Pathogenesis and Genomic Analysis of a Virulent Leptospira Interrogans Serovar Copenhageni Isolated from a Dog with Lethal Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Case
2.2. Isolation of Leptospires
2.3. Specific Antibodies Detection
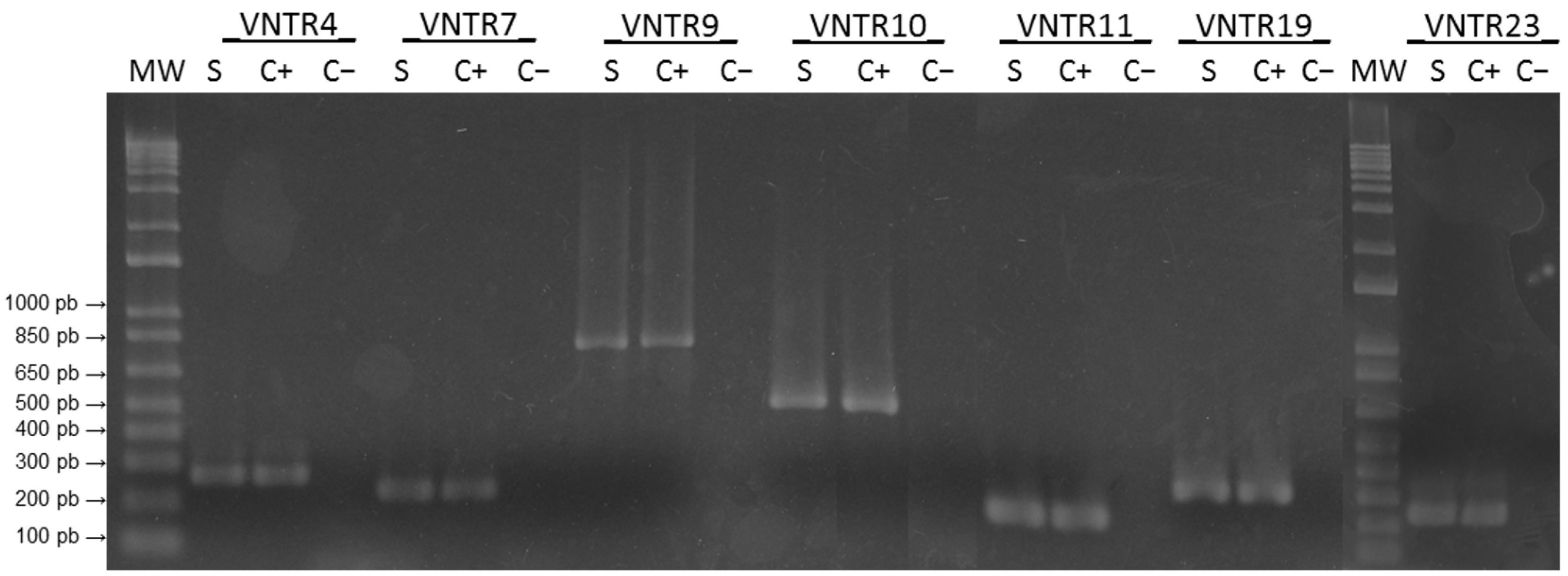
2.4. Molecular Analysis
2.5. Whole-Genome Sequencing
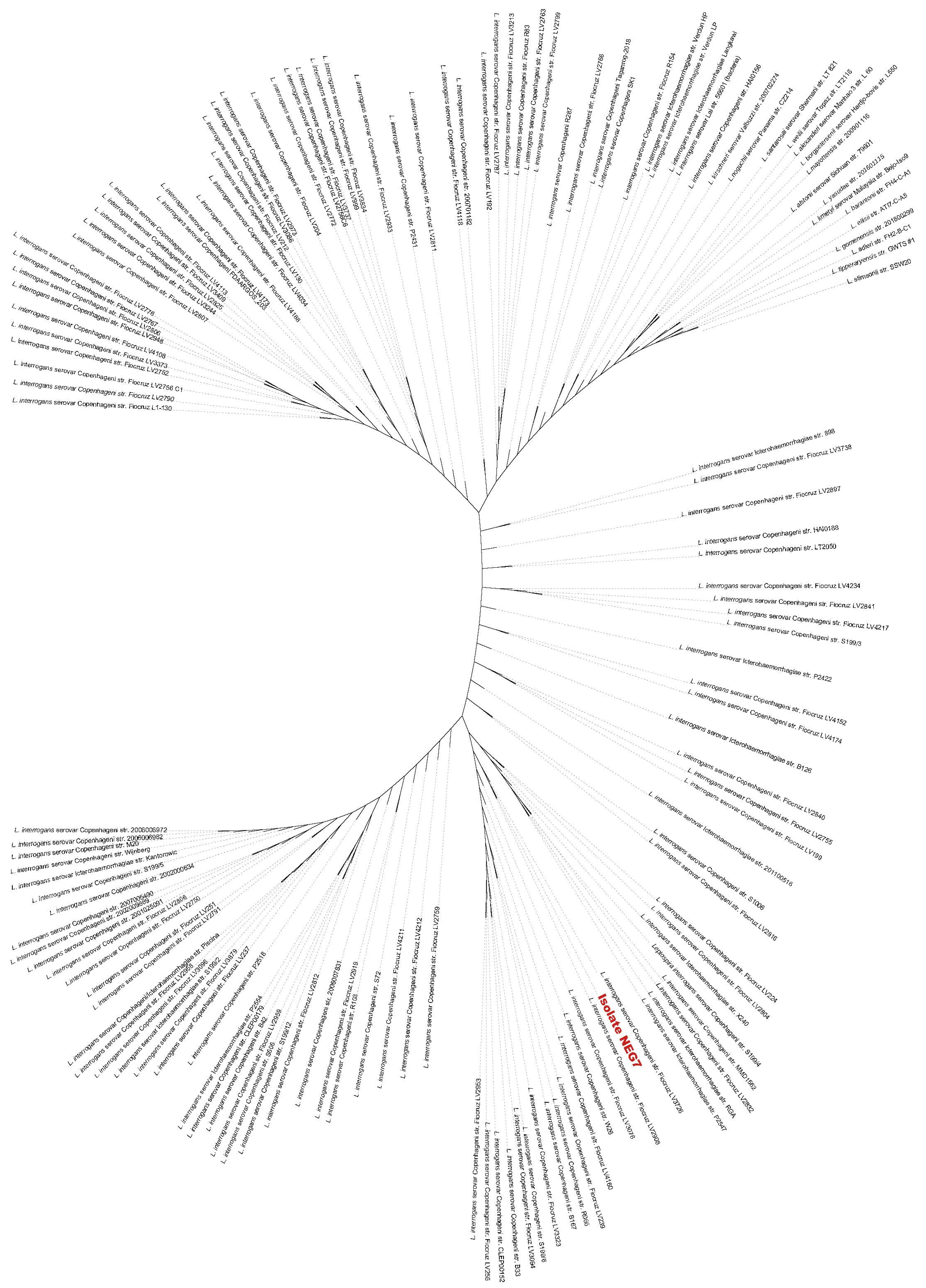
2.6. Comparative and Phylogenetic Analysis
3. Results
3.1. Hematological and Serodiagnosis
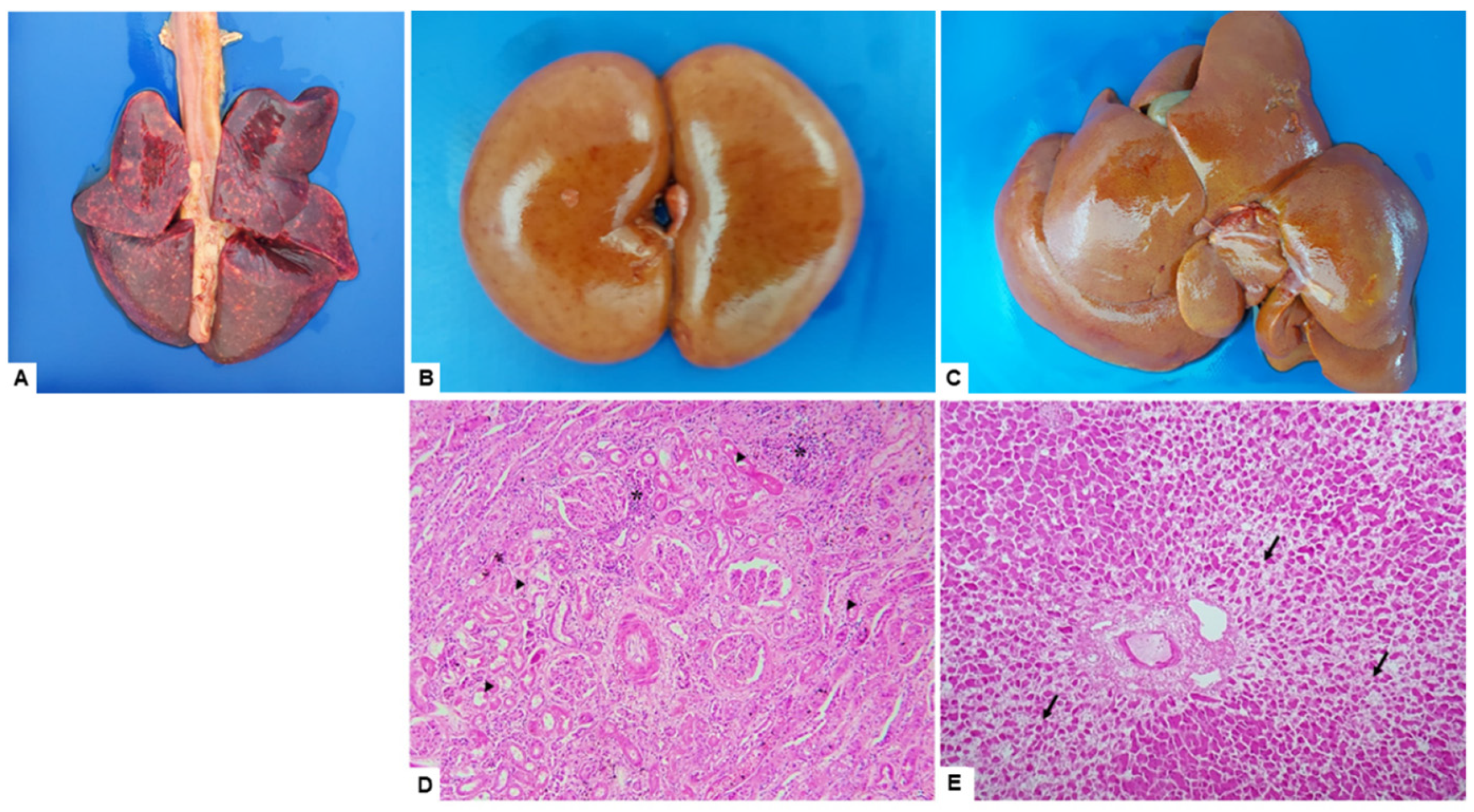
3.2. Macroscopic and Microscopic Findings
3.3. Isolation and Genomic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Validi, M.; Karkhah, A.; Prajapati, V.K.; Nouri, H.R. Immuno-Informatics Based Approaches to Design a Novel Multi Epitope-Based Vaccine for Immune Response Reinforcement against Leptospirosis. Mol. Immunol. 2018, 104, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, P.R.; Hagan, J.E.; Costa, F.; Calcagno, J.; Kane, M.; Martinez-Silveira, M.S.; Goris, M.G.A.; Stein, C.; Ko, A.I.; Abela-Ridder, B. Global Burden of Leptospirosis: Estimated in Terms of Disability Adjusted Life Years. PLoS Negl. Trop. Dis. 2015, 9, e0004122. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.M.; Schneider, M.C.; Munoz-Zanzi, C.; Costa, F.; Benschop, J.; Hartskeerl, R.; Julio Martinez, J.; Jancloes, M.; Bertherat, E. A Road Map for Leptospirosis Research and Health Policies Based on Country Needs in Latin America. Rev. Panam. Salud Pública 2017, 41, e131. [Google Scholar] [CrossRef]
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl. Trop. Dis. 2015, 9, e0003898. [Google Scholar] [CrossRef] [PubMed]
- Wunder, E.A.; Adhikarla, H.; Hamond, C.; Owers, K.A.; Liang, L.; Rodrigues, C.B.; Bisht, V.; Nally, J.E.; Alt, D.P.; Reis, M.G.; et al. A Live Attenuated Vaccine Model Confers Cross-Protective Immunity Against 2 Different Species of Leptospira Spp. bioRxiv 2020, 10, e64166. [Google Scholar] [CrossRef]
- Boey, K.; Shiokawa, K.; Rajeev, S. Leptospira Infection in Rats: A Literature Review of Global Prevalence and Distribution. PLoS Negl. Trop. Dis. 2019, 13, e0007499. [Google Scholar] [CrossRef] [PubMed]
- Minter, A.; Costa, F.; Khalil, H.; Childs, J.; Diggle, P.; Ko, A.I.; Begon, M. Optimal Control of Rat-Borne Leptospirosis in an Urban Environment. Front. Ecol. Evol. 2019, 7, 209. [Google Scholar] [CrossRef]
