. Consequently, modeling suggested that Ghana is among the countries where the interruption of transmission of soil-transmitted helminths may become challenging [46]. Animal reservoirs are elements of the transmission cycles as well [47].
To follow up with the decline of helminth infections in Ghana [44] with the aim of final eradication, information on historic prevalence values determined with up-to-date real-time PCR approaches, which were not available yet when the samples were collected, can be helpful to define baseline prevalence values. Accordingly, enteric helminth-specific real-time PCR from frozen residual DNA eluate samples derived from stool specimens of children with and without diarrhea collected in the Ghanaian Ashanti region between 2007 and 2008 [48,49,50,51,52,53] was performed in order to contribute to the available epidemiologic knowledge on historic regional intestinal helminth prevalence.
2. Materials and Methods
2.1. Study Type and Sample Collection
For the retrospective cross-sectional assessment, residual nucleic acid extractions from 2046 stool samples which were collected in the course of a study from 2007 till 2008 from Ghanaian children from the Ashanti Region with and without clinical diarrhea (defined by ≥3 unformed stools per day) were included in the assessment. As detailed elsewhere, multiple screenings for viral, bacterial, and protozoan enteropathogens were performed with those samples [48,49,50,51,52,53], while molecular helminth assessment had not yet been conducted so far. Nucleic acid extraction was performed with the QiaAMP DNA Stool Mini Kit (Qiagen, Hilden, Germany) as described by the manufacturer. Subsequently, the samples were stored frozen at −80 °C. As reported previously [48,49,50,51,52,53], children were ≤13 years of age with a median of less than 3 years in a left-shifted distribution, while the proportions of boys and girls as well as the proportions of individuals with and without diarrhea were nearly evenly distributed. Further, about one out of five children were diagnosed with malaria at the time of the assessment.
2.2. Applied Real-Time PCRs for the Detection of Helminth DNA in Stool Samples, Inclusion and Exclusion Criteria and Statistical Assessment
All nucleic acid extractions from human stool were subjected to in-house multiplex real-time PCR targeting Ascaris lumbricoides (ITS1, minimum detectable genomic equivalent: 1.3 × 102), Ancylostoma spp. (ITS2, minimum detectable genomic equivalent: 1.3 × 102), Enterobius vermicularis (ITS1, minimum detectable genomic equivalent: 1.6 × 101), Hymenolepis nana (ITS1, minimum detectable genomic equivalent: 1.4 × 101), Necator americanus (ITS2, minimum detectable genomic equivalent: 1.3 × 102), Schistosoma spp. (detecting S. haematobium, S. mansoni and S. intercalatum without discrimination on the species level, ITS2, minimum detectable genomic equivalent: 3.0 × 100), Strongyloides stercoralis (18S rRNA, minimum detectable genomic equivalent: 1.3 × 102), Taenia saginata (ITS1, minimum detectable genomic equivalent: 9.0 × 100), Taenia solium (ITS1, minimum detectable genomic equivalent: 1.3 × 101), and Trichuris trichiura (18S rRNA, minimum detectable genomic equivalent: 1.1 × 101), respectively. Plasmid-based positive controls and PCR-grade water-based negative controls were included in each real-time PCR run. The sequences of the primer and probe oligonucleotides as well as of the positive control plasmid inserts as published elsewhere [54] are shown in Appendix A Table A1. The real-time PCRs were performed on RotorGene Q thermocyclers exactly as described elsewhere; performance characteristics of the assays have been provided there as well [54]. Based on the experience of a previous multicentric evaluation study [54] and participation in the international external laboratory assessment scheme for helminth PCR [55], late real-time PCR signals with cycle threshold (Ct) values higher than 40 with typical sigmoid-shaped amplification curves still indicate specific amplification. All samples, for which sufficient residual nucleic acid material was available, were included in the assessment. The study samples were treated in the same way as patient samples in diagnostic routine use of the applied helminth PCR assays without technical replicates. There were no exclusion criteria. The results were descriptively demonstrated without further statistical analyses.
2.3. Ethics
Ethical clearance for the sample collection and the informed consent procedure was obtained from the Committee on Human Research, Publications and Ethics, School of Medical Science, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana (reference CHRPE/KNUST/KATH/01_10_08). To be included in the study, written informed consent was obtained from the parents or the legal guardian prior to the enrolment. In case of non-participation, medical treatment was nevertheless provided. Further, anonymous characterization of residual samples was granted by the medical association of Hamburg, Germany, (reference number: WF-011/19, obtained on 11 March 2019). The assessments were performed in line with the Declaration of Helsinki and its amendments.
3. Results
From a total of 2046 included residual sample materials, positive real-time PCR results were obtained from 3.8% (n = 77) samples. Prevalence values for detected target DNA of the different assessed helminths ranged from 0.0% (n = 0) to 2.0% (n = 41). Prevalence values > 0.5% were recorded for only three species with 2.0% Strongyloides stercoralis (n = 41), 0.8% Hymenolepis nana (n = 16), and 0.7% Necator americanus (n = 14). Solely individual cases were observed for other helminths with 0.1% Enterobius vermicularis (n = 2), 0.1% Schistosoma spp. (n = 2), 0.1% Taenia saginata (n = 1), and 0.1% Trichuris trichiura (n = 1). No cases at all were seen for Ascaris lumbricoides, Ancylostoma spp. and Taenia solium (Figure 1). Details including the recorded cycle threshold (Ct value) ranges are provided in Table 1. In 2.6% (2/77) of the positive samples and thus in 0.1% (2/2046) of the totally assessed samples, co-infections with different helminths were recorded. The co-infections comprised two different target helminths each, i.e., T. trichiura and T. saginata in one case as well as N. americanus and S. stercoralis in the other case.
