Prevalence and Determinants of COVID-19 Vaccine Acceptance in South East Asia: A Systematic Review and Meta-Analysis of 1,166,275 Respondents
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy and Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis
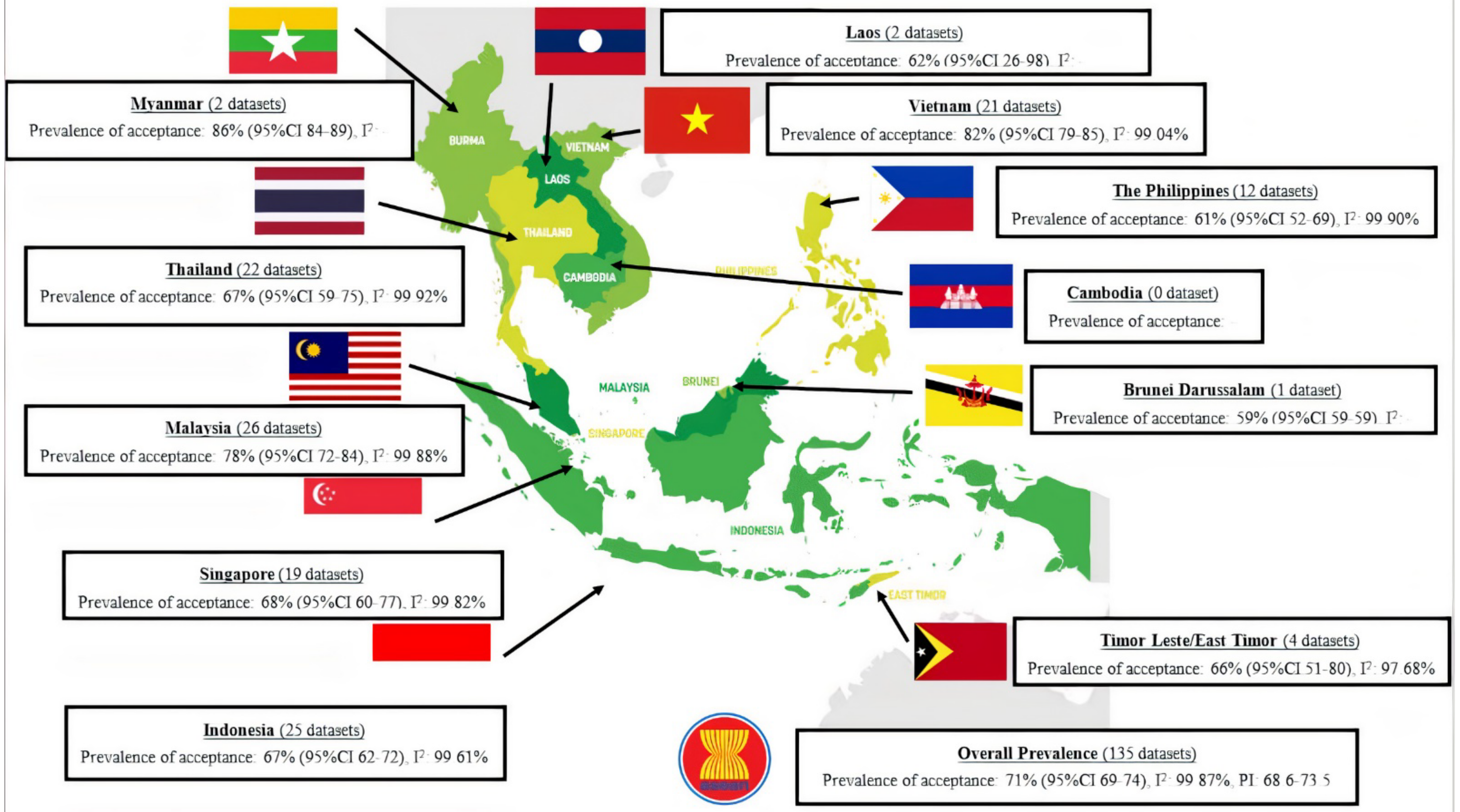
3. Results
4. Discussion
4.1. Comparison of COVID-19 Vaccination Acceptance Rate with Other Studies
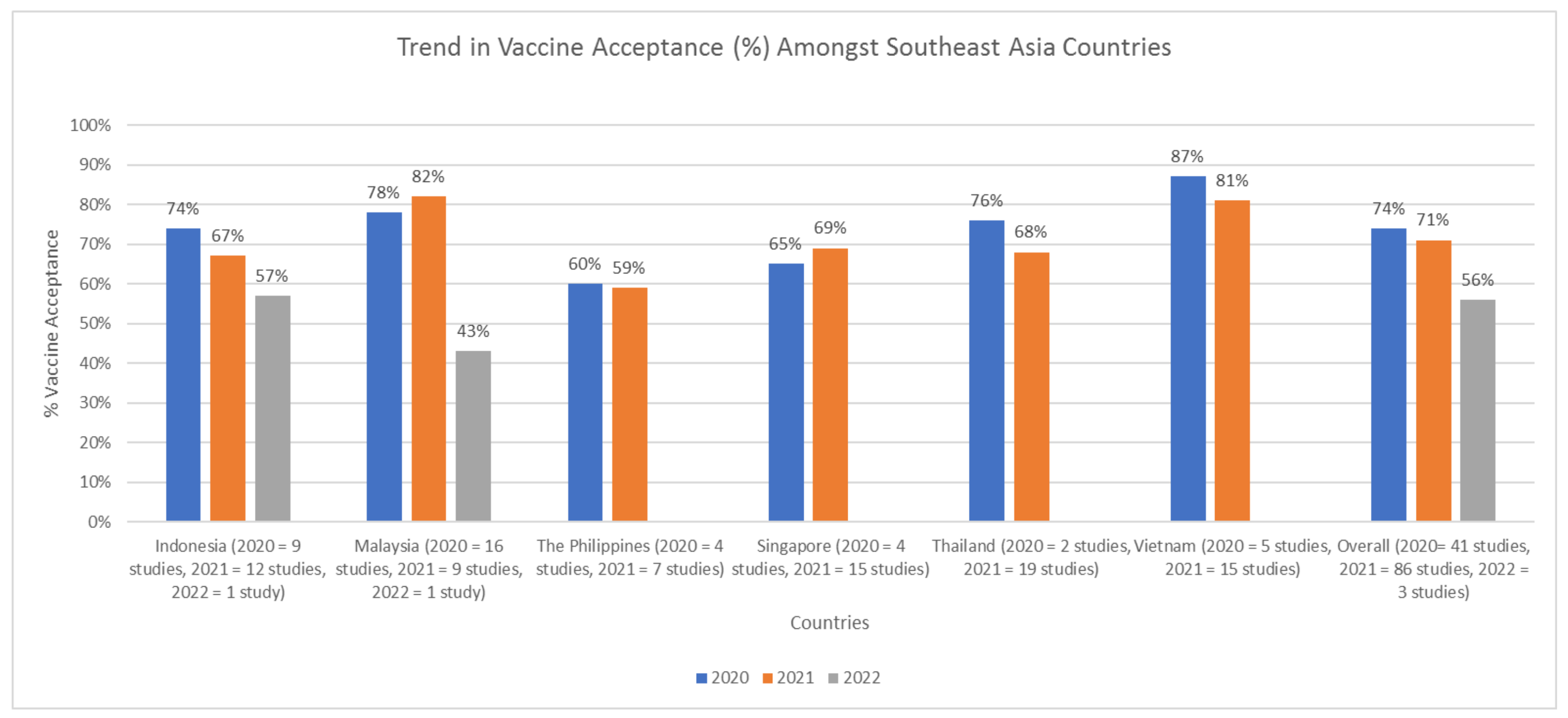
4.2. Trends in COVID-19 Vaccination Acceptance Rate in SEA
4.3. Findings of Sub-Group Analysis
4.4. Limitation
4.5. Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmes, E.C.; Goldstein, S.A.; Rasmussen, A.L.; Robertson, D.L.; Crits-Christoph, A.; Wertheim, J.O.; Anthony, S.J.; Barclay, W.S.; Boni, M.F.; Doherty, P.C.; et al. The origins of SARS-CoV-2: A critical review. Cell 2021, 184, 4848–4856. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.; Quiñones-Laveriano, D.M.; Azañero, J.; Chumpitaz, R.; Claros, J.; Salazar, L.; Rosales, O.; Nuñez, L.; Roca, D.; Alcantara, A. Mortality and associated risk factors in patients hospitalized due to COVID-19 in a Peruvian reference hospital. PLoS ONE 2022, 17, e0264789. [Google Scholar] [CrossRef] [PubMed]
- Dewi, R.; Kaswandani, N.; Karyanti, M.R.; Setyanto, D.B.; Pudjiadi, A.H.; Hendarto, A.; Djer, M.M.; Prayitno, A.; Yuniar, I.; Indawati, W.; et al. Mortality in children with positive SARS-CoV-2 polymerase chain reaction test: Lessons learned from a tertiary referral hospital in Indonesia. Int. J. Infect. Dis. 2021, 107, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Putri, N.D.; Prawira, Y.; Tartila, T.; Jasin, M.R.; Puspitasari, H.A.; Puspaningtyas, N.W.; Indawati, W.; Karyanti, M.R.; Setyanto, D.B.; Prayitno, A.; et al. Clinical Features of Multisystem Inflammatory Syndrome in Children Associated with COVID-19 in Indonesia. J. Trop. Pediatr. 2022, 68, fmac025. [Google Scholar] [CrossRef] [PubMed]