- Martins, G.; Lilenbaum, W. Control of Bovine Leptospirosis: Aspects for Consideration in a Tropical Environment. Res. Vet. Sci. 2017, 112, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.L.T.O.; Verjovski-Almeida, S.; Van Sluys, M.A.; Monteiro-Vitorello, C.B.; Digiampietri, L.A.; Harstkeerl, R.A.; Marques, M.V.; Oliveira, M.; Setubal, J.C.; Haake, D.A.; et al. Genome Features of Leptospira Interrogans Serovar Copenhageni. Braz. J. Med. Biol. Res. 2004, 37, 459–478. [Google Scholar] [CrossRef] [PubMed]
- Guglielmini, J.; Bourhy, P.; Schiettekatte, O.; Zinini, F.; Brisse, S.; Picardeau, M. Genus-Wide Leptospira Core Genome Multilocus Sequence Typing for Strain Taxonomy and Global Surveillance. PLoS Negl. Trop. Dis. 2019, 13, e0007374. [Google Scholar] [CrossRef]
- Polo, N.; Machado, G.; Rodrigues, R.; Hamrick, P.N.; Munoz-Zanzi, C.; Pereira, M.; Bercini, M.; Timm, L.; Schneider, M.; Polo, N.; et al. A One Health Approach to Investigating Leptospira Serogroups and Their Spatial Distributions among Humans and Animals in Rio Grande Do Sul, Brazil, 2013–2015. Trop. Med. Infect. Dis. 2019, 4, 42. [Google Scholar] [CrossRef]
- Schneider, M.C.; Najera, P.; Pereira, M.M.; Machado, G.; dos Anjos, C.B.; Rodrigues, R.O.; Cavagni, G.M.; Muñoz-Zanzi, C.; Corbellini, L.G.; Leone, M.; et al. Leptospirosis in Rio Grande Do Sul, Brazil: An Ecosystem Approach in the Animal-Human Interface. PLoS Negl. Trop. Dis. 2015, 9, e0004095. [Google Scholar] [CrossRef] [PubMed]
- Narkkul, U.; Thaipadungpanit, J.; Srisawat, N.; Rudge, J.W.; Thongdee, M.; Pawarana, R.; Pan-Ngum, W. Human, Animal, Water Source Interactions and Leptospirosis in Thailand. Sci. Rep. 2021, 11, 3215. [Google Scholar] [CrossRef]
- Bertasio, C.; Boniotti, M.B.; Lucchese, L.; Ceglie, L.; Bellinati, L.; Mazzucato, M.; Furlanello, T.; D’incau, M.; Natale, A. Detection of New Leptospira Genotypes Infecting Symptomatic Dogs: Is a New Vaccine Formulation Needed? Pathogens 2020, 9, 484. [Google Scholar] [CrossRef]
- Jorge, S.; Miotto, B.A.; Kremer, F.S.; Cagliari, R.; de Oliveira, N.R.; Heinemann, M.B.; da Silva Pinto, L.; Hagiwara, M.K.; Campos, V.F.; Dellagostin, O.A. Complete Genome Sequence and in Silico Analysis of L. Interrogans Canicola Strain DU114: A Virulent Brazilian Isolate Phylogenetically Related to Serovar Linhai. Genomics 2019, 111, 1651–1656. [Google Scholar] [CrossRef]
