Abstract
Background: Antimicrobial resistance (AMR) is a multidimensional phenomenon. The environment acts as a mixing pot of drug-resistant bacteria from many sources such as pharmaceutical, biomedical, veterinary, and agricultural sectors. In this study, we analysed the existing AMR-related policies/guidelines/legislations in India in the above domains and how the current practices are being guided by them. Methods: We used a convergent parallel mix method design. Quantitative data were collected through a review of policies/guidelines/legislations in the said domains and analysed using the SWOT tool parallelly supported by key informant interviews of domain-specific stakeholders. Results: Altogether, 19 existing AMR policies/guidelines/legislations were identified. The existence of few policies/guidelines in each domain indicated the evolving environment for policy interventions. However, the lack of capacity among farmers, inadequate provision for structured capacity building, high cost of alternatives to antimicrobials, and lack of provision of incentivisation in case of crop failure were identified as the major weaknesses prevalent across the domains. Opportunities for policy refinements/the introduction of new policies are ample. However, easy access to antimicrobials and injudicious use imposes threats to AMR containment in all sectors. Conclusions: Despite having a few policies for the containment of AMR, their implementation witnesses challenge due to the lack of collaborative approaches, the existence of policies disjointed from ground reality, infrastructural issues, and the lack of capacity and resources.
1. Introduction
Since the introduction of antibiotics in 1930, they have gained immense importance in global health and food production [1]. However, the irrational use of antibiotics fosters the process of the mutation, selection, and proliferation of bacteria in the environment, resulting in antimicrobial resistance (AMR), which is one of the major public health concerns of recent times [2]. The indiscriminate and extensive use of antimicrobials is known to be the biggest driver of AMR, and according to Klein et al., low- and middle-income countries (LMICs) tend to consume antibiotics more than high-income countries [3].
AMR is a multifaceted and complex global phenomenon, and the environment has been considered a mixing pot of drug-resistance bacteria from all sources [4]. The pharmaceutical, biomedical, veterinary, and agricultural sectors are considered potential sources of AMR pollution in the environment [5,6,7,8,9]. The application of antibiotics is not only restricted to therapeutics but is also used as prophylactics [10]. To meet the mounting demand for food for the growing population, the rampant use of antibiotics as prophylactics and growth promoters in animal husbandry, aquaculture, and agriculture is increasing in low- and middle-income countries, including India [11]. Unfortunately, India recorded the highest consumption of antibiotics, indicated by their increasing sales [12]. Prompt intervention is needed to address the AMR issue. Moreover, inadequate sanitation and poor hygiene practices amplify the propensity for increasing antimicrobial pollution in the environment [13]. AMR is being recognised as one of the barriers to the attainment of Sustainable Development Goals (SDG 3: good health and well-being and SDG 6: clean water and sanitation).
In recent times, AMR has been recognised as a “One Health” problem due to its interconnectedness between human and animal health and the environment. The burden of antimicrobial-resistant bacteria also varies with geographical location, further catalysed by different environmental variables [14] and seasonality [15]. Moreover, there are several social determinants such as poverty, illiteracy, and rapid urbanisation that perpetuate the spread of contagious bacterial diseases, resulting in increased susceptibility to infectious diseases in the Indian Subcontinent [16]. This interconnectedness of humans, animals, and the environment brings about the complexity of AMR, which needs to be addressed through multicentric, collaborative approaches.
As a global initiative to contain AMR, the WHO, along with the FAO and the OIE, developed a comprehensive Global Action Plan (GAP) on AMR in 2015 [17]. Additionally, in 2019, the WHO categorised antibiotics in the AWaRe groups to monitor their administration. However, its implementation across LMICs is still awaited [18]. Inadequate empirical evidence on AMR incidences in the different sectors and their interconnectedness poses a major hindrance to unravelling the environmental dimension of AMR and demands a review of the existing policies and guidelines, and the formulation of new ones if required. Against this backdrop, we conducted situation/SWOT analyses on the existing AMR-related policies/guidelines/legislations in India and how they are guiding the current practices.
2. Methodology of the Study
2.1. Study Design
We used a convergent parallel mix method design in the present study, where the quantitative data were collected through a review of policy-related documents/guidelines/legislations in various priority domains mentioned earlier, all of which are related to the environmental dimensions of AMR. The qualitative data were concurrently collected using key informant interviews of relevant stakeholders identified from similar priority domains and available during the period of study.
