Abstract
Tuberculosis (TB) and COVID-19 have become significant health problems globally, especially in countries with high prevalence. Therefore, this research aims to examine all possibilities and predict the impact of TB-SARS-CoV-2 co-infection to anticipate the cascade effect of both diseases in all sectors. The conceptual strategy of the algorithm in TB-COVID-19 is needed to create an integrated management system. It includes the stages of early detection with accurate and effective methods, as well as the synchronization of TB-COVID-19 health services, starting from primary health facilities to secondary and tertiary referral centers. The algorithm in TB-COVID-19 is crucial to prepare future strategies for PTB co-infection viral respiratory infections other than SARS-CoV-2, ILI, ARI, and SARI. Since the implementation involves all health services, there is a need to integrate the governance of TB-COVID-19 and other comorbidities in good health services based on research and multicentre design.
1. Introduction
Tuberculosis (TB) remains a leading menace in infectious disease, and COVID-19 has become a global pandemic since it was announced by World Health Organization (WHO) on 11 March 2020. This is a double burden for TB-endemic countries such as India, China, and Indonesia [1,2,3]. Furthermore, there has been an increase in TB-SARS-CoV-2 co-infection cases, which are reported as rare [4,5,6,7]. Due to the potentially worse outcome of co-infection, a comprehensive and intensive investigation is required from all sectors.
The understanding of all possibilities and predicting the effect of TB-COVID-19 is critical to anticipate the cascade effect of both diseases in all sectors and other communicable diseases such as Influenza-Like Illness (ILI), Severe Acute Respiratory Infections (SARI), and Acute Respiratory Infection (ARI). It is necessary to reinforce monitoring and evaluation of the implementation of TB control programs integrated with other communicable diseases. Awareness of the importance of overcoming problems encourages various strategic efforts to establish integrated management systems in the health systems in the future, which elevates the role of various aspects, including multicentre collaborations. Moreover, consistent research is also needed to design an efficient management system strategy for serious communicable diseases that can be implemented globally.
TB is a complicated infectious disease; therefore, several cases, such as antimicrobial resistance and Latent TB Infection (LTBI), need immediate solutions [8,9]. However, many health policies have weakened implementation due to the pandemic. The similarity of features in respiratory tract infections also complicates identification, delaying the process of handling and treatment [10,11]. This condition is exacerbated by the high population and TB cases in low-middle-income countries such as India, Indonesia, and Pakistan [2,12].
Since COVID-19 was declared a pandemic, communities and governments were mobilized to contain the further spread and reduce the effect on populations, health structures, and economies. This is because COVID-19 affects the vulnerable population and those affected by communicable diseases. For example, the stimulating effects of the pandemic will result in a global spread of a TB epidemic and the risk of its biological effects, such as the interaction of two diseases [13,14,15]. Therefore, this research was carried out to create the best strategy to implement integrated management systems and anticipate the complex problem in emerging or re-emerging communicable diseases for the global goal of suitable environments, good health, and welfare.
2. Occurrence of TB-SARS-CoV-2 Co-Infection
Data from meta-analysis and a literature study with extensive publication searches from various countries found 86 cases from 36 studies of TB-SARS-CoV-2 co-infection [6]. TB and COVID-19 have symptoms that tend to be the same because they attack the lungs [13,16,17]. Analytical data of lung imaging features showed the 10 most common similarity features respectively, cavities (32.58%), infiltrates (31.46%), ground-glass opacity (19.1%), nodules (16.85%), pleural effusion (11.24%), fibrosis (12.36%), patchy shadows (8.99%), consolidation (8.99%), military lesions (5.62%), and reticules (5.62%), and the most significantly different in laboratory examination is leucocyte count. The survivor showed a lower leucocyte count than the non-survivors (8.015 [4.8–8.97] vs. 12.9 [10.5–16.73] × 109/L, p = 0.007) [6]. A cohort study stated that patients with TB-COVID-19 have both higher mortality rates and severe symptoms than those with only COVID-19 [6,18]; data from a pooled study comparing the TB-COVID-19 group and non-TB group regarding odds ratios (ORs) showed the percentages of deaths was 3.46% (822/23,732) in the overall group, 5.69% (123/2161) in the TB-COVID-19 group, and 3.24% (699/21,571) in the non-TB group; data from WHO also showed the increased number of TB death between 2019–2020. An important factor that affected the mortality rate in TB cases is the lower TB detection rate which decreased by 18% during the pandemic [2,6]. Although the number of TB-COVID-19 cases is low, the severity and high mortality rate remain a threat [19,20,21]. It was also discovered that TB management is affected by the pandemic, such as decreasing the number of TB cases findings in several countries [2,22,23,24]. Meta-analyses show that TB-COVID-19 patients aged >65 years have a higher risk of death and comorbidity rate [6,25]. Some evidence indicates that gender also affects the risk of these diseases, where males have a more vulnerable risk than females [6,18] due to high cases of TB [26,27]. An important factor that affected the mortality rate in TB cases is the lower TB detection rate which decreased by 18% during the pandemic [2,6]. Although the number of TB-COVID-19 cases is low, the severity and high mortality rate remain a threat [19,20]. It was also discovered that TB management is affected by the pandemic, such as decreasing the number of TB cases findings in several countries [2,22,23,24]. Meta-analyses show that TB-COVID-19 patients aged >65 years have a higher risk of death and comorbidity rate [6,25]. Some evidence indicates that gender also affects the risk of these diseases, where males have a more vulnerable risk than females [6,18] due to high cases of TB [26,27].