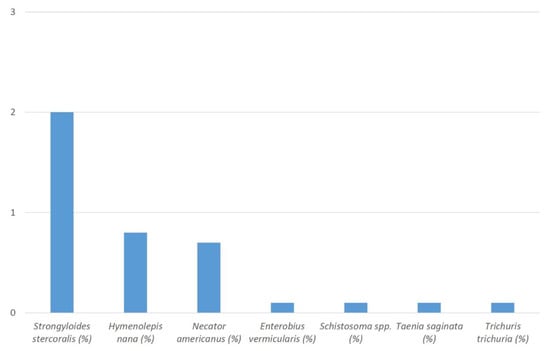
Figure 1.
Percentages (%) of recorded helminth DNA in the stool samples of the study participants.

Table 1.
Positive PCR results and recorded cycle threshold (Ct) value ranges. A total of 2046 samples were assessed.
Focusing on associations of helminth infections and malaria, the co-incidence of helminth infections and malaria was 22.7% (14/77). Helminth infections co-occurring with malaria comprised S. stercoralis (n = 6), H. nana (n = 5), E. vermicularis (n = 1), N. americanus (n = 1), and a co-infection with S. stercoralis and N. americanus (n = 1). A minority of 24.7% (19/77) of the recorded helminth infections was associated with reported diarrhea, comprising S. stercoralis (n = 12), H. nana (n = 3), N. americanus (n = 3), and Schistosoma spp. (n = 1). No significant differences were observed between cycle threshold (Ct) values of helminth infections in patients with and without diarrhea (Table 2).

Table 2.
Ct values in case of helminth infections in patients with and without reported diarrhea.
4. Discussion
A PCR-based assessment of helminth prevalences was performed with stool samples of Ghanaian children from the Ashanti Region. Residual sample material was used that was collected in the years 2007 and 2008 [48,49,50,51,52,53]. Therefore, baseline prevalence values for follow-up assessments were established. In line with ongoing intervention programs in Ghana [56], the overall prevalence of recorded helminth infections was low. In detail, the epidemiological coverage of anti-helminthic mass drug administration for Ghanaian pre-school children was estimated to be 98.37% in the study year 2008 [57], likely explaining the very low detection rates in the stool samples. Although socioeconomic and behavioral aspects specifically related to helminth infections had not been systematically recorded for the study population, malnourishment of no more than 10% and vaccination rates ranging between 80% and more than 90% as reported elsewhere [48] suggest little hints for neglect and a good general access of the assessed children to the country’s public health infrastructure.
In contrast to the low prevalence values as observed in the study here, estimates of the helminth prevalence on a Pan-African level in the decade of the study period were much higher. In a review from 2009, Sub-Saharan African prevalence estimates for enteric infections with hookworms, A. lumbricoides and T. trichiura but also for infections with Schistosoma spp. were higher than 20% each [58]. Another research group [6] argued that those estimates might have been too high, suggesting lower prevalence estimates of 16.5% for hookworms, 6.6% for A. lumbricoides, and 4.4% for T. trichiura instead. Still, those estimates were much higher than the proportions of infections observed in the assessed Ghanaian children.
In line with previous assessments in Ghana [20,21,22,23,42,43], nematodes such as S. stercoralis and hookworms quantitatively dominated. In comparison, the very low rate of Schistosoma spp. was less expected [25,59] but reflects the scattered distribution of S. mansoni as reported for Ghana [22,60,61]. Interestingly, the cestode H. nana was the second most frequent helminth within the assessed Ghanaian stool samples, although its prevalence was still low and well in line with previous scarcely available Ghanaian studies including this parameter [21,62]. Real-time PCR-based screening for H. nana is yet rarely applied in epidemiological studies compared to more frequently used assays targeting nematodes [54]. The protocol from this study was first introduced after evaluation in 2020 [54]. All other helminths included in the screening were only rarely identified or absent. Helminth co-infections, i.e., infections with more than one helminth species, were observed in two instances (0.1%) only.
Due to the very low overall detection rates, assessment of associations with demographic features or clinical features was not possible. It should be noted that such associations are difficult to interpret because of the high rates of co-infections with facultative enteropathogenic bacteria and protozoa [48,49]. While only a minority of 19 helminth infections with a distribution resembling the overall distribution of positive helminth real-time PCR results in this study was associated with reported diarrhea, a total of 27 co-infections with the bacterial and protozoan pathogens Campylobacter jejuni (n = 10), Giardia duodenalis (n = 7), Shigella spp./enteroinvasive Escherichia coli (not further discriminated, n = 6), Cryptosporidium parvum (n = 3) and Salmonella enterica (n = 1) had been previously detected in the same 19 samples [48]. Accordingly, any etiological relevance of the helminth detections with a focus on diarrhea is highly questionable for the donors of the respective stool samples, which is also in line with the seemingly paradox, non-significant finding of higher mean Ct values for H. nana and N. americanus in samples of patients with diarrhea compared to patients without diarrhea. In a similar way, the proportion of helminth detections in stool samples of patients with malaria just matched the overall proportion of malaria cases within the assessed population, not allowing for any further conclusions.
Interestingly, the abundance of the helminth species S. stercoralis and N. americanus in the assessed samples outnumbered orally transmitted helminths. The specific reasons are unknown because previous assessments of enteric pathogens other than helminths in the study population suggested frequent transmission events via the oral route [48,49]. So, it is likely that the finding more reflects a generally higher regional abundance of these species rather than a lower relevance of the oral transmission route.
The observed low abundance of Enterobius vermicularis is surprising in a cohort consisting of children. It remains unclear whether this finding was just a consequence of the Ghanaian anti-helminthic mass drug administration program [57]. Alternatively, sensitivity issues of the real-time PCR-based testing approach might have also played a role here, because scotch tape preparations were not performed but target DNA was just amplified from stool DNA extractions.