- Zakeri, S.; Khorami, N.; Ganji, Z.F.; Sepahian, N.; Malmasi, A.-A.; Mehdi Gouya, M.; Djadid, N.D. Leptospira Wolffii, a Potential New Pathogenic Leptospira Species Detected in Human, Sheep and Dog. Infect. Genet. Evol. 2010, 10, 273–277. [Google Scholar] [CrossRef]
- Goarant, C. Leptospirosis: Risk Factors and Management Challenges in Developing Countries. Res. Rep. Trop. Med. 2016, 7, 49–62. [Google Scholar] [CrossRef]
- Gay, N.; Soupé-Gilbert, M.-E.; Goarant, C. Though Not Reservoirs, Dogs Might Transmit Leptospira in New Caledonia. Int. J. Environ. Res. Public Health 2014, 11, 4316–4325. [Google Scholar] [CrossRef]
- Faine, S.; Adler, B.; Bolin, C.; Perolat, P. Leptospira and Leptospirosis, 2nd ed.; Medisci Press: Melbourne, Australia, 1999; ISBN 095863260X. [Google Scholar]
- Majed, Z.; Bellenger, E.; Postic, D.; Pourcel, C.; Baranton, G.; Picardeau, M. Identification of Variable-Number Tandem-Repeat Loci in Leptospira Interrogans Sensu Stricto Identification of Variable-Number Tandem-Repeat Loci in Leptospira Interrogans Sensu Stricto. J. Clin. Microbiol. 2005, 43, 539–545. [Google Scholar] [CrossRef]
- Simpson, J.T.; Wong, K.; Jackman, S.D.; Schein, J.E.; Jones, S.J.M.; Birol, İ. ABySS: A Parallel Assembler for Short Read Sequence Data. Genome Res. 2009, 19, 1117. [Google Scholar] [CrossRef]
- Boisvert, S.; Laviolette, F.; Corbeil, J. Ray: Simultaneous Assembly of Reads from a Mix of High-Throughput Sequencing Technologies. J. Comput. Biol. 2010, 17, 1519. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Zerbino, D.R.; Birney, E. Velvet: Algorithms for de Novo Short Read Assembly Using de Bruijn Graphs. Genome Res. 2008, 18, 821. [Google Scholar] [CrossRef]
- Lin, S.-H.; Liao, Y.-C. CISA: Contig Integrator for Sequence Assembly of Bacterial Genomes. PLoS ONE 2013, 8, e60843. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality Assessment Tool for Genome Assemblies. Bioinformatics 2013, 29, 1072. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Tatusova, T.; Dicuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI Prokaryotic Genome Annotation Pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef] [PubMed]