3. Tuberculosis and COVID-19 Integrated Management
The COVID-19 pandemic will impact TB management when the focus of attention is shifted to response and dealing with the prolonged spread of infection [28,29]. Therefore, integrated TB-COVID-19 management is needed to minimize the effect of the pandemic on the TB program. Due to the similarities of both diseases [30], integrated TB-COVID-19 management needs to be constructed quickly [17,31]. This can be developed with monitoring, surveillance systems, infrastructure, robust programs that have years of advanced TB end programs in many countries, and advanced diagnostic tools [13,32].
A standard algorithm that includes early detection stages with accurate and effective methods is required to determine the management of patients with suspected TB-COVID-19 and prevent disease progression from becoming severe. It also involves the synchronization of TB-COVID-19 health services, starting from primary health facilities, secondary as well as tertiary referral centers, and preparing future strategies for co-infection of pulmonary tuberculosis (PTB) respiratory virus infections other than SARS-CoV-2, ILI, ARI, and SARI [33,34,35,36,37]. Therefore, a standard algorithm is an integrated TB-COVID-19 case management activity based on the mechanism of lower respiratory tract infection, starting with the transmission of agents via airborne and considering similar factors that attack the primary lung tissue and interfere with the host immunity [13,38]. The implementation of the TB-COVID-19 management program needs consideration in many sectors. These include COVID-19 screening, testing, and adherence support of all HIV, TB, MDR-TB, other patients with chronic disease, and severe acute respiratory tract infection ILI, ARI, and SARI [39,40,41,42].
The U.S. Agency for International Development (USAID) statement regarding the guidelines for implementing the Tuberculosis Contact Investigation Program (PI-TBCI) stated that it is important to implement TBCI integrated with COVID-19 contact tracing at the household, health facility, and broader community levels. The integration approach in detecting TB-COVID-19 cases can increase efficiency in using human resources, strengthen the community, and sustain the implemented disease control and prevention strategy. The strategy for health system services includes the community-level early warning system, the primary healthcare early detection point, and a nationwide diagnostic network. To effectively locate active cases, a systematic contact investigation in households and among social contacts of active disease processes is required as the crucial guideline of TBCI and COVID-19 screening in households. This is related to the triage unit of health facilities and an integrated referral system. Based on the prepared guidelines, especially the community approach, the mapping of high-burden sites and diagnostic capacity mapping is recommended to sustain community-based treatment monitoring and digital adherence interventions [43]. This is because active case finding with a community approach is the potential strategy for sustained TB programs in the COVID-19 pandemic. Therefore, health facilities need consistency to anticipate the importance of this approach in terms of patients seeking care [44,45].
Meaningful actions must be implemented to cope with the complex disease problem that can spread quickly. Furthermore, controlling fear and stigma in the community regarding infectious diseases is no less critical. It starts from the One Health approach launched by WHO, which improves the management system and stakeholders in the health sector, from primary healthcare to referral hospitals [12,46,47]. LTBI problems were also reported to positively impact the severity of COVID-19 infection. Previous reports also showed that the role of LTBI can reduce COVID-19 mortality. However, LTBI policy guidelines must be taken seriously due to the high activation probability [48,49,50].
4. Algorithm TB-COVID-19 Design
A comprehensive framework for managing diseases, especially pulmonary infection, is essential to prevent contagious spreading in the community or population. An algorithm is an indispensable tool for standardizing the efficient management of patients with pulmonary disease, especially TB-COVID-19 co-infection.
Therefore, the TB-COVID-19 algorithm is based on TB incidence, history, contacts, and clinical manifestations. These include signs and symptoms, abnormal laboratory results and CXR abnormalities, active infiltrate and chronic infection characteristics, other comorbidities, and vulnerable people. Decision-making methods are also required to identify the causative Mycobacterium tuberculosis (MTB) or SARS-CoV-2, diagnosis, therapeutic, and referral system of TB-COVID-19. TB status needs to be evaluated carefully, starting from patient admission, application of good management, and therapeutic strategies to prevent the rapid development and the complication of severe COVID-19 [13,51,52,53].
The following recommendations are essential for managing and treating patients with a history of MTB infection, such as LTBI or TB disease and possible SARS-CoV-2 co-infection. Firstly, community and medical teams must be aware that latent and active TB is a risk factor for SARS-CoV-2 infection, and people at high risk must be given effective preventive measures [28,43]. This vulnerable group must be monitored regularly in medical, public health, or community-based settings when resources are available. Secondly, co-infection cases must be confirmed at the admission point, and the patient needs to be placed in an isolation ward with a standardized facility. Thirdly, medical resources must be prepared for co-infected patients to anticipate the possibility of severe symptom development. Fourthly, specific therapy must be designed for cases co-infected with TB—for example, treatment with immunosuppressive agents due to the potential for LTBI activation [54].
