Mycobacterium tuberculosis Drug Resistance in Ethiopia: An Updated Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Databases and Search Strategy
2.3. Selection Criteria and Data Extraction
2.4. Quality Assessment
2.5. Definitions
| Author/s | Year of Publication | Study Period | Study Region | Type of Patients | Study Design | Molecular Diagnostic Methods | Type of TB Cases | Total Number of Patients (n) | Total Positive Cases (n) | Total Isolates with Available DST Results (n) | Any Drug Resistance (n) | Any Anti-TB Drug Resistance, (n) | Mono-Resistance, (n) | MDR, (n) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retreated Cases | New Cases | Overall | ||||||||||||||||||||
| New (n) | Retreated (n) | INH, (n) | RIF, (n) | EMB, (n) | FLQ, (n) | INH, (n) | RIF, (n) | EMB, (n) | ||||||||||||||
| Zewdie et al. [31] | 2018 | 2014 | AA | EPTB | Cross-sectional | GenoType®MTBDRplus | 52 | 8 | 65 | 60 | 60 | 6 | 6 | 5 | NS | NS | 1 | 0 | NS | 3 | 2 | 5 |
| Workalemahu et al. [32] | 2013 | 2011 | OR | PTB | NR | GenoType®MTBRplus | NR | NR | 121 | 15 | 15 | 1 | 1 | NR | NS | NS | 1 | 0 | NS | 0 | 0 | 0 |
| Wondale et al. [33] | 2018 | 2014–2016 | SNNP | PTB and EPTB | NR | GenoType®MTBDRplus V.2. | 153 | 8 | 161 | 126 | 126 | 4 | 1 | 3 | NS | NS | NR | 2 | NS | 1 | 0 | 1 |
| Tessema et al. [34] | 2012 | 2009 | AM | PTB | Cross-sectional | GenoType®MTBDRplus and GenoType®MTBDRsl | 214 | 46 | 260 | 260 | 260 | 45 | 35 | 15 | 8 | NR | 22 | 2 | NR | 5 | 8 | 13 |
| Tadesse et al. [35] | 2016 | 2013–2014 | OR | PTB | Cross-sectional | GenoType®MTBDRplus V.2 | 41 | 71 | 122 | 118 | 112 | 44 | 41 | 34 | NS | NS | 10 | 3 | NS | 26 | 5 | 31 |
| Tadesse et al. [36] | 2017 | 2013–2015 | OR | EPTB | NR | GeneXpertMTB/RIF | NR | NR | 436 | 310 | 279 | 10 | NS | 10 | NS | NS | NS | NR | NS | NR | NR | NR |