The study has a number of limitations. First, storage of the nucleic acid eluates for about 13 years since the time of sample collection may have resulted in minor nucleic acid degradation, potentially resulting in decreased sensitivity with regard to samples with a priori low target DNA concentrations close to the technical detection limits of the real-time PCRs. To keep the probability of this type of bias low, the nucleic acids within the eluates had been optimally preserved by storing the samples deep frozen at −80 °C. Moreover, the recorded cycle threshold values were in the typical range as observed for infected individuals and sufficiently far away from the detection threshold, suggesting that the DNA was still widely intact. In addition, DNA preservation had been exemplarily controlled in the course of a recent test comparison assessment [63]. For the respective study [63], selected residual samples had been re-assessed with the same real-time PCR assays for DNA of enteric protozoa and entero-invasive bacteria which had also been applied with the same stool samples shortly after acquisition in Ghana. Obtained cycle threshold values had been in a comparable range, thus suggesting that deep freezing-based DNA preservation had been successful. Second, no microscopic results were available for correlation and confirmation of the PCR results. While real-time PCR from stool samples is a priori more sensitive than microscopy for protozoan pathogens, this is considerably less unambiguously true in the case of helminths [64], from which nucleic acids are more difficult to extract from eggs and cuticula cells [65]. So, microscopic results would have provided true additional value but the retrospective design of the study made this option unfeasible. Fourth, the study did not provide a comprehensive assessment of all helminth infections potentially occurring in Ghanaian individuals. For example, no serological screening for toxocariasis was conducted, although high seroprevalence rates have previously been reported from Ghana [66]. Fifth, the conducted stool assessment for Schistosoma spp. DNA did not exclude the shedding of Schistosoma haematobium eggs via the patients’ urine and so, it did not provide a comprehensive overview of schistosomiasis in the assessed Ghanaian population. Sixth, lacking systematic assessment of demographic, socioeconomic, and behavioral data related to helminth infections limits the interpretability of the study results.
5. Conclusions
In spite of the abovementioned limitations, the results of the study suggested a low overall infection rate of the assessed Ghanaian children from the Ashanti Region in 2007 and 2008 with enteric helminths. Next to the expected dominance of the nematodes S. stercoralis and N. americanus, the cestode H. nana was among the most frequently identified helminths. The assessment provides a small piece to the epidemiological puzzle and baseline values for future follow-up assessments in this geographic region.
Author Contributions
Conceptualization, U.L., H.F. and D.D.; methodology, F.W. and H.F.; software, F.W.; validation, F.W. and H.F.; formal analysis, F.W. and H.F.; investigation, F.W. and H.F.; resources, H.F., J.M., D.D., C.W.A., E.K.P., J.A. and D.F.; data curation, F.W.; writing—original draft preparation, F.W. and H.F.; writing—review and editing, F.W., C.W.A., E.K.P., J.A., D.F., U.L., J.M., H.F. and D.D.; visualization, F.W.; supervision, H.F., J.M., U.L. and D.D.; project administration. H.F.; funding acquisition, H.F., J.M. and D.D. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by grant 36K2-S-45 1922 “Evaluation and optimization of molecular diagnostic tests for tropical parasitic diseases for surveillance and risk assessment purposes in tropical deployment settings—a German–French cooperation project between the German Armed Forces Hospital Hamburg and the Military Hospital Laveran, Marseille” of the German Ministry of Defense (MoD) awarded to Hagen Frickmann. We acknowledge support from the Open Access Publication Funds of the Bernhard Nocht Institute for Tropical Medicine Hamburg. The publication of this article was funded by the Open Access Fund of the Leibniz Association.
Institutional Review Board Statement
Ethical clearance for the sample collection and the informed consent procedure was obtained from the Committee on Human Research, Publications and Ethics, School of Medical Science, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. To be included in the study, written informed consent was obtained from the parents or the legal guardian prior to the enrolment. In case of non-participation, medical treatment was nevertheless provided. Further, anonymous characterization of residual samples was granted by the medical association of Hamburg, Germany, (reference number: WF-011/19, obtained on 11 March 2019). The assessments were performed in line with the Declaration of Helsinki and its amendments.
Informed Consent Statement
To be included in the study, written informed consent was obtained from the parents or the legal guardian prior to the enrolment.
Data Availability Statement
All relevant data are provided within the manuscript. Raw data can be made available on reasonable request.
Acknowledgments
Annett Michel is gratefully acknowledged for excellent technical assistance.
Conflicts of Interest
The authors declare no conflict of interest The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A

Table A1.
Sequences of the primer and probe oligonucleotides (hybridization probes including the used reporter and quencher molecules) as well as of the positive control plasmid inserts of the applied helminth-specific real-time PCRs. The positive control plasmid inserts were included in pEX-A128 vector backbones (Eurofins Scientific SE, Luxembourg).
Table A1.