The integrated TB control program can be carried out by preparing exceptional staff for control and treatment management. This includes aspects of diagnosis, treatment, contact tracing, outbreak investigation, latent disease, dialogue with the community and establishment of collaboration with relevant organizations. Although there is a difference between the dynamics of TB infection and COVID-19, mitigation strategies can still be carried out to aid in COVID-19 community control. Effective scientific community interactions are highly beneficial for dealing with the COVID-19 pandemic and demonstrate actions when an emergency occurs. Scientists can investigate in collaboration, with profound research funding in artificial intelligence modeling linked to clinical algorithms to predict disease severity, forming an international clinical trial platform [5].
The right timing of diagnosis is an essential factor for aligning the integration of the management of both diseases. Due to the early symptoms and acute onset of SARS-CoV-2 infection, quick detection will help early diagnosis and follow with a rapid examination of Mycobacterium tuberculosis infection, discovery, and radiological examinations [55]. Previous reports stated that TB has a natural effect or “weight” in increasing the probability of death among COVID-19 patients. Therefore, patient management in health services and the pathogenesis of TB and COVID-19 can be analyzed based on references to case reports to determine the basic management strategies. This also involves respiratory infections, starting from the primary or priority prevention of disease progression and the prevention of illness or spread of disease to further treat TB-COVID-19 cases in the form of a standard algorithm [28,56].
Health services and all the elemental support assisted with integrated policy need to be developed and engaged to limit the contagious spreading of COVID-19 infection and maintain the TB service program. All elements and critical support of rapid TB diagnosis must be held simultaneously, even during pandemic emergencies [17]. In a health facility, triage is vital to implement simultaneous-integrated diagnostic testing for TB and COVID-19 detection. Diagnostic testing is crucial to detect and control pathogens in public health settings, including TB and COVID-19, as the guidance for appropriate treatment informs contact tracing and decides disease control strategy. Therefore, simultaneous-integrated testing of TB and COVID-19 is urgently implemented in the TB high-burden countries. An algorithm must consider that the triage unit of a health facility needs to include parallel testing simultaneously as specimen collection and integrated testing with a multiplex diagnostic platform [45,57].
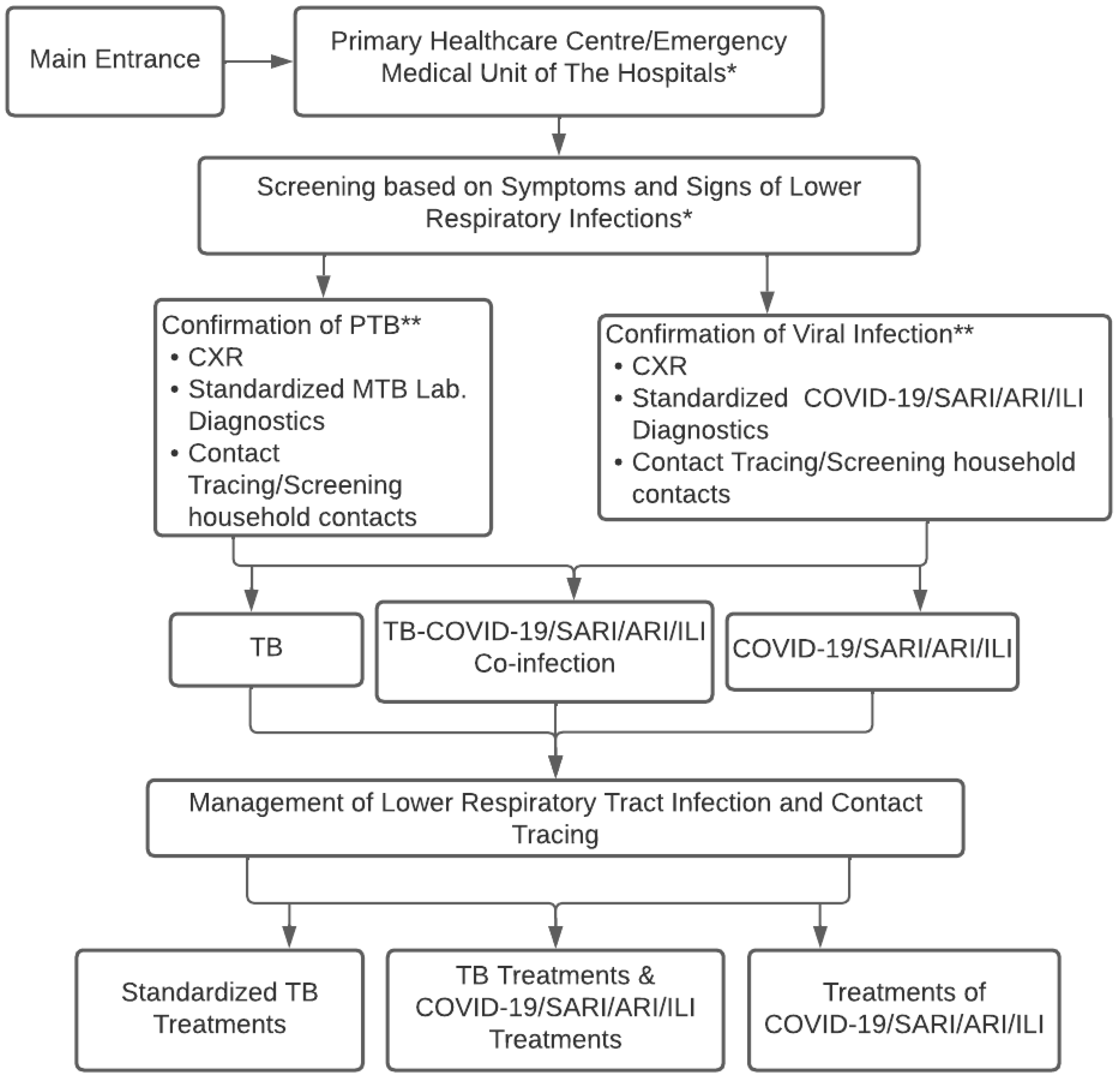
Figure 1 shows the conceptual strategies for controlling respiratory infections of TB-COVID-19/SARI/ARI/ILI to observe and design concepts appropriate to regional conditions. The concept starts from the beginning of the disease to the screening diagnosis of a “suspected” case of TB-COVID-19/SARI/ARI/ILI, as well as laboratory testing, CXR, determining diagnosis, treatment decisions, contact tracing, and infection control in the community.