2.2. Study Duration
The study was conducted during the period of April 2021–March 2022.
2.3. Study Population
We considered all the legislations, policies, and guidelines related to the use of antibiotics/antimicrobials; the allowable limits of antibiotics/antimicrobials in various items; the discharge limits of antibiotics from factories; waste management; and the monitoring of antimicrobial use in plant agriculture/animal husbandry/human health sector. Key informant interviews (KIIs) were conducted with stakeholders of the aforesaid priority domains at different levels, i.e., farmers, practitioners, executive or implementing officers/scientists, and policymakers.
2.4. Literature Review
We initially searched the official websites of various line departments of the Government of India for the policies/guidelines/legislations directly or indirectly related to the containment of antimicrobial resistance in India. The electronic databases of the published literature such as PubMed/Medline, Google Scholar, Scopus, Embase, and Index Medicus were also searched for this purpose for the last ten years (2010–2020) (Table S1). Simultaneously, we also received information about such existing policies during the KIIs and incorporated it into our studies.
2.5. Interview with Stakeholders
The KIIs were conducted to understand how the current AMR policy landscape in India is guiding the practices and what could be the possible way forward. The key informants were selected based on their expertise and willingness to share information. A total of 17 KIIs were conducted in this study. The process of KIIs was initiated with the development of an interview guide for each domain encompassing key questions in the following segments: the existing policies/guidelines/legislation in India related to AMR containment in various domains; the status of implementation of those policies/guidelines/legislations; the challenges in implementing them; the gaps in the existing intervention strategies; and the way forward to improve the environment to contain AMR in India. The interviews were audio-recorded. Data were transcribed into text data, then arranged systematically, and coded based on the predetermined scopes (the current landscape of policies/guidelines; implementation challenges; gaps if any; current practices; the area of intervention) identified through the literature review. The defined codes were then gathered together by eliminating redundancy to develop the key themes. Until this point, two investigators ran this process independently.
2.6. SWOT Analysis
The SWOT (strength, weakness, opportunity, and threat) analysis is one of the tools that can be used for strategic management to appraise policies. We performed a SWOT analysis in this study to strategically identify the scopes of intervention in the mentioned domains for containing AMR in the environment in India, as this analysis considers both the internal and external factors and generates information for prioritising and facilitating decision making.
3. Results
In this study, we found 19 existing AMR policies/guidelines/legislations in India. Two were from the plant/agricultural sector, eight from animal husbandry, five from the human health sector, and four from the pharmaceutical sector.
3.1. Policies/Guidelines/Legislation Reviewed in the Plant/Agricultural Domain
Two existing policies related to AMR were found in this domain. One was an act related to the use of insecticide along with antibiotics, which came into force in 1968 [19]. The act mandated the licensing of the production and trading of antibiotics. Another one was implemented by the Ministry of Agriculture and Farmers Welfare, Government of India, in 2021 [20], regarding the use of prescribed formulations of insecticides, fungicides, and antibiotics (Table 1).

Table 1.
Existing policies/guidelines/legislations for restricting antibiotic usage on plant agriculture in India.
Analysis of the Reviewed Policies in the Plant/Agricultural Domain (Table S2)
We conducted a SWOT analysis of the policies available in this sector. It is evident from the presence of current policies that the system has started to evolve. However, implementation challenges persist. The ignorance among farmers regarding the optimum use of antimicrobials and the desire to make a profit are major weaknesses. During the KIIs, one of the key informants (district-level agricultural officer, male) mentioned, “the farmer believes that application of antibiotic in large amount keep the crops healthy and increase their yield, though the disease occurred in crops can be manageable also without any economic loss”. Another key informant (scientist, male) mentioned, “the farmers cannot identify the acute stage of infection when the antibiotic formulation is needed to apply on crops”. However, these weaknesses can also be used as opportunities for new policy introduction focusing on the capacity building of farmers. However, the absence of surveillance on sales of such preparation at the market may currently act as a threat to optimising the use of antimicrobials in the plant/agricultural sector (Figure 1).