Sequences of the primer and probe oligonucleotides (hybridization probes including the used reporter and quencher molecules) as well as of the positive control plasmid inserts of the applied helminth-specific real-time PCRs. The positive control plasmid inserts were included in pEX-A128 vector backbones (Eurofins Scientific SE, Luxembourg).
| Ascaris lumbricoides-specific real-time PCR oligonucleotides | |
| forward primer | 5′-GTAATAGCAGTCGGCGGTTTCTT-3′ |
| reverse primer | 5′-GCCCAACATGCCACCTATTC-3′ |
| probe | 5′-ROX-TTGGCGGACAATTGCATGCGAT-BHQ2-3′ |
| positive control insert | 5′-GGTGATGTAATAGCAGTCGGCGGTTTCTTTTTTTTTGGCGGACAATTGCATGCGATTTGCTATGTGTTGAGGGAGAATAGGTGGCATGTTGGGCTTGTTA-3′ |
| Strongyloides stercoralis-specific real-time PCR oligonucleotides | |
| forward primer | 5′-GAATTCCAAGTAAACGTAAGTCATTAGC-3′ |
| reverse primer | 5′-TGCCTCTGGATATTGCTCAGTTC-3′ |
| probe | 5′-CY5-ACACACCGGCCGTCGCTGC-BHQ2-3′ |
| positive control insert | 5′-AACGAGGAATTCCAAGTAAACGTAAGTCATTAGCTTACATTGATTACGTCCCTGCCCTTTGTACACACCGGCCGTCGCTGCCCGGAACTGAGCAATATCCAGAGGCAGGAAGA-3′ |
| Ancyclostoma spp.-specific real-time PCR oligonucleotides | |
| forward primer | 5′-GAATGACAGCAAACTCGTTGTTG-3′ |
| reverse primer | 5′-ATACTAGCCACTGCCGAAACGT-3′ |
| probe | 5′-YAKYE-ATCGTTTACCGACTTTAG-MGBEQ-3′ |
| positive control insert | 5′-TGCGCTGAATGACAGCAAACTCGTTGTTGCTGCTGAATCGTTTACCGACTTTAGAACGTTTCGGCAGTGGCTAGTATAACAAC-3′ |
| Necator americanus-specific real-time PCR oligonucleotides | |
| forward primer | 5′-CTGTTTGTCGAACGGTACTTGC-3′ |
| reverse primer | 5′-ATAACAGCGTGCACATGTTGC-3′ |
| probe | 5′-FAM-CTGTACTACGCATTGTATAC-MGBEQ-3′ |
| positive control insert | 5′-GAACACTGTTTGTCGAACGGTACTTGCTCTGTACTACGCATTGTATACGTGTTCAGCAATTCCCGTTTAAGTGAAGAACACACGTGCAACATGTGCACGCTGTTATTCACTACG-3′ |
| Trichuris trichiura-specific real-time PCR oligonucleotides | |
| forward primer | 5′-TTGAAACGACTTGCTCATCAACTT-3′ |
| reverse primer | 5′-CTGATTCTCCGTTAACCGTTGTC-3′ |
| probe | 5′-YAKYE-CGATGGTACGCTACGTGCTTACCATGG-MGBEQ-3′ |
| positive control insert | 5′-CGACGATGCTTTGAAACGACTTGCTCATCAACTTTCGATGGTACGCTACGTGCTTACCATGGTGACAACGGTTAACGGAGAATCAGGGTTCGGCTC-3′ |
| Schistosoma spp.-specific real-time PCR oligonucleotides | |
| forward primer | 5′-GGTCTAGATGACTTGATYGAGATGCT-3′ |
| reverse primer | 5′-TCCCGAGCGYGTATAATGTCATTA-3′ |
| probe | 5′-FAM-TGGGTTGTGCTCGAGTCGTGGC-BHQ1-3′ |
| positive control insert | 5′-TAGTCTGGTCTAGATGACTTGATTGAGATGCTGCGGTGGGTTGTGCTCGAGTCGTGGCTTAATGACATTATACACGCTCGGGATAATTC-3′ |
| Taenia solium-specific real-time PCR oligonucleotides | |
| forward primer | 5′-ATGGATCAATCTGGGTGGAGTT-3′ |
| reverse primer | 5′-ATCGCAGGGTAAGAAAAGAAGGT-3′ |
| probe | 5′-Cy5-TGGTACTGCTGTGGCGGCGG-BHQ2-3′ |
| positive control insert | 5′-TTGACTGATGATGGATCAATCTGGGTGGAGTTGGTGGTACTGCTGTGGCGGCGGTATTGTCAACTTCTTCTGTACCTTCTTTTCTTACCCTGCGATGGGGTGCCTA-3′ |
| Taenia saginata-specific real-time PCR oligonucleotides | |
| forward primer | 5′-GCGTCGTCTTTGCGTTACAC-3′ |
| reverse primer | 5′-TGACACAACCGCGCTCTG-3′ |
| probe | 5′-ROX-CCACAGCACCAGCGACAGCAGCAA-BHQ2-3′ |
| positive control insert | 5′-GCCCCATCATGCGTCGTCTTTGCGTTACACGTGGCGATGTTGCTGCTGTCGCTGGTGCTGTGGTGGCGGCGCAGAGCGCGGTTGTGTCACCGTTGGTGG-3′ |
| Enterobius vermicularis-specific real-time PCR oligonucleotides | |
| forward primer | 5′CGGTGTAATTTTGTTGGTGTCTATG-3′ |
| reverse primer | 5′-TGGCAGCATTGCAAACTAATG-3′ |
| probe | 5′-FAM-TGTGCCAGTCAACGCCTAAACCGT-C-BHQ1-3′ |
| positive control insert | 5′-TGTAATATAACGGTGTAATTTTGTTGGTGTCTATGCTTTGTGCCAGTCAACGCCTAAACCGTCGTTGATGTGTGTATAAGATGAAGCATAAAGCAAAAGGTTTGCTACTTGTAGCAGA-CTAGACTTAATAAGCATTAGTTTGCAATGCTGCCAACTATGATAA-3′ |
| Hymenolepis nana-specific real-time PCR oligonucleotides | |
| forward primer | 5′-CATTGTGTACCAAATTGATGATGAGTA-3′ |
| reverse primer | 5′-CAACTGACAGCATGTTTCGATATG-3′ |
| probe | 5′-JOE-CGTGTGCGCCTCTGGCTTACCG-BHQ1-3′ |
| positive control insert | 5′-ACACTTATTACATTGTGTACCAAATTGATGATGAGTAGACGTGTGCGCCTCTGGCTTACCGTTTACTGCCTCGTCATATCGAAACATGCTGTCAGTTGCTGCTGCTCA-3′ |
References
- Ananthakrishnan, S.; Nalini, P.; Pani, S.P. Intestinal geohelminthiasis in the developing world. Natl. Med. J. India 1997, 10, 67–71. [Google Scholar] [PubMed]
- Markell, E.K. Intestinal nematode infections. Pediatr. Clin. N. Am. 1985, 32, 971–986. [Google Scholar] [CrossRef]