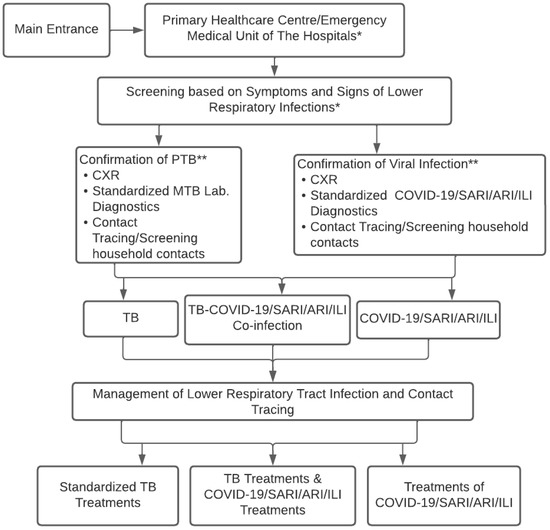
Figure 1.
Conceptual Algorithm of TB-COVID-19 Integrated Management in Healthcare Facilities. * Main entrance admission of the patient in the primary healthcare center or emergency medical unit/clinical unit of the hospitals to implement the screening tests; ** TB and COVID-19 contacts consider universal testing for TB and COVID-19 if resources permit.
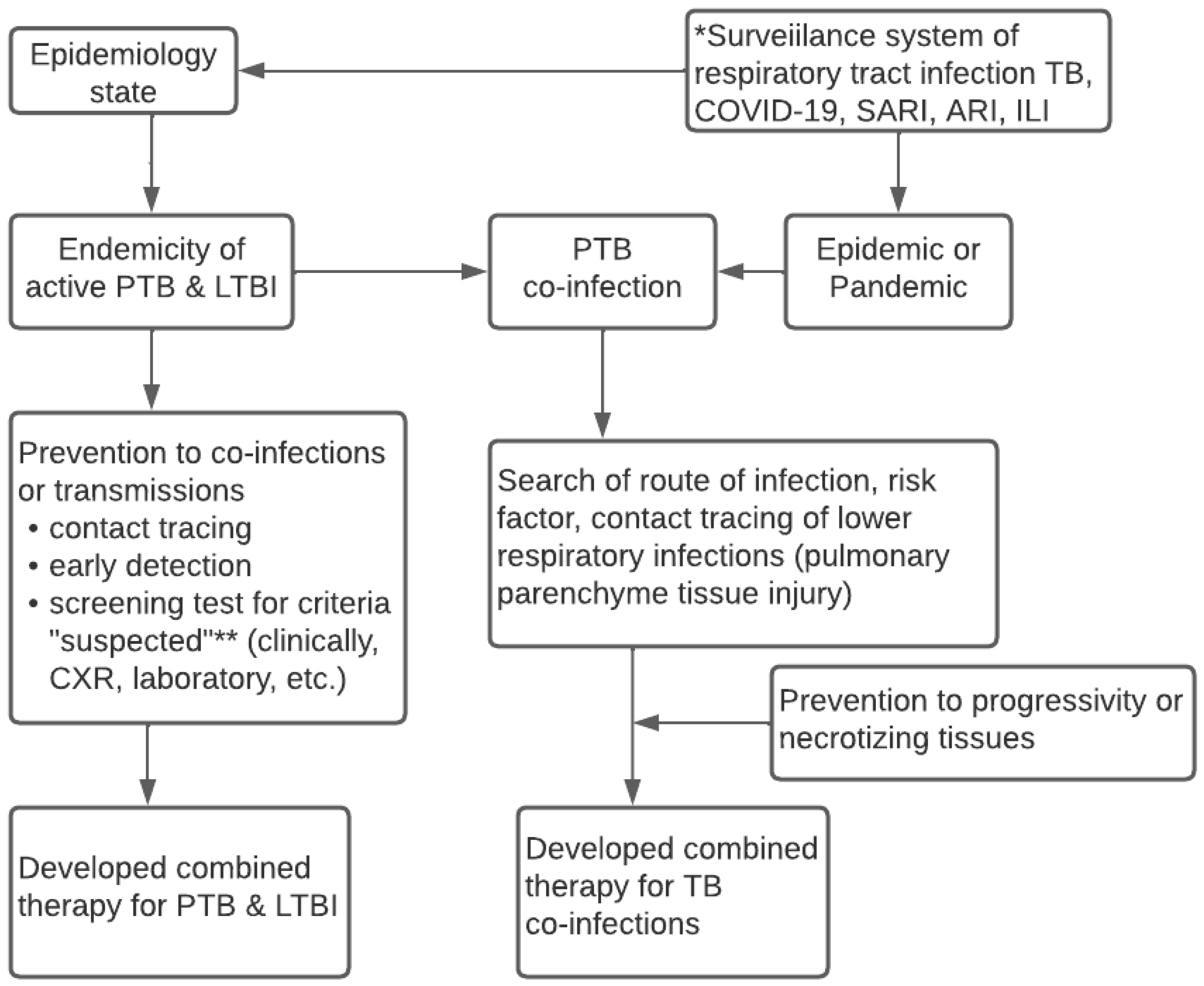
Figure 2 reveals the conceptual algorithm of the surveillance system for lower respiratory infections. It was discovered that the algorithm needs to consider the local state of epidemiologic data and carry out the integrated surveillance system of lower respiratory infections, namely TB, COVID-19, SARI, and ARI. Meanwhile, the system needs updated data on the endemicity of active TB, LTBI, and the epidemic and pandemic statuses in an area. Information on the health conditions of the local community is necessary to predict the occurrence of co-infections of pulmonary TB. The epidemiologic data or t-evidence serves as the basis to determine the design of the diagnosis, screening, and early detection, which is parallel to contact tracing. Furthermore, it is essential to prevent progressivity, transmissions, and decisions from appropriately combined therapeutics.
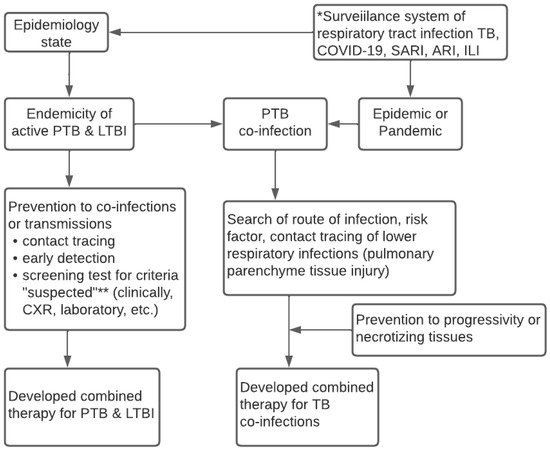
Figure 2.
Conceptual Algorithm of TB—COVID-19 Integrated Management based on The Surveillance System. * Surveillance epidemiology system for respiratory infection related to WHO guideline 2020; ** Suspected criteria of COVID-19 based on WHO case definition 2020; ** Case definition of suspected TB based on WHO guideline 2010.
Based on these results, future implementation needs to involve all health services to integrate the governance of TB-COVID-19 and comorbid. Implementing the TB-COVID-19 integration algorithm does not rule out ILI, ARI, and SARI or ignore HIV, comorbid diabetes mellitus, and other immunocompromised patients, as shown in Figure 2. Every time a patient with symptoms of respiratory tract infection visits a health care unit, a