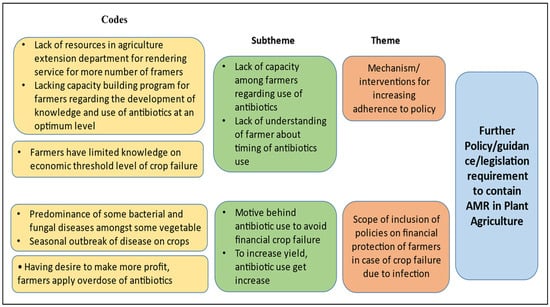
Figure 1.
Thematic areas for intervention in plant/agricultural sector.
3.2. Policies/Guidelines/Legislations Reviewed in the Veterinary Domain
We reviewed 14 policies/ guidelines/ legislations related to AMR in the veterinary sector (Table 2). Out of them, the majority are implemented by the Ministry of Health and Family Welfare, Government of India, and a few by the Ministry of Commerce and Industry, GOI, and the Department of Animal Husbandry and Dairying. Many of them are related to limiting the use of antibiotics for consumable animal products and processed animal food, and few are related to stringent surveillance mechanisms to regulate the use of antibiotics, monitor antibiotic residue levels in milk and honey, and monitor the presence of antibiotic residues in exportable aquaculture products [21]. The National Animal Diseases Control Programme (NADCP) was implemented in 2019 by the Department of Animal Husbandry and Dairying (DAHD) for controlling foot and mouth disease and brucellosis among farm animals (cattle, buffalo, sheep, goat, and pig) through vaccination [22]. The Indian Network of Fisheries and Animal Antimicrobial Resistance (INFAAR), founded in 2018, was designed for identifying AMR in various animal food production systems [22]. Apart from that, a surveillance mechanism known as the National Animal Disease Reporting System (NADRS) was developed by the DAHD [22].

Table 2.
Rules, regulations, policies, and guidelines to contain AMR in aquaculture and livestock products in India.
Analysis of the Reviewed Policies in the Veterinary Domain (Table S2)
Upon analysis, it is understood that the concerned ministries are aware of the problem and already have started acting on it. However, the lack of provisions for quality checks of domestic products even seeds, the absence of structured capacity-building programmes for farmers, the absence of surveillance mechanisms for regulation of the administration of antibiotics and mechanisms for the regular checking of antibiotic residues in domestic food animals and monitoring of critically important antibiotics for humans which are used in animals as growth promoters are identified to be major weaknesses. Moreover, the lack of coordination among different authorised bodies, such as the Bureau of Indian Standards (BIS) and the Food Standards and Safety Authority of India (FSSAI) regarding the list of antibiotics to be used within a limit, may mislead the stakeholders on the administration of antibiotics on farm animals. One of the key informants (representative of farmers, male) from this sector mentioned, “The farmers don’t get good quality seeds from agents and even farmers are completely unaware about the SPF (Specific Pathogen Free) testing reports of the seeds. Farmers do not have enough knowledge on farm management and treatment of animals, so they use antibiotics as preventive measures and apply those as they want”. However, the presence of strict surveillance on the use of antimicrobials for exportable food animals is the opportunity to introduce similar mechanisms for quality checks in domestic food animals. Presently, the absence of any provision for controlling the high cost of available growth promoters of animals acts as a threat, as it leaves no choice for the farmers but to use antibiotics as growth promoters (Figure 2). Another key informant (representative of farmers, male) from this sector mentioned, “As probiotics and prebiotics are very expensive to our farmers they tend to use antibiotics as a growth promoter of animals”. Hence, despite the presence of so many policies in this field related to the containment of AMR, animal food remains a major source of transmission of AMR bacteria in the environment.
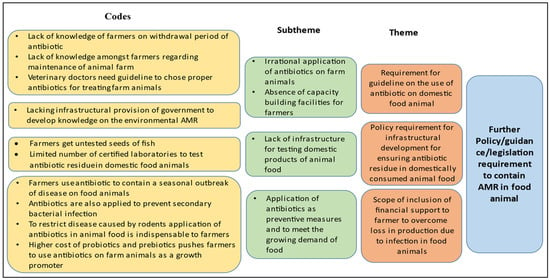
Figure 2.
Thematic areas for intervention identified through KIIs in animal health sector for containment of AMR.