- Boonsilp, S.; Thaipadungpanit, J.; Amornchai, P.; Wuthiekanun, V.; Bailey, M.S.; Holden, M.T.G.; Zhang, C.; Jiang, X.; Koizumi, N.; Taylor, K.; et al. A Single Multilocus Sequence Typing (MLST) Scheme for Seven Pathogenic Leptospira Species. PLoS Negl. Trop. Dis. 2013, 7, e1954. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and Applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Rutherford, K.; Parkhill, J.; Crook, J.; Horsnell, T.; Rice, P.; Rajandream, M.A.; Barrell, B. Artemis: Sequence Visualization and Annotation. Bioinformatics 2000, 16, 944–945. [Google Scholar] [CrossRef]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High Throughput ANI Analysis of 90K Prokaryotic Genomes Reveals Clear Species Boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef] [PubMed]
- Minkin, I.; Medvedev, P. Scalable Multiple Whole-Genome Alignment and Locally Collinear Block Construction with SibeliaZ. Nat. Commun. 2020, 11, 6327. [Google Scholar] [CrossRef] [PubMed]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of Acquired Antimicrobial Resistance Genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.L.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic Resistome Surveillance with the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2020, 48, D517. [Google Scholar] [CrossRef] [PubMed]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A Better, Faster Version of the PHAST Phage Search Tool. Nucleic Acids Res. 2016, 44, 16–21. [Google Scholar] [CrossRef]
- Vincent, A.T.; Schiettekatte, O.; Goarant, C.; Neela, V.K.; Bernet, E.; Thibeaux, R.; Ismail, N.; Khalid, M.K.N.M.; Amran, F.; Masuzawa, T.; et al. Revisiting the Taxonomy and Evolution of Pathogenicity of the Genus Leptospira through the Prism of Genomics. PLoS Negl. Trop. Dis. 2019, 13, e0007270. [Google Scholar] [CrossRef]
- Ellis, W.A. Animal Leptospirosis. In Leptospira and Leptospirosis; Adler, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 99–138. [Google Scholar]
- Knöpfler, S.; Mayer-Scholl, A.; Luge, E.; Klopfleisch, R.; Gruber, A.D.; Nöckler, K.; Kohn, B. Evaluation of Clinical, Laboratory, Imaging Findings and Outcome in 99 Dogs with Leptospirosis. J. Small Anim. Pract. 2017, 58, 582–588. [Google Scholar] [CrossRef]