standardized management algorithm for patients with suspected PTB, PTB co-infection of COVID-19/SARI/ARI/ILI is carried out to implement the standardized prevention, treatment, patient care, and control of infectious diseases in the community. Similarly, it involves the standard flow of patient management at every regular visit of PTB patients in the Health care unit. The integrated treatment of TB-COVID-19 must also consider information from WHO 2020, TB, and COVID-19 treatment guidelines.
Implementing the algorithm of TB-COVID-19 integrated management could achieve good patient care with outcomes decreasing the morbidity and mortality of respiratory tract infections and contribute to TB control, especially in endemic countries.
5. Conclusions
TB is more complicated and crucial to managing when co-infection with COVID-19/SARI/ARI/ILI or other respiratory viral infections. Therefore, based on implementation research and multicentre design, there is a need to create an updated guideline and algorithm for the integrated management of TB-COVID-19/SARI/ARI/ILI.
Author Contributions
N.M.M., S.S., T.T.P., Z.N. and M.A. designed the content and wrote this review. All authors have read and agreed to the published version of the manuscript.
Funding
The present study was supported by a grant from the Research Group, Universitas Airlangga, Indonesia (number: 345/UN3.14/PT/2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Universitas Airlangga for providing research funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization (WHO). Listings of WHO’s Response to COVID-19. 2020. Available online: https://www.who.int/news/item/29-06-2020-covidtimeline (accessed on 29 October 2021).
- World Health Organization (WHO). Global Tuberculosis Report 2021; WHO: Geneva, Switzerland, 2021. Available online: https://www.who.int/publications/i/item/9789240037021 (accessed on 29 October 2021).
- Centers for Disease Control and Prevention (CDC). Tuberculosis. Global Health. 2020. Available online: https://www.cdc.gov/globalhealth/newsroom/topics/tb/index.html (accessed on 7 October 2021).