- Octavius, G.S.; Wijaya, J.H.; Tan, A.O.; Muljono, M.P.; Chandra, S.; Juliansen, A. Autopsy findings of pediatric COVID-19: A systematic review. Egypt. J. Forensic Sci. 2022, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Octavius, G.S.; Tan, R.; Pratama, T.A.; Budiputri, C.L.; Meliani, F.; Heriyanto, R.S.; Muljadi, R.; Juliansen, A. Cardiac manifestations and diagnostic imaging in pediatric inflammatory multisystem syndrome temporally associated with COVID-19: A systematic review. Med. J. Indones. 2021, 31, 20–37. [Google Scholar] [CrossRef]
- Octavius, G.S.; Silviani, F.R.; Lesmandjaja, A.; Angelina; Juliansen, A. Impact of COVID-19 on adolescents’ mental health: A systematic review. Middle East Curr. Psychiatry 2020, 27, 72. [Google Scholar] [CrossRef]
- Pak, A.; Adegboye, O.A.; Adekunle, A.I.; Rahman, K.M.; McBryde, E.S.; Eisen, D.P. Economic Consequences of the COVID-19 Outbreak: The Need for Epidemic Preparedness. Front. Public Health 2020, 8, 241. [Google Scholar] [CrossRef]
- Timotius, E.; Octavius, G.S. Global Changing of Consumer Behavior to Retail Distribution due to Pandemic of COVID-19: A Systematic Review. J. Distrib. Sci. 2021, 19, 69–80. [Google Scholar]
- Kelly, K.; Hwei, L.R.Y.; Octavius, G.S. Coronavirus outbreaks including COVID-19 and impacts on medical education: A systematic review. J. Community Empower. Health 2020, 3, 130–140. [Google Scholar] [CrossRef]
- Ali, M.J.; Hanif, M.; Haider, M.A.; Ahmed, M.U.; Sundas, F.; Hirani, A.; Khan, I.A.; Anis, K.; Karim, A.H. Treatment Options for COVID-19: A Review. Front. Med. 2020, 7, 480. [Google Scholar] [CrossRef]
- Octavius, G.S.; Pardede, C.S.B.R.; Thandy, C.C.; Fisca, C.A.L.; Juliansen, A. Convalescent plasma therapy in critically ill pediatric COVID-19 patients: A systematic review. Infect. Dis. Trop. Med. (IDTM) 2022, 8, e868–e878. [Google Scholar]
- Iezadi, S.; Azami-Aghdash, S.; Ghiasi, A.; Rezapour, A.; Pourasghari, H.; Pashazadeh, F.; Gholipour, K. Effectiveness of the non-pharmaceutical public health interventions against COVID-19; a protocol of a systematic review and realist review. PLoS ONE 2020, 15, e0239554. [Google Scholar] [CrossRef]
- Sayed, A.A. The Progressive Public Measures of Saudi Arabia to Tackle COVID-19 and Limit Its Spread. Int. J. Environ. Res. Public Health 2021, 18, 783. [Google Scholar] [CrossRef]
- Gupta, B.; Gupta, A. Will COVID vaccine be a game changer in current pandemic situation? IP J. Surg. Allied Sci. 2021, 3, 34–38. [Google Scholar] [CrossRef]