- Shrestha, A.; Schindler, C.; Odermatt, P.; Gerold, J.; Erismann, S.; Sharma, S.; Koju, R.; Utzinger, J.; Cissé, G. Intestinal parasite infections and associated risk factors among schoolchildren in Dolakha and Ramechhap districts, Nepal: A cross-sectional study. Parasit. Vectors 2018, 11, 532. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.E.; Werkman, M.; Dunn, J.C.; Anderson, R.M. Current epidemiological evidence for predisposition to high or low intensity human helminth infection: A systematic review. Parasit. Vectors 2018, 11, 65. [Google Scholar] [CrossRef]
- Anderson, R.M. The population dynamics and epidemiology of intestinal nematode infections. Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 686–696. [Google Scholar] [CrossRef]
- Karagiannis-Voules, D.A.; Biedermann, P.; Ekpo, U.F.; Garba, A.; Langer, E.; Mathieu, E.; Midzi, N.; Mwinzi, P.; Polderman, A.M.; Raso, G.; et al. Spatial and temporal distribution of soil-transmitted helminth infection in sub-Saharan Africa: A systematic review and geostatistical meta-analysis. Lancet Infect. Dis. 2015, 15, 74–84. [Google Scholar] [CrossRef]
- Basavaraju, S.V.; Schantz, P. Soil-transmitted helminths and Plasmodium falciparum malaria: Epidemiology, clinical manifestations, and the role of nitric oxide in malaria and geohelminth co-infection. Do worms have a protective role in P. falciparum infection? Mt. Sinai. J. Med. 2006, 73, 1098–1105. [Google Scholar]
- Eziefula, A.C.; Brown, M. Intestinal nematodes: Disease burden, deworming and the potential importance of co-infection. Curr. Opin. Infect. Dis. 2008, 21, 516–522. [Google Scholar] [CrossRef]
- Yatich, N.J.; Yi, J.; Agbenyega, T.; Turpin, A.; Rayner, J.C.; Stiles, J.K.; Ellis, W.O.; Funkhouser, E.; Ehiri, J.E.; Williams, J.H.; et al. Malaria and intestinal helminth co-infection among pregnant women in Ghana: Prevalence and risk factors. Am. J. Trop. Med. Hyg. 2009, 80, 896–901. [Google Scholar] [CrossRef]
- Hartgers, F.C.; Obeng, B.B.; Boakye, D.; Yazdanbakhsh, M. Immune responses during helminth-malaria co-infection: A pilot study in Ghanaian school children. Parasitology 2008, 135, 855–860. [Google Scholar] [CrossRef]
- Donohue, R.E.; Cross, Z.K.; Michael, E. The extent, nature, and pathogenic consequences of helminth polyparasitism in humans: A meta-analysis. PLoS Negl. Trop. Dis. 2019, 13, e0007455. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.R.; Turkeltaub, J.A.; Hotez, P.J. Global progress towards eliminating gastrointestinal helminth infections. Curr. Opin. Gastroenterol. 2014, 30, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Watkins, W.E.; Pollitt, E. “Stupidity or worms”: Do intestinal worms impair mental performance? Psychol. Bull. 1997, 121, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, H. Do intestinal nematodes affect productivity in adulthood? Parasitol. Today 2000, 16, 153–158. [Google Scholar] [CrossRef]
- Yatich, N.J.; Jolly, P.E.; Funkhouser, E.; Agbenyega, T.; Rayner, J.C.; Ehiri, J.E.; Turpin, A.; Stiles, J.K.; Ellis, W.O.; Jiang, Y.; et al. The effect of malaria and intestinal helminth coinfection on birth outcomes in Kumasi, Ghana. Am. J. Trop. Med. Hyg. 2010, 82, 28–34. [Google Scholar] [CrossRef]
- Roberts, T.; Gravett, C.A.; Velu, P.P.; Theodoratou, E.; Wagner, T.A.; Zhang, J.S.; Campbell, H.; Rubens, C.E.; Gravett, M.G.; Rudan, I. Epidemiology and aetiology of maternal parasitic infections in low- and middle-income countries. J. Glob. Health 2011, 1, 189–200. [Google Scholar]
- Orr, A.R.; Quagraine, J.E.; Suwondo, P.; George, S.; Harrison, L.M.; Dornas, F.P.; Evans, B.; Caccone, A.; Humphries, D.; Wilson, M.D.; et al. Genetic Markers of Benzimidazole Resistance among Human Hookworms (Necator americanus) in Kintampo North Municipality, Ghana. Am. J. Trop. Med. Hyg. 2019, 100, 351–356. [Google Scholar] [CrossRef]
- Humphries, D.; Nguyen, S.; Kumar, S.; Quagraine, J.E.; Otchere, J.; Harrison, L.M.; Wilson, M.; Cappello, M. Effectiveness of Albendazole for Hookworm Varies Widely by Community and Correlates with Nutritional Factors: A Cross-Sectional Study of School-Age Children in Ghana. Am. J. Trop. Med. Hyg. 2017, 96, 347–354. [Google Scholar] [CrossRef]
- Humphries, D.; Simms, B.T.; Davey, D.; Otchere, J.; Quagraine, J.; Terryah, S.; Newton, S.; Berg, E.; Harrison, L.M.; Boakye, D.; et al. Hookworm infection among school age children in Kintampo north municipality, Ghana: Nutritional risk factors and response to albendazole treatment. Am. J. Trop. Med. Hyg. 2013, 89, 540–548. [Google Scholar] [CrossRef]