- Shrinivasan, R.; Rane, S.; Pai, M. India’s syndemic of tuberculosis and COVID-19. BMJ Glob. Health 2020, 5, e003979. [Google Scholar] [CrossRef] [PubMed]
- Togun, T.; Kampmann, B.; Stoker, N.G.; Lipman, M. Anticipating the impact of the COVID-19 pandemic on TB patients and TB control programmes. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Song, W.-M.; Zhao, J.-Y.; Zhang, Q.-Y.; Liu, S.-Q.; Zhu, X.-H.; An, Q.-Q.; Xu, T.-T.; Li, S.-J.; Liu, J.-Y.; Tao, N.-N.; et al. COVID-19 and Tuberculosis Coinfection: An Overview of Case Reports/Case Series and Meta-Analysis. Front. Med. 2021, 8, 657006. [Google Scholar] [CrossRef] [PubMed]
- Tadolini, M.; García-García, J.-M.; Blanc, F.-X.; Borisov, S.; Goletti, D.; Motta, I.; Codecasa, L.; Tiberi, S.; Sotgiu, G.; Migliori, G.B. On tuberculosis and COVID-19 co-infection. Eur. Respir. J. 2020, 56, 2002328. [Google Scholar] [CrossRef] [PubMed]
- Sekaran, S.; Jagani, R.P.; Waleed, M.S.; Celi, C.S.V.; Marzban, S. Latent tuberculosis and COVID-19 disease. Discov. Rep. 2021, 4, e26. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial Resistance–Tuberculosis. An Agency of the European Union. 2022. Available online: https://www.ecdc.europa.eu/en/tuberculosis/antimicrobial-resistance (accessed on 14 June 2022).
- Bardhan, M.; Hasan, M.M.; Ray, I.; Sarkar, A.; Chahal, P.; Rackimuthu, S.; Essar, M.Y. Tuberculosis amidst COVID-19 pandemic in India: Unspoken challenges and the way forward. Trop. Med. Health 2021, 49, 84. [Google Scholar] [CrossRef]
- Bouaddi, O.; Hasan, M.M.; Sahito, A.M.; Shah, P.A.; Mohammed, A.Z.A.; Essar, M.Y. Tuberculosis in the middle of COVID-19 in Morocco: Efforts, challenges and recommendations. Trop. Med. Health 2021, 49, 98. [Google Scholar] [CrossRef]
- Awan, H.A.; Sahito, A.M.; Sukaina, M.; Khatri, G.; Waheed, S.; Sohail, F.; Hasan, M.M. Tuberculosis amidst COVID-19 in Pakistan: A massive threat of overlapping crises for the fragile healthcare systems. Epidemiol. Infect. 2022, 150, e41. [Google Scholar] [CrossRef]
- Dara, M.; Sotgiu, G.; Reichler, M.R.; Chiang, C.-Y.; Chee, C.B.E.; Migliori, G.B. New diseases and old threats: Lessons from tuberculosis for the COVID-19 response. Int. J. Tuberc. Lung Dis. 2020, 24, 544–545. [Google Scholar] [CrossRef]
- Ong, C.W.M.; Goletti, D. Impact of the global COVID-19 outbreak on the management of other communicable diseases. Int. J. Tuberc. Lung Dis. 2020, 24, 547–548. [Google Scholar] [CrossRef]
- Sheerin, D.; Abhimanyu Wang, X.; Johnson, W.E.; Coussens, A. Systematic evaluation of transcriptomic disease risk and diagnostic biomarker overlap between COVID-19 and tuberculosis: A patient-level meta-analysis. medRxiv 2020. [Google Scholar] [CrossRef]
- Zhou, S.; Van Staden, Q.; Toska, E. Resource reprioritisation amid competing health risks for TB and COVID-19. Int. J. Tuberc. Lung Dis. 2020, 24, 1215–1216. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, G.; Espinoza, W.; de Waard, J.H. How TB and COVID-19 compare: An opportunity to integrate both control programmes. Int. J. Tuberc. Lung Dis. 2020, 24, 971–974. [Google Scholar] [CrossRef] [PubMed]
- TB/COVID-19 Global Study Group. Tuberculosis and COVID-19 co-infection: Description of the global cohort. Eur. Respir. J. 2021, 59, 2102538. [Google Scholar]
- Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases, South Africa, Risk Factors for Coronavirus Disease 2019 (COVID-19) Death in a Population Cohort Study from the Western Cape Province, South Africa. Clin. Infect. Dis. 2021, 73, e2005-15. [CrossRef]