- Tian, F.; Yang, R.; Chen, Z. Safety and efficacy of COVID-19 vaccines in children and adolescents: A systematic review of randomized controlled trials. J. Med. Virol. 2022, 94, 4644–4653. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Fan, Y.J.; Chan, K.H.; Hung, I.F. Safety and Efficacy of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Different Vaccines at Phase 3. Vaccines 2021, 9, 989. [Google Scholar] [CrossRef]
- Razai, M.S.; Chaudhry, U.A.R.; Doerholt, K.; Bauld, L.; Majeed, A. Covid-19 vaccination hesitancy. BMJ 2021, 373, n1138. [Google Scholar] [CrossRef]
- Thomson, A.; Robinson, K.; Vallée-Tourangeau, G. The 5As: A practical taxonomy for the determinants of vaccine uptake. Vaccine 2016, 34, 1018–1024. [Google Scholar] [CrossRef]
- MacDonald, N.E. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef] [PubMed]
- Gargano, L.M.; Painter, J.E.; Sales, J.M.; Morfaw, C.; Jones, L.M.; Murray, D.; Wingood, G.M.; DiClemente, R.J.; Hughes, J.M. Seasonal and 2009 H1N1 influenza vaccine uptake, predictors of vaccination, and self-reported barriers to vaccination among secondary school teachers and staff. Hum. Vaccines 2011, 7, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Dubé, E.; Vivion, M.; MacDonald, N.E. Vaccine hesitancy, vaccine refusal and the anti-vaccine movement: Influence, impact and implications. Expert Rev. Vaccines 2015, 14, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, C.; Wilson, R.; O’Leary, M.; Eckersberger, E.; Larson, H.J. Strategies for addressing vaccine hesitancy—A systematic review. Vaccine 2015, 33, 4180–4190. [Google Scholar] [CrossRef] [PubMed]
- Kamali, K.; Hoseinzade, Z.; Hajimiri, K.; Hoveidamanesh, S.; Zahraei, S.M.; Gouya, M.M.; Bavandpouri, S.M.; Mohamadi, T.; Mohamadi, S.; Bigdeli, Z.; et al. Determinants of COVID-19 vaccine acceptance in healthcare workers in Iran: National Survey. BMC Infect. Dis. 2022, 22, 703. [Google Scholar] [CrossRef]
- Zhang, J.; Dean, J.; Yin, Y.; Wang, D.; Sun, Y.; Zhao, Z.; Wang, J. Determinants of COVID-19 Vaccine Acceptance and Hesitancy: A Health Care Student-Based Online Survey in Northwest China. Front. Public Health 2022, 9, 777565. [Google Scholar] [CrossRef]