- Kohn, B.; Steinicke, K.; Arndt, G.; Gruber, A.D.; Guerra, B.; Jansen, A.; Kaser-Hotz, B.; Klopfleisch, R.; Lotz, F.; Luge, E.; et al. Pulmonary Abnormalities in Dogs with Leptospirosis. J. Vet. Intern. Med. 2010, 24, 1277–1282. [Google Scholar] [CrossRef]
- Athapattu, T.; Fernando, R.; Abayawansha, R.; Fernando, P.; Fuward, M.; Samarakoon, N.; Koizumi, N.; Gamage, C. Carrier Status of Leptospira Spp. in Healthy Companion Dogs in Sri Lanka. Vector-Borne Zoonotic Dis. 2022, 22, 93–100. [Google Scholar] [CrossRef]
- Putz, E.J.; Nally, J.E. Investigating the Immunological and Biological Equilibrium of Reservoir Hosts and Pathogenic Leptospira: Balancing the Solution to an Acute Problem? Front. Microbiol. 2020, 11, 2005. [Google Scholar] [CrossRef]
- Jaeger, L.H.; Pestana, C.P.; Carvalho-Costa, F.A.; Medeiros, M.A.; Lilenbaum, W. Characterization of the Clonal Subpopulation Fiocruz L1-130 of Leptospira Interrogans in Rats and Dogs from Brazil. J. Med. Microbiol. 2018, 67, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- da Costa, R.S.; Di Azevedo, M.I.N.; Borges, A.L.D.S.B.; Carvalho-Costa, F.A.; Martins, G.; Lilenbaum, W. Persistent High Leptospiral Shedding by Asymptomatic Dogs in Endemic Areas Triggers a Serious Public Health Concern. Animals 2021, 11, 937. [Google Scholar] [CrossRef] [PubMed]
- Larson, C.R.; Dennis, M.; Nair, R.V.; Llanes, A.; Peda, A.; Welcome, S.; Rajeev, S. Isolation and Characterization of Leptospira Interrogans Serovar Copenhageni from a Dog from Saint Kitts. JMM Case Rep. 2017, 4, e005120. [Google Scholar] [CrossRef]
- Paz, L.N.; Dias, C.S.; Almeida, D.S.; Balassiano, I.T.; Medeiros, M.A.; Costa, F.; Silva, D.N.; Reis, J.N.; Estrela-Lima, A.; Hamond, C.; et al. Multidisciplinary Approach in the Diagnosis of Acute Leptospirosis in Dogs Naturally Infected by Leptospira Interrogans Serogroup Icterohaemorrhagiae: A Prospective Study. Comp. Immunol. Microbiol. Infect. Dis. 2021, 77, 101664. [Google Scholar] [CrossRef]