- Liu, J.; Zhang, S.; Wu, Z.; Shang, Y.; Dong, X.; Li, G.; Zhang, L.; Chen, Y.; Ye, X.; Du, H.; et al. Clinical outcomes of COVID-19 in Wuhan, China: A large cohort study. Ann. Intensive. Care 2020, 10, 99. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Deng, A.P.; Hu, T.; Chen, X.G.; Zhuang, Y.L.; Tan, X.H.; Zhen, H.Z.; Sun, L.M.; Li, Y.; Zhong, H.J.; et al. Clinical outcomes of COVID-19 cases and influencing factors in Guangdong province. Zhonghua Liu Xing Bing Xue Za Zhi 2020, 41, 1999–2004. [Google Scholar]
- Ding, W.; Li, Y.; Bai, Y.; Li, Y.; Wang, L.; Wang, Y. Estimating the Effects of the COVID-19 Outbreak on the Reductions in Tuberculosis Cases and the Epidemiological Trends in China: A Causal Impact Analysis. Infect. Drug Resist. 2021, 14, 4641–4655. [Google Scholar] [CrossRef]
- Pai, M.; Kasaeva, T.; Swaminathan, S. Covid-19′s Devastating Effect on Tuberculosis Care–A Path to Recovery. N. Engl. J. Med. 2022, 386, 1490–1493. [Google Scholar] [CrossRef]
- Soko, R.N.; Burke, R.M.; Feasey, H.R.; Sibande, W.; Nliwasa, M.; Henrion, M.Y.; Khundi, M.; Dodd, P.J.; Ku, C.C.; Kawalazira, G.; et al. Effects of Coronavirus Disease Pandemic on Tuberculosis Notifications, Malawi. Emerg. Infect. Dis. 2021, 27, 1831–1839. [Google Scholar] [CrossRef]
- Guan, W.-J.; Liang, W.-H.; Zhao, Y.; Liang, H.-R.; Chen, Z.-S.; Li, Y.-M.; Liu, X.-Q.; Chen, R.-C.; Tang, C.-L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef] [PubMed]
- Borgdorff, M.W.; Nagelkerke, N.J.; Dye, C.; Nunn, P. Gender and tuberculosis: A comparison of prevalence surveys with notification data to explore sex differences in case detection. Int. J. Tuberc. Lung Dis. 2000, 4, 123–132. [Google Scholar] [PubMed]
- Horton, K.C.; MacPherson, P.; Houben, R.M.G.J.; White, R.G.; Corbett, E.L. Sex Differences in Tuberculosis Burden and Notifications in Low- and Middle-Income Countries: A Systematic Review and Meta-analysis. PLoS Med. 2016, 13, e1002119. [Google Scholar] [CrossRef] [PubMed]
- Cilloni, L.; Fu, H.; Vesga, J.F.; Dowdy, D.; Pretorius, C.; Ahmedov, S.; Nair, S.A.; Mosneaga, A.; Masini, E.; Sahu, S.; et al. The potential impact of the COVID-19 pandemic on the tuberculosis epidemic a modelling analysis. EClinicalMedicine 2020, 28, 100603. [Google Scholar] [CrossRef]
- Xu, C.; Li, T.; Hu, D.; Zhang, H.; Zhao, Y.; Liu, J. Predicted Impact of the COVID-19 Responses on Deaths of Tuberculosis–China, 2020. China CDC Wkly 2021, 3, 21–24. [Google Scholar] [CrossRef]
- Visca, D.; Ong, C.; Tiberi, S.; Centis, R.; D’Ambrosio, L.; Chen, B.; Mueller, J.; Duarte, R.; Dalcolmo, M.; Sotgiu, G.; et al. Tuberculosis and COVID-19 interaction: A review of biological, clinical and public health effects. Pulmonology 2020, 27, 151–165. [Google Scholar] [CrossRef]
- O’Leary, T.J. Relative Sensitivity of Saliva and Upper Airway Swabs for Initial Detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Ambulatory Patients: Rapid Review. J. Mol. Diagn. 2021, 23, 265–273. [Google Scholar] [CrossRef]
- Chapman, H.J.; Veras-Estévez, B.A. Lessons Learned During the COVID-19 Pandemic to Strengthen TB Infection Control: A Rapid Review. Glob. Health Sci. Pract. 2021, 9, 964–977. [Google Scholar] [CrossRef]
- Awasthi, A.K.; Singh, P.K. Tuberculosis management in India during COVID-19 crisis. J. Public Health Policy 2021, 42, 185–189. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Tuberculosis Report 2020; WHO: Geneva, Switzerland, 2020.
- Abdool Karim, Q.; Abdool Karim, S.S. COVID-19 affects HIV and tuberculosis care. Science 2020, 369, 366–368. [Google Scholar] [CrossRef]
- Klinton, J.S.; Oga-Omenka, C.; Heitkamp, P. TB and COVID–Public and private health sectors adapt to a new reality. J. Clin. Tuberc. Other Mycobact. Dis. 2020, 21, 100199. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Public Health Surveillance for COVID-19: Interim Guidance. WHO. 2020. Available online: https://www.who.int/publications/i/item/who-2019-nCoV-surveillanceguidance-2020.8 (accessed on 1 December 2021).