- Patwary, M.M.; Bardhan, M.; Haque, M.Z.; Sultana, R.; Alam, M.A.; Browning, M. COVID-19 Vaccine Acceptance Rate and Its Factors among Healthcare Students: A Systematic Review with Meta-Analysis. Vaccines 2022, 10, 806. [Google Scholar] [CrossRef]
- Patwary, M.M.; Bardhan, M.; Disha, A.S.; Hasan, M.; Haque, M.Z.; Sultana, R.; Hossain, M.R.; Browning, M.H.E.M.; Alam, M.A.; Sallam, M. Determinants of COVID-19 Vaccine Acceptance among the Adult Population of Bangladesh Using the Health Belief Model and the Theory of Planned Behavior Model. Vaccines 2021, 9, 1393. [Google Scholar] [CrossRef]
- Mose, A.; Wasie, A.; Shitu, S.; Haile, K.; Timerga, A.; Melis, T.; Sahle, T.; Zewdie, A. Determinants of COVID-19 vaccine acceptance in Ethiopia: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0269273. [Google Scholar] [CrossRef]
- Youssef, D.; Abou-Abbas, L.; Berry, A.; Youssef, J.; Hassan, H. Determinants of acceptance of Coronavirus disease-2019 (COVID-19) vaccine among Lebanese health care workers using health belief model. PLoS ONE 2022, 17, e0264128. [Google Scholar] [CrossRef]
- Shakeel, C.S.; Mujeeb, A.A.; Mirza, M.S.; Chaudhry, B.; Khan, S.J. Global COVID-19 Vaccine Acceptance: A Systematic Review of Associated Social and Behavioral Factors. Vaccines 2022, 10, 110. [Google Scholar] [CrossRef]
- Robinson, E.; Jones, A.; Lesser, I.; Daly, M. International estimates of intended uptake and refusal of COVID-19 vaccines: A rapid systematic review and meta-analysis of large nationally representative samples. Vaccine 2021, 39, 2024–2034. [Google Scholar] [CrossRef]
- Kazeminia, M.; Afshar, Z.M.; Rajati, M.; Saeedi, A.; Rajati, F. Evaluation of the Acceptance Rate of COVID-19 Vaccine and its Associated Factors: A Systematic Review and Meta-analysis. J. Prev. 2022, 43, 421–467. [Google Scholar] [CrossRef]
- Worldometer. South-Eastern Asia Population (LIVE). Available online: https://www.worldometers.info/world-population/south-eastern-asia-population/#:~:text=The%20current%20population%20of%20South,of%20the%20total%20world%20population (accessed on 15 September 2022).