| Sinshaw et al. [37] | 2019 | 2017–2018 | AA | PTB | Cross-sectional | GenoType®MTBDRplus V.2 and Genotype®MTBDRsl V.2 | 345 | 73 | 418 | 26 | 26 | 10 | 10 | 3 | NR | 0 | 7 | 0 | NR | 1 | 2 | 3 |
| Mulu et al. [38] | 2017 | 2014–2015 | AM | PTB and EPTB | Cross-sectional | GeneXpertMTB/RIF | 373 | 132 | 505 | 117 | 117 | 12 | NS | 12 | NS | NS | NS | NS | NS | NR | NR | NR |
| Jaleta et al. [39] | 2017 | 2013–2015 | AM | PTB | Cross-sectional (Retro) | GeneXpert MTB/RIF | 305 | 1515 | 1820 | 448 | 448 | 71 | NS | 71 | NS | NS | NS | NS | NS | NR | NR | NR |
| Haile et al. [40] | 2020 | 2015–2016 | OR | PTB | NR | GenoType®MTBDRplus | 105 | 6 | 111 | 92 | 92 | 6 | 5 | 1 | NS | NS | 5 | 1 | NS | 0 | 0 | 0 |
| Habte et al. [41] | 2016 | 2013–2015 | AM | PTB | Cross-sectional | GeneXpert MTB/RIF | 119 | 0 | 119 | 111 | 111 | 5 | NS | 5 | NS | NS | NS | NR | NS | NR | NR | NR |
| Gizachew Beza et al. [42] | 2017 | 2016 | AM | PTB | Cross-sectional | GeneXpert MTB/RIF | 243 | 22 | 265 | 9 | 9 | 1 | NS | 1 | NS | NS | NS | NR | NS | NR | NR | NR |