- Woodhead, M.; Blasi, F.; Ewig, S.; Garau, J.; Huchon, G.; Ieven, M.; Ortqvist, A.; Schaberg, T.; Torres, A.; van der Heijden, G.; et al. Guidelines for the management of adult lower respiratory tract infections—Full version. Clin. Microbiol. Infect. 2011, 17 (Suppl. 6), E1–E59. [Google Scholar] [CrossRef]
- Ongole, J.J.; Rossouw, T.M.; Fourie, P.B.; Stoltz, A.C.; Hugo, J.; Marcus, T.S. Sustaining essential healthcare in Africa during the COVID-19 pandemic. Int. J. Tuberc. Lung Dis. 2020, 24, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; George, A.A.; Sahu, K.K.; Lal, A.; Abraham, G. Tuberculosis and COVID-19 Co-infection: An Updated Review. Acta Biomed. 2020, 92, e2021025. [Google Scholar] [PubMed]
- Kielmann, K.; Karat, A.; Zwama, G.; Colvin, C.; Swartz, A.; Voce, A.S.; Yates, T.A.; MacGregor, H.; McCreesh, N.; Kallon, I.; et al. Tuberculosis infection prevention and control: Why we need a whole systems approach. Infect. Dis. Poverty 2020, 9, 56. [Google Scholar] [CrossRef]
- Ilesanmi, O.S.; Afolabi, A.A.; Akande, A.; Raji, T.; Mohammed, A. Infection prevention and control during COVID-19 pandemic: Realities from health care workers in a north central state in Nigeria. Epidemiol. Infect. 2021, 149, e15. [Google Scholar] [CrossRef]
- USAID. COVID-19 Contact Tracing and TB Contact Investigation: An Integrated Implementation Approach. 2021. Available online: https://www.stoptb.org/file/9146/download (accessed on 19 December 2021).
- Chan, G.; Triasih, R.; Nababan, B.; du Cros, P.; Wilks, N.; Main, S.; Huang, G.K.L.; Lin, D.; Graham, S.M.; Majumdar, S.S.; et al. Adapting active case-finding for TB during the COVID-19 pandemic in Yogyakarta, Indonesia. Public Health Action 2021, 11, 41–49. [Google Scholar] [CrossRef]
- Wingfield, T.; Karmadwala, F.; MacPherson, P.; A Millington, K.; Walker, N.F.; E Cuevas, L.; Squire, S.B. Challenges and opportunities to end tuberculosis in the COVID-19 era. Lancet Respir. Med. 2021, 9, 556–558. [Google Scholar] [CrossRef]
- World Health Organization (WHO). One Health. WHO. 2017. Available online: https://www.who.int/news-room/questions-and-answers/item/one-health (accessed on 14 June 2022).
- Elmahi OK, O.; Uakkas, S.; Olalekan, B.Y.; Damilola, I.A.; Adedeji, O.J.; Hasan, M.M.; Thomson, D.J. Antimicrobial resistance and one health in the post COVID-19 era: What should health students learn? Antimicrob. Resist. Infect. Control 2022, 11, 58. [Google Scholar] [CrossRef]
- Takahashi, H. Role of latent tuberculosis infections in reduced COVID-19 mortality: Evidence from an instrumental variable method analysis. Med. Hypotheses 2020, 144, 110214. [Google Scholar] [CrossRef]
- Madan, M.; Baldwa, B.; Raja, A.; Tyagi, R.; Dwivedi, T.; Mohan, A.; Guleria, R. Impact of Latent Tuberculosis on Severity and Outcomes in Admitted COVID-19 Patients. Cureus 2021, 13, e19882. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines on the Management of Latent Tuberculosis Infection; WHO: Geneva, Switzerland, 2015.
- Stochino, C.; Villa, S.; Zucchi, P.; Parravicini, P.; Gori, A.; Raviglione, M.C. Clinical characteristics of COVID-19 and active tuberculosis co-infection in an Italian reference hospital. Eur. Respir. J. 2020, 56, 2001708. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). COVID-19: Considerations for Tuberculosis (TB) Care. World Health Organization (WHO) Information Note. 2020. Available online: www.who.int/tb/COVID_19considerations_tuberculosis_services.pdf (accessed on 15 December 2021).
- Lung, T.; Marks, G.; Nhung, N.V.; Anh, N.T.; Hoa, N.L.P.; Anh, L.T.N.; Hoa, N.B.; Britton, W.J.; Bestrashniy, J.; Jan, S.; et al. Household contact investigation for the detection of tuberculosis in Vietnam: Economic evaluation of a cluster-randomised trial. Lancet Glob. Health 2019, 7, e376–e384. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Fleming, J.; Yu, Y.; Gu, Y.; Liu, C.; Liu, Y. Active or latent tuberculosis increases susceptibility to COVID-19 and disease severity. medRxiv 2020. Available online: https://www.medrxiv.org/content/10.1101/2020.03.10.20033795v1 (accessed on 15 December 2021).
- Khurana, A.K.; Aggarwal, D. The (in)significance of TB and COVID-19 co-infection. Eur. Respir. J. 2020, 56, 2002105. [Google Scholar] [CrossRef] [PubMed]
- Tamuzi, J.L.; Ayele, B.T.; Shumba, C.S.; Adetokunboh, O.O.; Uwimana-Nicol, J.; Haile, Z.T.; Inugu, J.; Nyasulu, P.S. Implications of COVID-19 in high burden countries for HIV/TB: A systematic review of evidence. BMC Infect. Dis. 2020, 20, 744. [Google Scholar] [CrossRef]
- USAID. Simultaneous, Integrated Diagnostic Testing Approach to Detect COVID-19 and TB in High TB Burden Countries. Stop TB Partnership. 2021. Available online: https://www.stoptb.org/file/9145/download (accessed on 14 June 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).