- Norhayati, M.N.; Che Yusof, R.; Azman, Y.M. Systematic Review and Meta-Analysis of COVID-19 Vaccination Acceptance. Front. Med. 2021, 8, 783982. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Britto, C.; Pollard, A.J.; Voysey, M.; Blohmke, C.J. An Appraisal of the Clinical Features of Pediatric Enteric Fever: Systematic Review and Meta-analysis of the Age-Stratified Disease Occurrence. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017, 64, 1604–1611. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Jukkrit, W.; Nattapong, A.; Nuttida, K. Knowledge, Attitude, Practice and Acceptance of COVID-19 Vaccine among Elderly in Chiang Mai, Thailand. J. Educ. Community Health 2021, 8, 245–251. [Google Scholar]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch; Losos, M.; Tugwell, P.; Ga, S.W.; Zello, G.; Petersen, J. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 18 July 2022).
- The Joanna Briggs Institute. Checklist for Prevalence Studies. Available online: https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Prevalence_Studies2017_0.pdf (accessed on 18 July 2022).
- Noordzij, M.; Dekker, F.W.; Zoccali, C.; Jager, K.J. Measures of disease frequency: Prevalence and incidence. Nephron. Clin. Pract. 2010, 115, c17–c20. [Google Scholar] [CrossRef]
- Nyaga, V.N.; Arbyn, M.; Aerts, M. Metaprop: A Stata command to perform meta-analysis of binomial data. Arch. Public Health 2014, 72, 39. [Google Scholar] [CrossRef]
- Borenstein, M. Research Note: In a meta-analysis, the I(2) index does not tell us how much the effect size varies across studies. J. Physiother. 2020, 66, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yang, L.; Zhao, J.; Wang, L.; Zou, J.; Wang, C.; Fan, X. Comparison of problem-based learning and traditional teaching methods in medical psychology education in China: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0243897. [Google Scholar] [CrossRef] [PubMed]
- Page, M.; Higgins, J.; Sterne, J. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Chichester, UK, 2019; pp. 349–374. [Google Scholar]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- IntHout, J.; Ioannidis, J.P.A.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol. 2014, 14, 25. [Google Scholar] [CrossRef]
- Harbord, R.M.; Whiting, P. metandi: Meta-analysis of diagnostic accuracy using hierarchical logistic regression. Stata J. 2009, 9, 211–229. [Google Scholar] [CrossRef]
- Rücker, G.; Schwarzer, G.; Carpenter, J.; Olkin, I. Why add anything to nothing? The arcsine difference as a measure of treatment effect in meta-analysis with zero cells. Stat. Med. 2009, 28, 721–738. [Google Scholar] [CrossRef]
- Alimohamadi, Y.; Hosamirudsari, H.; Hesari, E.; Sepandi, M. Global COVID-19 vaccine acceptance rate: A systematic review and meta-analysis. J. Public Health 2022, 1–13. [Google Scholar] [CrossRef]
- Mahmud, S.; Mohsin, M.; Hossain, S.; Islam, M.M.; Muyeed, A. The acceptance of COVID-19 vaccine at early stage of development and approval: A global systematic review and meta-analysis. Heliyon 2022, 8, e10728. [Google Scholar] [CrossRef]
- Sallam, M. COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates. Vaccines 2021, 9, 160. [Google Scholar] [CrossRef]
- Greco, T.; Zangrillo, A.; Biondi-Zoccai, G.; Landoni, G. Meta-analysis: Pitfalls and hints. Heart Lung Vessel. 2013, 5, 219–225. [Google Scholar]
- Minh, L.H.N.; Khoi Quan, N.; Le, T.N.; Khanh, P.N.Q.; Huy, N.T. COVID-19 Timeline of Vietnam: Important Milestones Through Four Waves of the Pandemic and Lesson Learned. Front. Public Health 2021, 9, 1578. [Google Scholar] [CrossRef]
- UN Chronicles. Game-Changers in Viet Nam’s Successful COVID-19 Response. Available online: https://www.un.org/ar/node/97757 (accessed on 15 September 2022).