- Miotto, B.A.; Tozzi, B.F.; de Penteado, M.S.; Guilloux, A.G.A.; Moreno, L.Z.; Heinemann, M.B.; Moreno, A.M.; Lilenbaum, W.; Hagiwara, M.K. Diagnosis of Acute Canine Leptospirosis Using Multiple Laboratory Tests and Characterization of the Isolated Strains. BMC Vet. Res. 2018, 14, 222. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.M.; Dias, G.C.D.R.S.; Saldanha, A.V.P.; Esteves, S.B.; Cortez, A.; Guedes, I.B.; Heinemann, M.B.; Gonçales, A.P.; Miotto, B.A. Molecular and Serological Characterization of Pathogenic Leptospira Spp. Isolated from Symptomatic Dogs in a Highly Endemic Area, Brazil. BMC Vet. Res. 2021, 17, 221. [Google Scholar] [CrossRef]
- Lavinsky, M.O.; Said, R.A.; Marcelo, G.; Strenzel, R.; Langoni, H. Seroprevalence of Anti-Leptospira Spp. Antibodies in Dogs in Bahia, Brazil. Prev. Vet. Med. 2012, 106, 79–84. [Google Scholar] [CrossRef]
- da Cunha, G.R.; Pellizzaro, M.; Martins, C.M.; Rocha, S.M.; Yamakawa, A.C.; da Silva, E.C.; dos Santos, A.P.; Morikawa, V.M.; Langoni, H.; Biondo, A.W. Serological Survey of Anti-Leptospira Spp. Antibodies in Individuals with Animal Hoarding Disorder and Their Dogs in a Major City of Southern Brazil. Vet. Med. Sci. 2022, 8, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, F.; De Morais, Z.M.; Dellagostina, O.A.; Seixas, F.K.; Freitas, J.C.; Zacarias, F.G.S.; Delbem, A.C.; Ferreira, T.S.; Souza, G.O.; Hartskeerl, R.A.; et al. Molecular and Serological Characterization of Leptospira Interrogans Serovar Canicola Isolated from Dogs, Swine, and Bovine in Brazil. Trop. Anim. Health Prod. 2013, 45, 117–121. [Google Scholar] [CrossRef]
- Hartskeerl, R.A.; Smythe, L.D. The Role of Leptospirosis Reference Laboratories. In Leptospira and Leptospirosis; Adler, B., Ed.; Springer: New York, NY, USA, 2014; pp. 273–288. ISBN 978-3-662-45059-8. [Google Scholar]
- Santos, L.A.; Adhikarla, H.; Yan, X.; Wang, Z.; Fouts, D.E. Genomic Comparison Among Global Isolates of L. Interrogans Serovars Copenhageni and Icterohaemorrhagiae Identified Natural Genetic Variation Caused by an Indel. Front. Cell. Infect. Microbiol. 2018, 8, 193. [Google Scholar] [CrossRef]

| Assembly | |||||||
|---|---|---|---|---|---|---|---|
| Size (Mb) | CG% | #Contigs | N50 (pb) | CDSs a | tRNAs | rRNAs | tmRNA |
| 4.64 | 35.13 | 60 | 134,612 | 3756 | 39 | 6 | 1 |
| Gene | Function | NEG7 Locus Tag | L1-130 Locus Tag | Identity (%) |
|---|---|---|---|---|
| ClpB | Molecular chaperone-Resist heat and oxidative stress | MPF80_10065 | LIC_12017 | 100.00 |
| ColA | Collagenase A-Dissemination | MPF80_13785 | LIC_12760 | 100.00 |