| Gebrehiwet et al. [43] | 2019 | 2016–2017 | AF | PTB | Cross-sectional | GeneXpertMTB/RIF | 321 | 63 | 384 | 94 | 94 | 4 | NS | 4 | NS | NS | NS | NR | NS | NR | NR | NR |
| Fanosie et al. [44] | 2016 | 2015 | AM | PTB | Cross-sectional | GenoType®MTBDRplus and GeneXpertMTB/RIF | 141 | NR | 141 | 37 | 37 | 1 | 1 | 1 | NS | NS | NR | NR | NS | NR | NR | 1 |
| Ejeta et al. [45] | 2018 | 2017 | GA | PTB | Cross-sectional | GeneXpertMTB/RIF | 465 | 530 | 995 | 193 | 193 | 9 | NS | 9 | NS | NS | NS | NR | NS | NR | NR | NR |
| Diriba et al. [46] | 2019 | 2017–2018 | Tig, HR, SNNP, AA, AM, OR | PTB | Cross-sectional | GenoType®MTBDRplus | NR | NR | 10,134 | 1183 | 329 | 40 | 19 | 21 | NS | NS | 19 | 22 | NS | 22 | 16 | 38 |
| Damena et al. [47] | 2019 | 2015–2016 | AA | PTB | Cross-sectional | GenoType®MTBDRplus V.2 and<break/>GenoType®MTBDRsl | 98 | 115 | 213 | 150 | 150 | 20 | 20 | 16 | 12 | 1 | 4 | 0 | 0 | 11 | 5 | 16 |