- World Health Organization. Viet Nam—Scaling up COVID-19 Vaccination Rates in Viet Nam through Vaccine Diplomacy, Efficient Vaccine Rollout and Enhancing Effective Service Delivery. Available online: https://www.who.int/about/accountability/results/who-results-report-2020-mtr/country-story/2021/vietnam (accessed on 15 September 2022).
- Nguyen, L.H.; Hoang, M.T.; Nguyen, L.D.; Ninh, L.T.; Nguyen, H.T.T.; Nguyen, A.D.; Vu, L.G.; Vu, G.T.; Doan, L.P.; Latkin, C.A.; et al. Acceptance and willingness to pay for COVID-19 vaccines among pregnant women in Vietnam. Trop. Med. Int. Health 2021, 26, 1303–1313. [Google Scholar] [CrossRef]
- The Asia Foundation. Timor-Leste Covid-19 Survey Round 5 February 2021. Available online: https://asiafoundation.org/publication/timor-leste-covid-19-survey-round-5-february-2021/ (accessed on 15 September 2022).
- Migriño, J., Jr.; Gayados, B.; Birol, K.R.J.; De Jesus, L.; Lopez, C.W.; Mercado, W.C.; Tolosa, J.C.; Torreda, J.; Tulagan, G. Factors affecting vaccine hesitancy among families with children 2 years old and younger in two urban communities in Manila, Philippines. West. Pac. Surveill. Response J. 2020, 11, 20–26. [Google Scholar] [CrossRef]
- Landicho-Guevarra, J.; Reñosa, M.D.C.; Wachinger, J.; Endoma, V.; Aligato, M.F.; Bravo, T.A.; Landicho, J.; Bärnighausen, K.; McMahon, S.A. Scared, powerless, insulted and embarrassed: Hesitancy towards vaccines among caregivers in Cavite Province, the Philippines. BMJ Glob. Health 2021, 6, e006529. [Google Scholar] [CrossRef]
- Côté, J.; Aita, M.; Chouinard, M.-C.; Houle, J.; Lavoie-Tremblay, M.; Lessard, L.; Rouleau, G.; Gélinas, C. Psychological distress, depression symptoms and fatigue among Quebec nursing staff during the COVID-19 pandemic: A cross-sectional study. Nurs. Open 2022, 9, 1744–1756. [Google Scholar] [CrossRef]
- Morgul, E.; Bener, A.; Atak, M.; Akyel, S.; Aktaş, S.; Bhugra, D.; Ventriglio, A.; Jordan, T.R. COVID-19 pandemic and psychological fatigue in Turkey. Int. J. Soc. Psychiatry 2020, 67, 128–135. [Google Scholar] [CrossRef]
- Gerace, A.; Rigney, G.; Anderson, J.R. Predicting attitudes towards easing COVID-19 restrictions in the United States of America: The role of health concerns, demographic, political, and individual difference factors. PLoS ONE 2022, 17, e0263128. [Google Scholar] [CrossRef]
- Cunha, R.; Ochoa-Leite, C.; Pires, L.; Morais, M.; Costa, R.; Rocha, L. COVID-19 vaccine booster in healthcare workers—Reasons for refusing. Pulmonology 2022, 28, 476–477. [Google Scholar] [CrossRef]
- Fieselmann, J.; Annac, K.; Erdsiek, F.; Yilmaz-Aslan, Y.; Brzoska, P. What are the reasons for refusing a COVID-19 vaccine? A qualitative analysis of social media in Germany. BMC Public Health 2022, 22, 846. [Google Scholar] [CrossRef] [PubMed]
- Dalton, J.E.; Bolen, S.D.; Mascha, E.J. Publication Bias: The Elephant in the Review. Anesth. Analg. 2016, 123, 812–813. [Google Scholar] [CrossRef]
- Martínez-Mesa, J.; González-Chica, D.A.; Duquia, R.P.; Bonamigo, R.R.; Bastos, J.L. Sampling: How to select participants in my research study? An. Bras. Dermatol. 2016, 91, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Boni, R.B. Web surveys in the time of COVID-19. Cad. Saúde Pública 2020, 36, e00155820. [Google Scholar] [CrossRef] [PubMed]
- Jüni, P.; Altman, D.G.; Egger, M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001, 323, 42–46. [Google Scholar] [CrossRef]
- Jung, R.G.; Di Santo, P.; Clifford, C.; Prosperi-Porta, G.; Skanes, S.; Hung, A.; Parlow, S.; Visintini, S.; Ramirez, F.D.; Simard, T.; et al. Methodological quality of COVID-19 clinical research. Nat. Commun. 2021, 12, 943. [Google Scholar] [CrossRef]
- Smith, G.D.; Ebrahim, S. Data dredging, bias, or confounding. BMJ 2002, 325, 1437–1438. [Google Scholar] [CrossRef]
- Our World in Data. Coronavirus (COVID-19) Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations?country=OWID_WRL (accessed on 15 September 2022).