3.3. Policies/Guidelines Reviewed and Analysed in Human Health Sector
The Ministry of Health and Family Welfare, Government of India, along with its line departments such as the Indian Council of Medical Research (ICMR) and the National Centre for Disease Control (NCDC), have adopted some approaches regarding the rational use of antimicrobials in humans to diminish the emergence of multidrug-resistant bacteria and their propensity of transmission in humans (Table 3).

Table 3.
Guidelines on the rational use of antibiotics on human beings.
The presence of a national action plan for AMR (2017–2021), few guidelines for infection control, and judicious antimicrobial use sets the field to develop policies to streamline the problem of AMR in the human health sector which will eventually reflect in other sectors too. However, the lack of policies mandating the rational use of antibiotics, weak regulatory control on the over-the-counter (OTC) sale of antibiotics, and the absence of structured utilisation of available platforms for generating public awareness on the consumption behaviour of antimicrobials are major weaknesses. The pressing demand for using information technology-based monitoring and surveillance on the administration of antibiotics in humans and AMR incidents in hospitals can be used as opportunities to create national-level data on it. Moreover, addressing the determinants of AMR in a national programme mode may create an environment in the country where all the states give due importance to the problem of AMR and bring out meaningful public health action. However, its absence in the country is currently acting as a major threat.
3.4. Policies/Guidelines Reviewed for the Biomedical and Pharmaceutical Sector
We reviewed four policies/guidelines/legislations related to the pharmaceutical and biomedical waste management sector (Table 4). However, the current guidelines/policies/legislations are mainly related to the disposal and management of hazardous substances and other solid and liquid wastes. Only one guideline mentions the permissible level of antibiotic residues [36].

Table 4.
Regulations and guidelines for the management of environmental pollutants to curb spread of AMR in the environment in India.
Analysis of the Reviewed Policies in the Biomedical and Pharmaceutical Sector (Table S2)
Upon analysis, it is evident that newer policies can be introduced in both of these sectors, as few policies already exist, and there is a felt need for more. However, it is observed that the policies are mostly related to the disposal and management of hazardous substances and other solid and liquid wastes. This is a weak area where more clarity is required on the process of the disposal and management of antimicrobial drugs and related waste generated from hospitals, communities, and manufacturing units. In this regard, a hospital management personnel said, “All the discarded drugs are kept in the same place and sent to Central store and then outsourced further by a third party agency”. The Ministry of Environment, Forest, and Climate Change (MoEFCC) has very recently published the permissible level of antibiotic residues in effluent [36], and this may work as an opportunity to control the spread of AMR through the discharge of effluents from pharmaceutical companies. Moreover, some pharmaceutical companies have also targeted zero liquid discharge. A key informant (scientist of a pharma company, male) mentioned, “Presently our company and all other manufacturing units aim at zero liquid discharge to recycle effluents discharged in pharmaceutical manufacturing units”. However, the lack of capacity among professionals across sectors to recognise the containment of AMR as a multisector initiative poses a major threat in the current scenario (Figure 3).
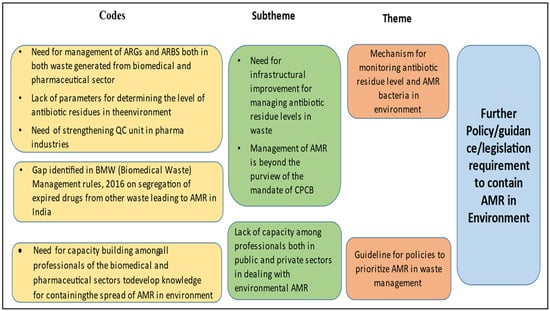
Figure 3.
Thematic areas for intervention in biomedical waste management and pharmaceutical waste management.
4. Discussion
The findings of the present study depicted the current status of AMR-related policies, guidelines, and regulations in India across different sectors and the major strengths, weaknesses, and threats present in the context of the available ones. However, the study also identified domain-specific opportunities for new policy frameworks or refinement of the existing ones.