| FcpA | Flagella coling protein-Dissemination | MPF80_15850 | LIC_13166 | 100.00 |
| FlaA2 | Flagella assembly-Dissemination | MPF80_04025 | LIC_10787 | 100.00 |
| FliM | Flagella motor switch protein-Dissemination | MPF80_09170 | LIC_11836 | 100.00 |
| FliN | Flagella motor switch protein-Dissemination | MPF80_06905 | LIC_11370 | 100.00 |
| HemO | Heme oxygenase-Iron acquisition | MPF80_18235 | LIC_20148 | 100.00 |
| HtpG | High-temperature protein G-associated with acute disease | MPF80_17695 | LIC_20044 | 100.00 |
| KatE | Catalase-Resist oxidative stress | MPF80_10140 | LIC_12032 | 100.00 |
| Unknown | Associated with host colonization | MPF80_14995 | LIC_12791 | 99.74 |
| Unknown | LPS biosynthesis associated with acute disease | MPF80_10675 | LIC_12142 | 100.00 |
| Unknown | Associated with host colonization | MPF80_06245 | LIC_11235 | 98.15 |
| Unknown | Associated with host colonization | MPF80_14995 | LIC_20153 | 100.00 |
| LenA | Endostatin-like protein-Adhesion to ECM | MPF80_10440 | LIC_12096 | 100.00 |
| LenB | Endostatin-like protein-Adhesion to ECM | MPF80_05070 | LIC_10997 | 100.00 |
| LenC | Endostatin-like protein-Adhesion to ECM | MPF80_15100 | LIC_13006 | 100.00 |
| LenD | Endostatin-like protein-Adhesion to ECM | MPF80_11560 | LIC_12315 | 100.00 |
| LenE | Endostatin-like protein-Adhesion to ECM | MPF80_17370 | LIC_13467 | 100.00 |
| Unknown | Gene regulation | MPF80_18045 | LIC_20111 | 100.00 |
| LigA | Immonoglobulin-like repeat protein Adhesion to ECM | MPF80_02420 | LIC_10465 | 100.00 |
| LigB | Immonoglobulin-like repeat protein Adhesion to ECM | MPF80_02410 | LIC_10464 | 100.00 |
| LipL21 | Neutrophil myeloperoxidase inhibitor | MPF80_00115 | LIC_10011 | 100.00 |
| LipL32 | Most abundant outer-membrane lipoprotein in pathogenic leptospires | MPF80_06820 | LIC_11352 | 100.00 |
| LipL36 | Abundant outer-membrane lipoprotein | MPF80_15375 | LIC_13060 | 100.00 |
| LipL41 | TonB-dependent receptor-Iron acquisition | MPF80_14890 | LIC_12966 | 99.72 |
| LipL45 | Adhesion to ECM | MPF80_08230 | LIC_11643 | 99.74 |
| Loa22 | Associated with acute disease | MPF80_01045 | LIC_10191 | 100.00 |
| LpxD1 | Lipid A biosynthesis protein-Temperature adaptation | MPF80_15300 | LIC_13046 | 100.00 |
| LruA | Associated with acute disease | MPF80_05095 | LIC_11003 | 100.00 |