| Brhane et al. [48] | 2017 | NR | SO | PTB | Cross-sectional | GenoType®MTBDRplus V.2 | 67 | 31 | 105 | 98 | 98 | 18 | 18 | 10 | NS | NS | 8 | 0 | NS | 7 | 3 | 10 |
| Biadglegne et al. [49] | 2013 | 2012 | AM | EPTB | Cross-sectional | GenoType®MTBDRplus and GenoType® MTBDRsl | 213 | 13 | 226 | 226 | 226 | 13 | 8 | 3 | 2 | NS | 6 | 1 | NR | 0 | 2 | 2 |
| Biadglegne et al. [50] | 2014 | NR | AM | EPTB | Cross-sectional | GeneXpertMTB/RIF & GenoType®MTBDRplus | 231 | 0 | 231 | 32 | 32 | 3 | NS | 3 | NS | NS | NS | NR | NS | NR | NR | NR |
| Bekele et al. [51] | 2018 | 2006–2010 | AA, AM,<break/>OR, SNNPR | PTB & TBLN | Cross-sectional | GenoType®MTBDRplus | NR | NR | 950 | 161 | 161 | 14 | 12 | 7 | NS | NS | 7 | 2 | NS | NR | NR | 5 |
| Bedewi Omer et al. [52] | 2016 | 2012–2013 | OR | PTB | Cross-sectional | GenoType®MTBDRplus | 268 | 11 | 279 | 279 | 279 | 31 | 25 | 9 | NS | Ns | 22 | 6 | NS | 0 | 3 | 3 |