A mechanism for the continuous capacity building of farmers remains a major requirement in all sectors. For example, it has been revealed by the Centre for Science and Environment (CSE) that the farmers in Delhi and Punjab rampantly use streptocycline as a fungicide for crop production [40]. In a few sectors, not only the lack of knowledge but also the threat of impending economic loss creates an environment for excess antimicrobial use. Despite having guidelines in a few sectors, the overuse of antibiotic formulations cannot be checked due to their easy availability in the market and the prevailing misperception of antibiotics being growth promoters. However, with the worldwide advancement of technologies for disease control in agriculture, a trend has recently been commenced in India to facilitate farmers with disease-resistant cultivators [41], but it needs to be utilised extensively to reduce the use of antibiotics. To maintain the sustainability of agriculture and food quality, the application of Trichoderma sp. as biopesticide and plant growth promoter agents [42] and Pseudomonas fluorescens [43] as biocontrol in place of antibiotic formulations can be accepted as cost-effective methods. Specifically, in the context of plant/agriculture, the adoption of biosecurity approaches such as purchasing plant materials from reputed sources, certified fertilisers, the use of disinfectant equipment, and the regular monitoring of crops [44] may present alternative strategies for better crop production.
Research and development are required in all sectors regarding the social implication of antibiotic use and to design interventions for behavioural change among stakeholders [45]. However, to encourage small-scale and marginalised farmers to comply with rules and guidelines, provisions for subsidies or incentives for them may be created within the scope of the existing policies or new policies for each sector.
Policies regarding investment in the research and development of cost-effective newer methods of cultivation are required to provide some alternatives to the current practice of antimicrobials as therapeutics or growth promoters, and this need has been felt across sectors.
In the veterinary sector, through the reinforcement of the existing policies, domestically consumed products must also be regulated for antibiotic residues, as it is carried out for exportable products, and the FSSAI may be identified as the agency to bring this change. However, regarding biomedical waste management, it is time to mandate the establishment of effluent treatment plants (ETPs) at hospitals and assess them to generate data on antimicrobial residues in effluents. Guidelines on maintaining a database on the usage of antibiotics in hospitals and other healthcare units, along with AMR cases, have become important for promoting the judicious use of antibiotics. The 2016 and 2018 Biomedical Waste Management (BWM) Rules did not incorporate the segregation of antibiotics from other solid waste. Therefore, antibiotic segregation from other solid wastes should also be given priority in the revised guidelines.
In the pharmaceutical industry, policies are required for measuring the magnitude of AMR bacteria before the effluents undergo the recycling process. Continuous monitoring for priority pathogens in environmental samples can guide policymakers regarding policy refinements on antimicrobials.
This study suffers the limitation of evaluating the gamut of policies/guidelines present in the important domains that contribute to environmental AMR in India which may be further investigated in each domain. However, this overall analysis generates evidence on how the existing policies guide the current practices and prepares the field for further discussion on policy requirements for the containment of environmental AMR in India.
We conclude that though public health is the prime development indicator of our country, and the government allocates abundant funds for the accomplishment of national health policies, their implementation witnesses challenges due to the lack of collaborative approaches, the existence of policies disjointed from ground reality, infrastructural issues, and the lack of capacity and resources.
This study highlighted the requirement of effective policies and implementable guidelines as well as coordination between state and central government agencies to address the present knowledge gaps on AMR to boost India’s potential towards leadership for limiting AMR across sectors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/tropicalmed7110336/s1, Table S1: Search strategies of literature; Table S2: SWOT analysis findings from Plant agriculture; Veterinary; Biomedical waste & Pharmaceutical domains.
Author Contributions
F.D., D.C., S.D., A.M. and A.K.D. conceptualised the study. F.D., D.C., A.K.D., S.D., A.M. and S.G. contributed to the study design. F.D., D.C., S.G., S.S., S.P. and R.C. carried out the study. F.D., D.C., S.G., S.S., S.P. and R.C. performed the analysis. F.D. wrote the first draft. S.D., R.B., V.D., A.K.D., A.M. and D.C. critically reviewed the manuscript and provided scientific inputs. All authors have read and agreed to the published version of the manuscript.
Funding
This study was commissioned by the United Nations Environment Programme (No.1/SSFA/3879/Asia and Pacific Office/2021), India Country Office, with funding support from the Government of Norway.
Institutional Review Board Statement
The study was approved by the Institutional Review Board (or Ethics Committee) of ICMR-National Institute of Cholera & Enteric Diseases, Kolkata, India. The approval number is No.A-1/2021-IEC dated 22 February 2021.
Informed Consent Statement
Prior to conducting the key informant interviews, written verbal consent was obtained.
Data Availability Statement
Data specific to this study are archived with the study team. It can be produced if necessary.