- Orish, V.N.; Ofori-Amoah, J.; Amegan-Aho, K.H.; Osisiogu, E.U.; Osei-Yeboah, J.; Lokpo, S.Y.; Allotey, E.A.; Adu-Amankwaah, J.; Azuma, D.E.; Agordoh, P.D. Eosinophilia in school-going children with Plasmodium falciparum and helminth infections in the Volta Region of Ghana. Pan. Afr. Med. J. 2021, 38, 277. [Google Scholar] [CrossRef]
- Adu-Gyasi, D.; Asante, K.P.; Frempong, M.T.; Gyasi, D.K.; Iddrisu, L.F.; Ankrah, L.; Dosoo, D.; Adeniji, E.; Agyei, O.; Gyaase, S.; et al. Epidemiology of soil transmitted Helminth infections in the middle-belt of Ghana, Africa. Parasite Epidemiol. Control 2018, 3, e00071. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, L.J.; Odoom, J.; Pratt, D.; Boatemaa, L.; Asante-Ntim, N.; Attiku, K.; Banahene, B.; Osei-Atweneboana, M.; Verweij, J.J.; Molyneux, D.; et al. Expanding molecular diagnostics of helminthiasis: Piloting use of the GPLN platform for surveillance of soil transmitted helminthiasis and schistosomiasis in Ghana. PLoS Negl. Trop. Dis. 2018, 12, e0006129. [Google Scholar] [CrossRef] [PubMed]
- Mirisho, R.; Neizer, M.L.; Sarfo, B. Prevalence of Intestinal Helminths Infestation in Children Attending Princess Marie Louise Children’s Hospital in Accra, Ghana. J. Parasitol. Res. 2017, 2017, 8524985. [Google Scholar] [CrossRef] [PubMed]
- Ayeh-Kumi, P.F.; Addo-Osafo, K.; Attah, S.K.; Tetteh-Quarcoo, P.B.; Obeng-Nkrumah, N.; Awuah-Mensah, G.; Abbey, H.N.; Forson, A.; Cham, M.; Asare, L.; et al. Malaria, helminths and malnutrition: A cross-sectional survey of school children in the South-Tongu district of Ghana. BMC Res. Notes 2016, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Armoo, S.; Cunningham, L.J.; Campbell, S.J.; Aboagye, F.T.; Boampong, F.K.; Hamidu, B.A.; Osei-Atweneboana, M.Y.; Stothard, J.R.; Adams, E.R. Detecting Schistosoma mansoni infections among pre-school-aged children in southern Ghana: A diagnostic comparison of urine-CCA, real-time PCR and Kato-Katz assays. BMC Infect. Dis. 2020, 20, 301. [Google Scholar] [CrossRef]
- Kulinkina, A.V.; Kosinski, K.C.; Adjei, M.N.; Osabutey, D.; Gyamfi, B.O.; Biritwum, N.K.; Bosompem, K.M.; Naumova, E.N. Contextualizing Schistosoma haematobium transmission in Ghana: Assessment of diagnostic techniques and individual and community water-related risk factors. Acta Trop. 2019, 194, 195–203. [Google Scholar] [CrossRef]
- Kosinski, K.C.; Kulinkina, A.V.; Tybor, D.; Osabutey, D.; Bosompem, K.M.; Naumova, E.N. Agreement among Four Prevalence Metrics for Urogenital Schistosomiasis in the Eastern Region of Ghana. Biomed. Res. Int. 2016, 2016, 7627358. [Google Scholar] [CrossRef]
- Kulinkina, A.V.; Walz, Y.; Koch, M.; Biritwum, N.K.; Utzinger, J.; Naumova, E.N. Improving spatial prediction of Schistosoma haematobium prevalence in southern Ghana through new remote sensors and local water access profiles. PLoS Negl. Trop. Dis. 2018, 12, e0006517. [Google Scholar] [CrossRef]
- Tetteh, I.K.; Adjei, R.O.; Sasu, S.; Appiah-Kwakye, L. Index of potential contamination: Schistosoma haematobium infections in school children in the Ashanti Region of Ghana. East Afr. Med. J. 2004, 81, 520–523. [Google Scholar] [CrossRef]
- Klumpp, R.K.; Webbe, G. Focal, seasonal and behavioural patterns of infection and transmission of Schistosoma haematobium in a farming village at the Volta Lake, Ghana. J. Trop. Med. Hyg. 1987, 90, 265–281. [Google Scholar]
- Scott, D.; Senker, K.; England, E.C. Epidemiology of human Schistosoma haematobium infection around Volta Lake, Ghana, 1973-75. Bull. World Health Organ. 1982, 60, 89–100. [Google Scholar] [PubMed]
- Lyons, G.R. Schistosomiasis in north-western Ghana. Bull. World Health Organ. 1974, 51, 621–632. [Google Scholar] [PubMed]
- Bozdĕch, V. Das Vorkommen von Schistosoma haematobium (Bilharz) und Schistosoma mansoni (Sambon) in städtische Populationen von Accra-Ghana und Kaduna-Nigeria [The incidence of Schistosoma haematobium (Bilharz) and Schistosoma mansoni (Sambon) in urban populations of Accra-Ghana and of Kaduna-Nigeria (author’s transl)]. Zentralbl. Bakteriol. Orig. A 1973, 224, 264–269. [Google Scholar]