| Lsa21 | Adhesion to ECM; potent TLR2 and TLR4 agonist | MPF80_01940 | LIC_10368 | 100.00 |
| LvrA | hybrid histidine kinase-Leptospira virulence regulator system | MPF80_08530 | LIC_11709 | 100.00 |
| LvrB | hybrid response regulator-Leptospira virulence regulator system | MPF80_08525 | LIC_11708 | 100.00 |
| Mce | Mammary cell entry protein-Cell entry | MPF80_09280 | LIC_11859 | 100.00 |
| OmpL1 | OmpA family protein-Adhesion to ECM | MPF80_04940 | LIC_10973 | 100.00 |
| OmpL37 | Adhesion to ECM | MPF80_11285 | LIC_12263 | 100.00 |
| OmpL47 | Adhesion to ECM | MPF80_15320 | LIC_13050 | 100.00 |
| Sph2 | Sphingomyelase 2-Hemolytic activity | MPF80_13140 | LIC_12631 | 100.00 |
| TlyA | Hemolysin A-Hemolytic activity | MPF80_01505 | LIC_10284 | 100.00 |
| TlyC | Hemolysin-Hemolytic activity | MPF80_15755 | LIC_13143 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, N.R.; Kremer, F.S.; de Brito, R.S.A.; Zamboni, R.; Dellagostin, O.A.; Jorge, S. Pathogenesis and Genomic Analysis of a Virulent Leptospira Interrogans Serovar Copenhageni Isolated from a Dog with Lethal Infection. Trop. Med. Infect. Dis. 2022, 7, 333. https://doi.org/10.3390/tropicalmed7110333
de Oliveira NR, Kremer FS, de Brito RSA, Zamboni R, Dellagostin OA, Jorge S. Pathogenesis and Genomic Analysis of a Virulent Leptospira Interrogans Serovar Copenhageni Isolated from a Dog with Lethal Infection. Tropical Medicine and Infectious Disease. 2022; 7(11):333. https://doi.org/10.3390/tropicalmed7110333
Chicago/Turabian Stylede Oliveira, Natasha Rodrigues, Frederico Schmitt Kremer, Risciela Salardi Alves de Brito, Rosimeri Zamboni, Odir Antônio Dellagostin, and Sérgio Jorge. 2022. "Pathogenesis and Genomic Analysis of a Virulent Leptospira Interrogans Serovar Copenhageni Isolated from a Dog with Lethal Infection" Tropical Medicine and Infectious Disease 7, no. 11: 333. https://doi.org/10.3390/tropicalmed7110333
APA Stylede Oliveira, N. R., Kremer, F. S., de Brito, R. S. A., Zamboni, R., Dellagostin, O. A., & Jorge, S. (2022). Pathogenesis and Genomic Analysis of a Virulent Leptospira Interrogans Serovar Copenhageni Isolated from a Dog with Lethal Infection. Tropical Medicine and Infectious Disease, 7(11), 333. https://doi.org/10.3390/tropicalmed7110333