Acknowledgments
We are extremely thankful to Divya Datt, Programme Manager, UNEP; Sonia Devi Henam, Programme Officer, UNEP for their effort to facilitate the funding and cooperation throughout the entire project period for its smooth accomplishment. We especially thank the Government of Norway for funding this important report. We also expand our wholehearted thanks to all the anonymous experts from different organisations, medical practitioners, and farmers for their cooperation and suggestions during the key informant interviews.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kirchhelle, C.; Atkinson, P.; Broom, A.; Chuengsatiansup, K.; Ferreira, J.P.; Fortané, N.; Frost, I.; Gradmann, C.; Hinchliffe, S.; Hoffman, S.J.; et al. Setting the standard: Multidisciplinary hallmarks for structural, equitable and tracked antibiotic policy. BMJ Glob. Health 2020, 5, e00309. [Google Scholar] [CrossRef] [PubMed]
- Liguori, K.; Keenum, I.; Davis, B.C.; Calarco, J.; Milligan, E.; Harwood, V.J.; Pruden, A. Antimicrobial Resistance Monitoring of Water Environments: A Framework for Standardized Methods and Quality Control. Environ Sci Technol. Environ. Sci. Technol. 2022, 56, 9149–9160. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- Radhouani, H.; Silva, N.; Poeta, P.; Torres, C.; Correia, S.; Igrejas, G. Potential impact of antimicrobial resistance in wildlife, environment and human health. Front. Microbiol. 2014, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Sudha, S.; Mridula, C.; Silvester, R.; Hatha, A.A. Prevalence and antibiotic resistance of pathogenic Vibrios in shellfishes from Cochin market. PLoS ONE 2014, 9, 1–11. [Google Scholar]
- Tahrani, L.; Soufi, L.; Mehri, I.; Najjari, A.; Hassan, A.; Van Loco, J.; Reyns, T.; Cherif, A.; Mansour, H.B. Isolation and characterization of antibiotic-resistant bacteria from pharmaceutical industrial wastewaters. Microb. Pathog. 2015, 89, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.L.; Asakura, M.; Shiramaru, S.; Pal, A.; Hinenoya, A.; Yamasaki, S. Molecular identification and antimicrobial resistance profiles of Campylobacter strains of poultry origin in India with special emphasis on fluoroquinolone resistance. Asian J. Med. Health Res. 2015, 1, 1–8. [Google Scholar] [CrossRef]
- Bardhan, T.; Chakraborty, M.; Bhattacharjee, B. Prevalence of colistin-resistant, carbapenem-hydrolyzing proteobacteria in hospital water bodies and out-falls of West Bengal, India. Int. J. Environ. Res. Public Health 2020, 17, 1007. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.; Reeder, R. Antibiotic use on crops in low and middle-income countries based on recommendations made by agricultural advisors. CABI Agric. Biosci. 2020, 1, 1. [Google Scholar] [CrossRef]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A review of antibiotic use in food animals: Perspective, policy, and potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Mutua, F.; Sharma, G.; Grace, D.; Bandyopadhyay, S.; Shome, B.; Lindahl, J. A review of animal health and drug use practices in India, and their possible link to antimicrobial resistance. Antimicrob. Resist. Infect. Control 2020, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Patel, I.; Hussain, R.; Khan, A.; Ahmad, A.; Khan, M.U.; Hassalai, M.A. Antimicrobial resistance in India. J. Pharm. Policy Pract. 2017, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Bürgmann, H.; Frigon, D.; Gaze, W.H.; Manaia, C.M.; Pruden, A.; Singer, A.C.; Smets, B.F.; Zhang, T. Water and sanitation: An essential battlefront in the war on antimicrobial resistance. FEMS Microbiol. Ecol. 2018, 94, fiy101. [Google Scholar] [CrossRef] [PubMed]
- Aarti, C.; Khusro, A.; Arasu, M.V.; Agastian, P.; Al-Dhabi, N.A. Biological potency and characterization of antibacterial substances produced by Lactobacillus pentosus isolated from Hentak, a fermented fish product of North-East India. Springerplus 2016, 5, 1743. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.; Purohit, M.; Diwan, V.; Chandran, S.P.; Riggi, E.; Parashar, V.; Tamhankar, A.J.; Lundborg, C.S. Monitoring of water quality, antibiotic residues, and antibiotic-resistant escherichia coli in the kshipra river in india over a 3-year period. Int. J. Environ. Res. Public Health. 2020, 17, 7706. [Google Scholar] [CrossRef] [PubMed]
- Bishwajit, G.; Ide, S.; Ghosh, S. Social determinants of infectious diseases in South Asia. Int. Sch. Res. Notices 2014, 2014, 135243. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Monitoring and Evaluation of the Global Action Plan on Antimicrobial Resistance: Framework and Recommended Indicators. 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/325006/9789241515665eng.pdf?sequence=1&isAllowed=y (accessed on 10 March 2022).