- Kretchy, J.P.; Dzodzomenyo, M.; Ayi, I.; Dwomoh, D.; Agyabeng, K.; Konradsen, F.; Dalsgaard, A. The Incidence, Intensity, and Risk Factors for Soil Transmissible Helminthes Infections among Waste Handlers in a Large Coastal Periurban Settlement in Southern Ghana. J. Environ. Public Health 2021, 2021, 5205793. [Google Scholar] [CrossRef] [PubMed]
- Squire, S.A.; Yang, R.; Robertson, I.; Ayi, I.; Squire, D.S.; Ryan, U. Gastrointestinal helminths in farmers and their ruminant livestock from the Coastal Savannah zone of Ghana. Parasitol, Res. 2018, 117, 3183–3194. [Google Scholar] [CrossRef] [PubMed]
- Amoah, I.D.; Abubakari, A.; Stenström, T.A.; Abaidoo, R.C.; Seidu, R. Contribution of Wastewater Irrigation to Soil Transmitted Helminths Infection among Vegetable Farmers in Kumasi, Ghana. PLoS Negl. Trop. Dis. 2016, 10, e0005161. [Google Scholar] [CrossRef]
- Zoli, A.; Shey-Njila, O.; Assana, E.; Nguekam, J.P.; Dorny, P.; Brandt, J.; Geerts, S. Regional status, epidemiology and impact of Taenia solium cysticercosis in Western and Central Africa. Acta Trop. 2003, 87, 35–42. [Google Scholar] [CrossRef]
- Addy, F.; Romig, T.; Wassermann, M. Genetic characterisation of Fasciola gigantica from Ghana. Vet. Parasitol. Reg. Stud. Rep. 2018, 14, 106–110. [Google Scholar] [CrossRef]
- Ofori, M.; Bogoch, I.I.; Ephraim, R.K. Prevalence of Dicrocoelium dendriticum ova in Ghanaian school children. J. Trop. Pediatr. 2015, 61, 229–230. [Google Scholar] [CrossRef]
- Asare, K.K.; Boampong, J.N.; Ameyaw, E.O.; Thomford, A.K.; Afoakwah, R.; Kwakye-Nuako, G.; Thomford, K.P.; Quashie, N.B. Microscopic identification of possible Clonorchis/Opisthorchis infection in two Ghanaian women with undiagnosed abdominal discomfort: Two case reports. J. Med. Case Rep. 2014, 8, 369. [Google Scholar] [CrossRef]
- Boyko, R.H.; Harrison, L.M.; Humphries, D.; Galvani, A.P.; Townsend, J.P.; Otchere, J.; Wilson, M.D.; Cappello, M. Dogs and pigs are transport hosts of Necator americanus: Molecular evidence for a zoonotic mechanism of human hookworm transmission in Ghana. Zoonoses Public Health 2020, 67, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Egbi, G.; Steiner-Asiedu, M.; Kwesi, F.S.; Ayi, I.; Ofosu, W.; Setorglo, J.; Klobodu, S.S.; Armar-Klemesu, M. Anaemia among school children older than five years in the Volta Region of Ghana. Pan Afr. Med. J. 2014, 17 (Suppl. 1), 10. [Google Scholar] [CrossRef] [PubMed]
- Bakker, H. Ancylostomiasis in the Dormaa area, Ghana. Trop. Geogr. Med. 1969, 21, 84–87. [Google Scholar] [PubMed]
- Osei, F.B.; Stein, A. Spatio-temporal analysis of small-area intestinal parasites infections in Ghana. Sci. Rep. 2017, 7, 12217. [Google Scholar] [CrossRef] [PubMed]
- Amoah, A.S.; Boakye, D.A.; Yazdanbakhsh, M.; van Ree, R. Influence of Parasitic Worm Infections on Allergy Diagnosis in Sub-Saharan Africa. Curr. Allergy Asthma Rep. 2017, 17, 65. [Google Scholar] [CrossRef]
- Brooker, S.J.; Nikolay, B.; Balabanova, D.; Pullan, R.L. Global feasibility assessment of interrupting the transmission of soil-transmitted helminths: A statistical modelling study. Lancet Infect. Dis. 2015, 15, 941–950. [Google Scholar] [CrossRef]
- Agyei, A.D. Epidemiological observations on helminth infections of calves in southern Ghana. Trop. Anim. Health Prod. 1991, 23, 134–140. [Google Scholar] [CrossRef]
- Krumkamp, R.; Sarpong, N.; Schwarz, N.G.; Adlkofer, J.; Loag, W.; Eibach, D.; Hagen, R.M.; Adu-Sarkodie, Y.; Tannich, E.; May, J. Gastrointestinal infections and diarrheal disease in Ghanaian infants and children: An outpatient case-control study. PLoS Negl. Trop. Dis. 2015, 9, e0003568. [Google Scholar]
- Eibach, D.; Krumkamp, R.; Hahn, A.; Sarpong, N.; Adu-Sarkodie, Y.; Leva, A.; Käsmaier, J.; Panning, M.; May, J.; Tannich, E. Application of a multiplex PCR assay for the detection of gastrointestinal pathogens in a rural African setting. BMC Infect. Dis. 2016, 16, 150. [Google Scholar] [CrossRef]
- Eibach, D.; Krumkamp, R.; Al-Emran, H.M.; Sarpong, N.; Hagen, R.M.; Adu-Sarkodie, Y.; Tannich, E.; May, J. Molecular characterization of Cryptosporidium spp. among children in rural Ghana. PLoS Negl. Trop. Dis. 2015, 9, e0003551. [Google Scholar] [CrossRef]
- Leva, A.; Eibach, D.; Krumkamp, R.; Käsmaier, J.; Rubbenstroth, D.; Adu-Sarkodie, Y.; May, J.; Tannich, E.; Panning, M. Diagnostic performance of the Luminex xTAG gastrointestinal pathogens panel to detect rotavirus in Ghanaian children with and without diarrhoea. Virol. J. 2016, 13, 132. [Google Scholar] [CrossRef] [PubMed]