- Yanto, T.A.; Octavius, G.S.; Heriyanto, R.S.; Ienawi, C.; Nisa, H.; Pasai, H.E. Psychological factors affecting COVID-19 vaccine acceptance in Indonesia. Egypt. J. Neurol. Psychiatry Neurosurg. 2021, 57, 177. [Google Scholar] [CrossRef]
- Murphy, J.; Vallières, F.; Bentall, R.P.; Shevlin, M.; McBride, O.; Hartman, T.K.; McKay, R.; Bennett, K.; Mason, L.; Gibson-Miller, J.; et al. Psychological characteristics associated with COVID-19 vaccine hesitancy and resistance in Ireland and the United Kingdom. Nat. Commun. 2021, 12, 29. [Google Scholar] [CrossRef]
- Rahman, M.M.; Chisty, M.A.; Alam, M.A.; Sakib, M.S.; Quader, M.A.; Shobuj, I.A.; Halim, M.A.; Rahman, F. Knowledge, attitude, and hesitancy towards COVID-19 vaccine among university students of Bangladesh. PLoS ONE 2022, 17, e0270684. [Google Scholar] [CrossRef]
- Fedorov, V.; Mannino, F.; Zhang, R. Consequences of dichotomization. Pharm. Stat. 2009, 8, 50–61. [Google Scholar] [CrossRef]
- Paez, A. Gray literature: An important resource in systematic reviews. J. Evid. Based Med. 2017, 10, 233–240. [Google Scholar] [CrossRef]
- Vallejo, B.M.; Ong, R.A.C. OCTA as an independent science advice provider for COVID-19 in the Philippines. Humanit. Soc. Sci. Commun. 2022, 9, 104. [Google Scholar] [CrossRef]
- IntHout, J.; Ioannidis, J.P.A.; Rovers, M.M.; Goeman, J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016, 6, e010247. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanto, T.A.; Lugito, N.P.H.; Hwei, L.R.Y.; Virliani, C.; Octavius, G.S. Prevalence and Determinants of COVID-19 Vaccine Acceptance in South East Asia: A Systematic Review and Meta-Analysis of 1,166,275 Respondents. Trop. Med. Infect. Dis. 2022, 7, 361. https://doi.org/10.3390/tropicalmed7110361
Yanto TA, Lugito NPH, Hwei LRY, Virliani C, Octavius GS. Prevalence and Determinants of COVID-19 Vaccine Acceptance in South East Asia: A Systematic Review and Meta-Analysis of 1,166,275 Respondents. Tropical Medicine and Infectious Disease. 2022; 7(11):361. https://doi.org/10.3390/tropicalmed7110361
Chicago/Turabian StyleYanto, Theo Audi, Nata Pratama Hardjo Lugito, Lie Rebecca Yen Hwei, Cindy Virliani, and Gilbert Sterling Octavius. 2022. "Prevalence and Determinants of COVID-19 Vaccine Acceptance in South East Asia: A Systematic Review and Meta-Analysis of 1,166,275 Respondents" Tropical Medicine and Infectious Disease 7, no. 11: 361. https://doi.org/10.3390/tropicalmed7110361
APA StyleYanto, T. A., Lugito, N. P. H., Hwei, L. R. Y., Virliani, C., & Octavius, G. S. (2022). Prevalence and Determinants of COVID-19 Vaccine Acceptance in South East Asia: A Systematic Review and Meta-Analysis of 1,166,275 Respondents. Tropical Medicine and Infectious Disease, 7(11), 361. https://doi.org/10.3390/tropicalmed7110361