- 2019 WHO AWaRe Classifcation Database of Antibiotics for Evaluation and Monitoring of Use. Available online: https://www.who.int/publications/i/item/WHOEMPIAU2019.11 (accessed on 20 March 2022).
- The Insecticide Act 1968. 46 (Ind.), Adopted on 2 September 1968. Available online: https://legislative.gov.in/sites/default/files/A1968-46.pdf (accessed on 18 January 2022).
- Central Insecticide Board & Registration Committee. Major Uses of Pesticides. Ministry of Agriculture & Farmers Welfare: Government of India. 2021. Available online: http://ppqs.gov.in/sites/default/files/2._mup_fungicide_upto_30.06.2021.pdf (accessed on 2 February 2022).
- Center for Disease Dynamics, Economics & Policy. Antibiotic Use and Resistance in Food Animals Current Policy and Recommendations. Available online: https://cddep.org/wp-content/uploads/2017/06/india_abx_report-2.pdf (accessed on 12 September 2021).
- Kumar, H.C.; Hiremath, J.; Yogisharadhya, R.; Balamurugan, V.; Jacob, S.S.; Reddy, G.M.; Suresh, K.P.; Shome, R.; Nagalingam, M.; Sridevi, R.; et al. Animal disease surveillance: Its importance & present status in India. Indian J. Med. Res. 2021, 153, 299–310. [Google Scholar] [CrossRef]
- Coastal Aquaculture Authority. Guidelines for regulating Coastal Aquaculture (Annexure-I), CAA Rules. 2005. Available online: http://caa.gov.in/uploaded/doc/Guidelines-Englishnew.pdf (accessed on 25 October 2021).
- Export Inspection Council. Milk Product: Residue Monitoring Plan (RMP) for Export to EU, Year 2011-12; Export Inspection Council, Ministry of Commerce & Industry, Government of India: New Delhi, India, 2011.
- Export Inspection Council. Honey: Residue Monitoring Plan (RMP), Year 2019-20; Export Inspection Council, Ministry of Commerce & Industry, Government of India: New Delhi, India, 2019.
- Marine Products Export Development Authority (MPEDA). National Residue Control Plan (NRCP). Available online: https://mpeda.gov.in/?page_id=1088 (accessed on 17 September 2021).
- Srivastava, R.K. National Policy for Containment of Antimicrobial Resistance India; Ministry of Health & Family Welfare: New Delhi, India, 2011. Available online: https://main.mohfw.gov.in/sites/default/files/3203490350abpolicy%20%281%29.pdf (accessed on 23 September 2021).
- Central Pollution Control Board. Guidelines for Environmental Management of Dairy Farms and Gaushalas; Parivesh Bhawan: New Delhi, India, 2021; Available online: http://www.uppcb.com/pdf/Reviesd-Guidelines_290721.pdf (accessed on 4 October 2021).
- Central Pollution Control Board. Environmental Guidelines for Poultry Farms; Parivesh Bhawan: New Delhi, India, 2021; Available online: https://cpcb.nic.in/openpdffile.php?id=TGF0ZXN0RmlsZS8zMzFfMTYyOTA5OTQwMF9tZWRpYXBob3RvMjg2MjcucGRm (accessed on 17 September 2021).
- Vijayan, K.K. CIBA’S Initiatives in promoting the responsible use of drugs and chemicals in Indian aquaculture. In Responsible Use of Antimicrobials in Indian Aquaculture: Opportunities and Challenges; ICAR—Central Institute of Brackishwater Aquaculture: Chennai, India, 2016; pp. 10–12. Available online: http://www.ciba.res.in/Books/cibaspl83.pdf (accessed on 20 September 2021).