- Graul, S.; Böttcher, S.; Eibach, D.; Krumkamp, R.; Käsmaier, J.; Adu-Sarkodie, Y.; May, J.; Tannich, E.; Panning, M. High diversity of human parechovirus including novel types in stool samples from Ghanaian children. J. Clin. Virol. 2017, 96, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Vinnemeier, C.D.; Klupp, E.M.; Krumkamp, R.; Rolling, T.; Fischer, N.; Owusu-Dabo, E.; Addo, M.M.; Adu-Sarkodie, Y.; Käsmaier, J.; Aepfelbacher, M.; et al. Tropheryma whipplei in children with diarrhoea in rural Ghana. Clin. Microbiol. Infect. 2016, 22, e1–e65. [Google Scholar] [CrossRef] [PubMed]
- Köller, T.; Hahn, A.; Altangerel, E.; Verweij, J.J.; Landt, O.; Kann, S.; Dekker, D.; May, J.; Loderstädt, U.; Podbielski, A.; et al. Comparison of commercial and in-house real-time PCR platforms for 15 parasites and microsporidia in human stool samples without a gold standard. Acta Trop. 2020, 207, 105516. [Google Scholar] [CrossRef]
- Cools, P.; van Lieshout, L.; Koelewijn, R.; Addiss, D.; Ajjampur, S.S.R.; Ayana, M.; Bradbury, R.S.; Cantera, J.L.; Dana, D.; Fischer, K.; et al. First international external quality assessment scheme of nucleic acid amplification tests for the detection of Schistosoma and soil-transmitted helminths, including Strongyloides: A pilot study. PLoS Negl. Trop. Dis. 2020, 14, e0008231. [Google Scholar] [CrossRef]
- Ahiadorme, M.; Morhe, E. Soil transmitted helminth infections in Ghana: A ten year review. Pan. Afr. Med. J. 2020, 35, 131. [Google Scholar] [CrossRef]
- Harhay, M.O.; Horton, J.; Olliaro, P.L. Epidemiology and control of human gastrointestinal parasites in children. Expert Rev. Anti. Infect. Ther. 2010, 8, 219–234. [Google Scholar] [CrossRef]
- Hotez, P.J.; Kamath, A. Neglected tropical diseases in sub-saharan Africa: Review of their prevalence, distribution, and disease burden. PLoS Negl. Trop. Dis. 2009, 3, e412. [Google Scholar] [CrossRef]
- Anyan, W.K.; Abonie, S.D.; Aboagye-Antwi, F.; Tettey, M.D.; Nartey, L.K.; Hanington, P.C.; Anang, A.K.; Muench, S.B. Concurrent Schistosoma mansoni and Schistosoma haematobium infections in a peri-urban community along the Weija dam in Ghana: A wake up call for effective National Control Programme. Acta Trop. 2019, 199, 105116. [Google Scholar] [CrossRef]
- Wen, S.T.; Chu, K.Y. Preliminary schistosomiasis survey in the lower Volta River below Akosombo Dam, Ghana. Ann. Trop. Med. Parasitol. 1984, 78, 129–133. [Google Scholar] [CrossRef]
- Amankwa, J.A.; Bloch, P.; Meyer-Lassen, J.; Olsen, A.; Christensen, N.O. Urinary and intestinal schistosomiasis in the Tono Irrigation Scheme, Kassena/Nankana District, upper east region, Ghana. Trop. Med. Parasitol. 1994, 45, 319–323. [Google Scholar] [PubMed]
- Abaka-Yawson, A.; Sosu, S.Q.; Kwadzokpui, P.K.; Afari, S.; Adusei, S.; Arko-Mensah, J. Prevalence and Determinants of Intestinal Parasitic Infections among Pregnant Women Receiving Antenatal Care in Kasoa Polyclinic, Ghana. J. Environ. Public Health 2020, 2020, 9315025. [Google Scholar] [CrossRef] [PubMed]
- Weinreich, F.; Hahn, A.; Eberhardt, K.A.; Kann, S.; Köller, T.; Warnke, P.; Dupke, S.; Dekker, D.; May, J.; Frickmann, H.; et al. Multicentric Evaluation of SeeGene Allplex Real-Time PCR Assays Targeting 28 Bacterial, Microsporidal and Parasitic Nucleic Acid Sequences in Human Stool Samples. Diagnostics 2022, 12, 1007. [Google Scholar] [CrossRef] [PubMed]
- Loderstädt, U.; Hagen, R.M.; Hahn, A.; Frickmann, H. New Developments in PCR-Based Diagnostics for Bacterial Pathogens Causing Gastrointestinal Infections-A Narrative Mini-Review on Challenges in the Tropics. Trop. Med. Infect. Dis. 2021, 6, 96. [Google Scholar] [CrossRef]
- Hoffmann, T.; Hahn, A.; Verweij, J.J.; Leboulle, G.; Landt, O.; Strube, C.; Kann, S.; Dekker, D.; May, J.; Frickmann, H.; et al. Differing Effects of Standard and Harsh Nucleic Acid Extraction Procedures on Diagnostic Helminth Real-Time PCRs Applied to Human Stool Samples. Pathogens 2021, 10, 188. [Google Scholar] [CrossRef]
- Kyei, G.; Ayi, I.; Boampong, J.N.; Turkson, P.K. Sero-Epidemiology of Toxocara Canis Infection in Children Attending Four Selected Health Facilities in the Central Region of Ghana. Ghana Med. J. 2015, 49, 77–83. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).