- National Programme on AMR Containment (2012-17). Available online: https://ncdc.gov.in/index1.php?lang=1&level=2&sublinkid=384&lid=344 (accessed on 17 September 2021).
- Walia, K.; Madhumathi, J.; Veeraraghavan, B.; Chakrabarti, A.; Kapil, A.; Ray, P.; Singh, H.; Sistla, S.; Ohri, V.C. Establishing antimicrobial resistance surveillance & research network in India: Journey so far. Indian J. Med. Res. 2019, 149, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Stewardship Program (AMSP) Guideline. Available online: https://main.icmr.nic.in/sites/default/files/guidelines/AMSP_0.pdf (accessed on 9 January 2022).
- Indian Council of Medical Research (ICMR). Treatment Guidelines for Antimicrobial Use in Common Syndromes; Indian Council of Medical Research: New Delhi, India, 2019. Available online: https://main.icmr.nic.in/sites/default/files/guidelines/Treatment_Guidelines_2019_Final.pdf (accessed on 20 March 2022).
- National Centre for Disease Control, Directorate General of Health Services. National Guidelines for Infection Prevention and Control in Healthcare Facilities; Ministry of Health and Family Welfare, Government of India: New Delhi, India, 2020. Available online: https://www.mohfw.gov.in/pdf/National%20Guidelines%20for%20IPC%20in%20HCF%20-%20final%281%29.pdf (accessed on 20 March 2022).
- Gazette of India. Gazette Notification G.S.R. 44(E); Gazette of India: New Delhi, India, 2020. Available online: http://moef.gov.in/wp-content/uploads/2020/01/finalization.pdf (accessed on 15 March 2022).
- The Environmental (Protection) Act, 1986. 29 (Ind.). Available online: https://www.indiacode.nic.in/bitstream/123456789/4316/1/ep_act_1986.pdf (accessed on 23 November 2021).
- Ministry of Drinking Water and Sanitation. Guidelines for Swachh Bharat Mission (Gramin). 2017. Available online: https://swachhbharatmission.gov.in/sbmcms/writereaddata/images/pdf/Guidelines/Complete-set-guidelines.pdf (accessed on 20 December 2021).
- Gazette of India. Gazette Notification G.S.R. 343(E). Gazette of India: New Delhi. 2016. Available online: https://dhr.gov.in/sites/default/files/Bio-medical_Waste_Management_Rules_2016.pdf (accessed on 20 March 2022).
- Centre for Science and Environment. Antibiotic Misuse in Crops. Available online: https://www.cseindia.org/antibiotic-misuse-in-crops-9797 (accessed on 8 February 2022).
- Department of Agriculture, Cooperation & Farmers’ Welfare. Annual Report 2020–2021. Ministry of Agriculture & Farmers’ Welfare, Government of India, Krishi Bhawan: New Delhi, India. Available online: https://agricoop.nic.in/sites/default/files/Web%20copy%20of%20AR%20%28Eng%29_7.pdf (accessed on 17 February 2022).
- Rai, N.; Limbu, A.K.; Joshi, A. Impact of Trichoderma sp. in agriculture: A mini-review. J. Biol. Today’s World 2020, 9, 7. [Google Scholar]
- Lally, R.D.; Galbally, P.; Moreira, A.S.; Spink, J.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Application of endophytic Pseudomonas fluorescens and a bacterial consortium to Brassica napus can increase plant height and biomass under greenhouse and field conditions. Front. Plant Sci. 2017, 8, 2193. [Google Scholar] [CrossRef] [PubMed]
- Biosecurity Manual for Sugarcane Producers—A Guide to Farm Biosecurity Measures to Reduce the Risks of Weeds, Pests and Diseases Impacting Production (Version 1.0); Sugar Research Australia Limited: Canberra, Australia, 2017; Available online: https://www.farmbiosecurity.com.au/wp-content/uploads/2019/03/Biosecurity-Manual-for-Sugarcane-Producers.pdf (accessed on 10 January 2022).
- Gandra, S.; Joshi, J.; Trett, A.; Lamkang, A.S.; Laxminarayan, R. Scoping Report on Antimicrobial Resistance in India; Center for Disease Dynamics, Economics & Policy: Washington, DC, USA, 2017; pp. 1–30. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).