| Amir Alelign et al. [53] | 2019 | 2015–2017 | AM | PTB & EPTB | Cross-sectional | GenoType®MTBDRplus | 90 | 21 | 111 | 111 | 111 | 20 | 20 | 2 | NS | NS | 18 | 0 | NS | 2 | 0 | 2 |
| Abate et al. [54] | 2014 | 2012–2013 | AA | PTB | Cross-sectional (Retro) | GenoType®MTBDRplus | 0 | 736 | 736 | 736 | 736 | 523 | 481 | 470 | NS | NS | 54 | 42 | NS | 427 | 0 | 427 |
| Total | 3844 | 3401 | 18,908 | 4992 | 4101 | 911 | 703 | 715 | 22 | 1 | 184 | 81 | 0 | 505 | 46 | 557 | ||||||
2.6. Statistical Analysis
2.7. Study Outcomes
3. Results
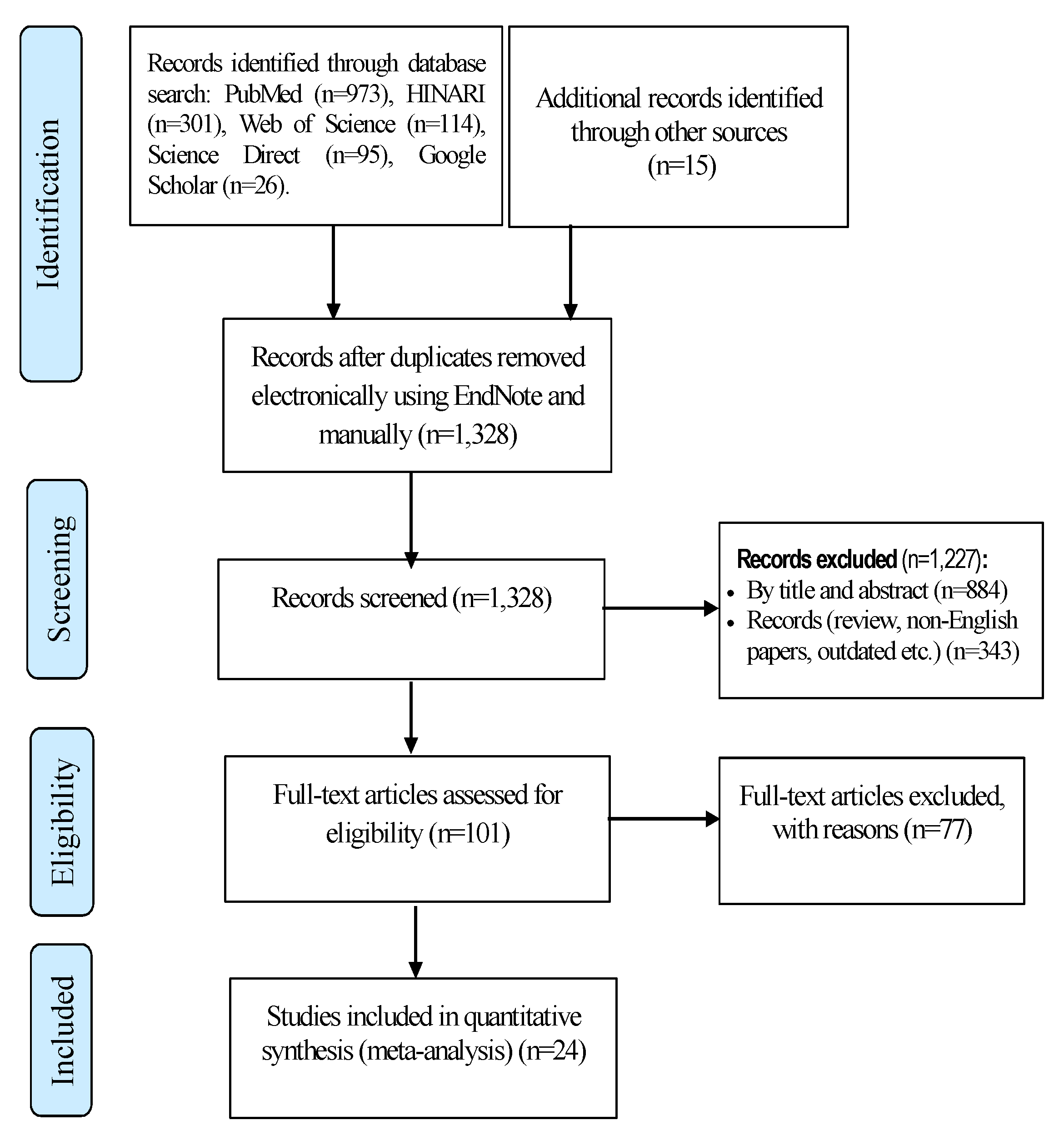
3.1. Search Results
3.2. Characteristics of Included Studies
3.3. Meta-Analysis Results
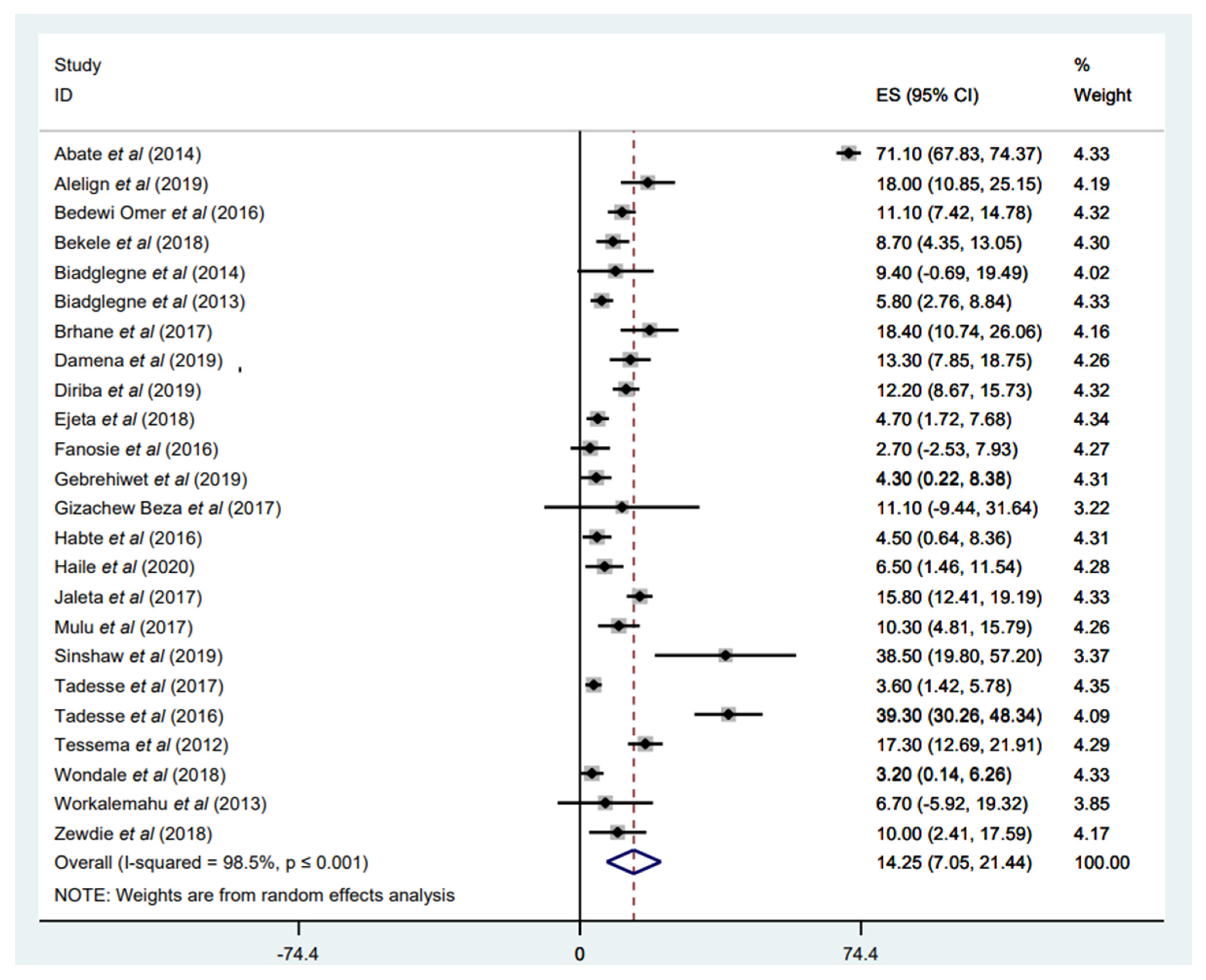
3.3.1. Prevalence of Any Anti-TB Drug Resistance
3.3.2. Prevalence of Any INH Resistance
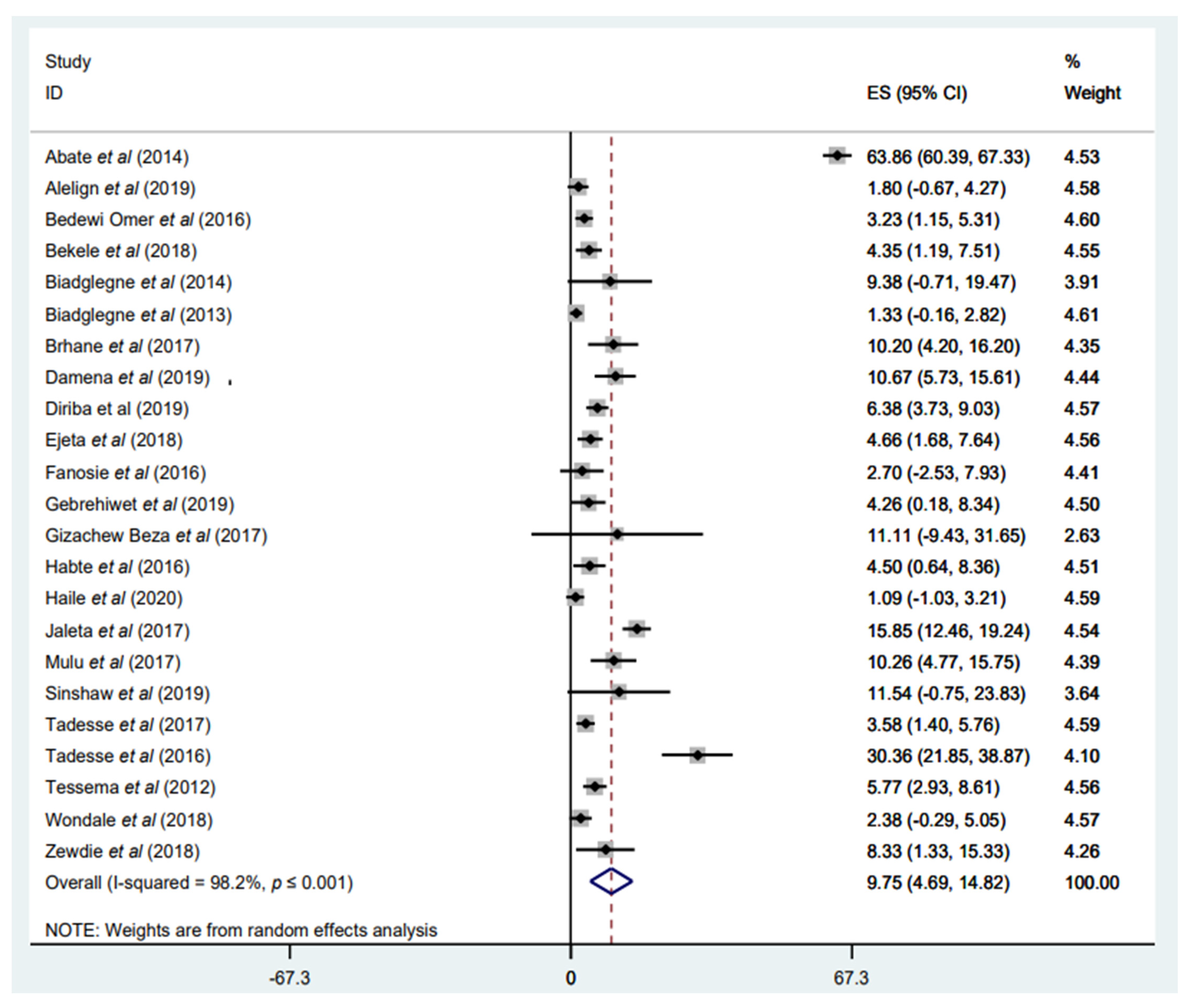
3.3.3. Prevalence of Any RIF Resistance
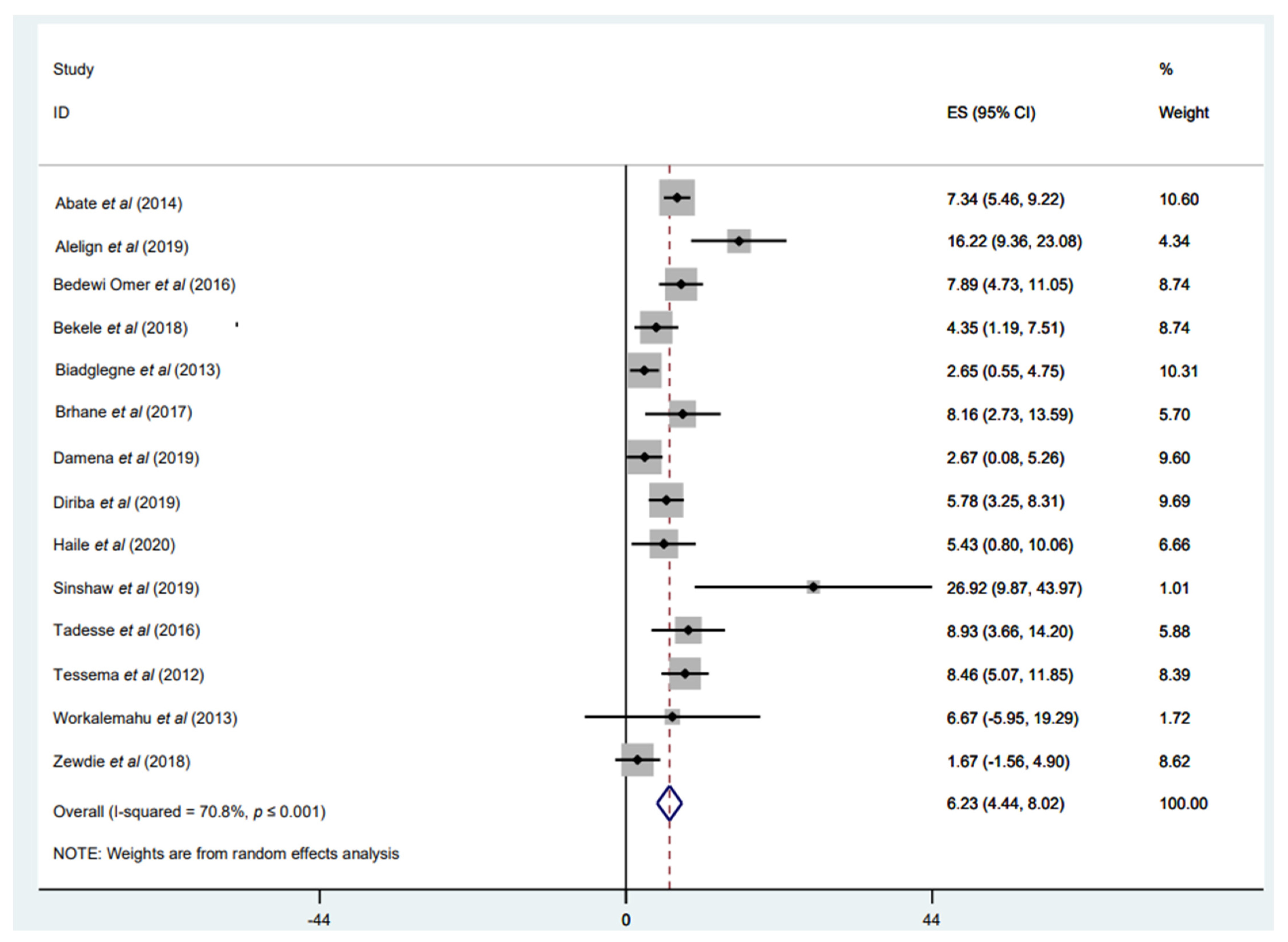
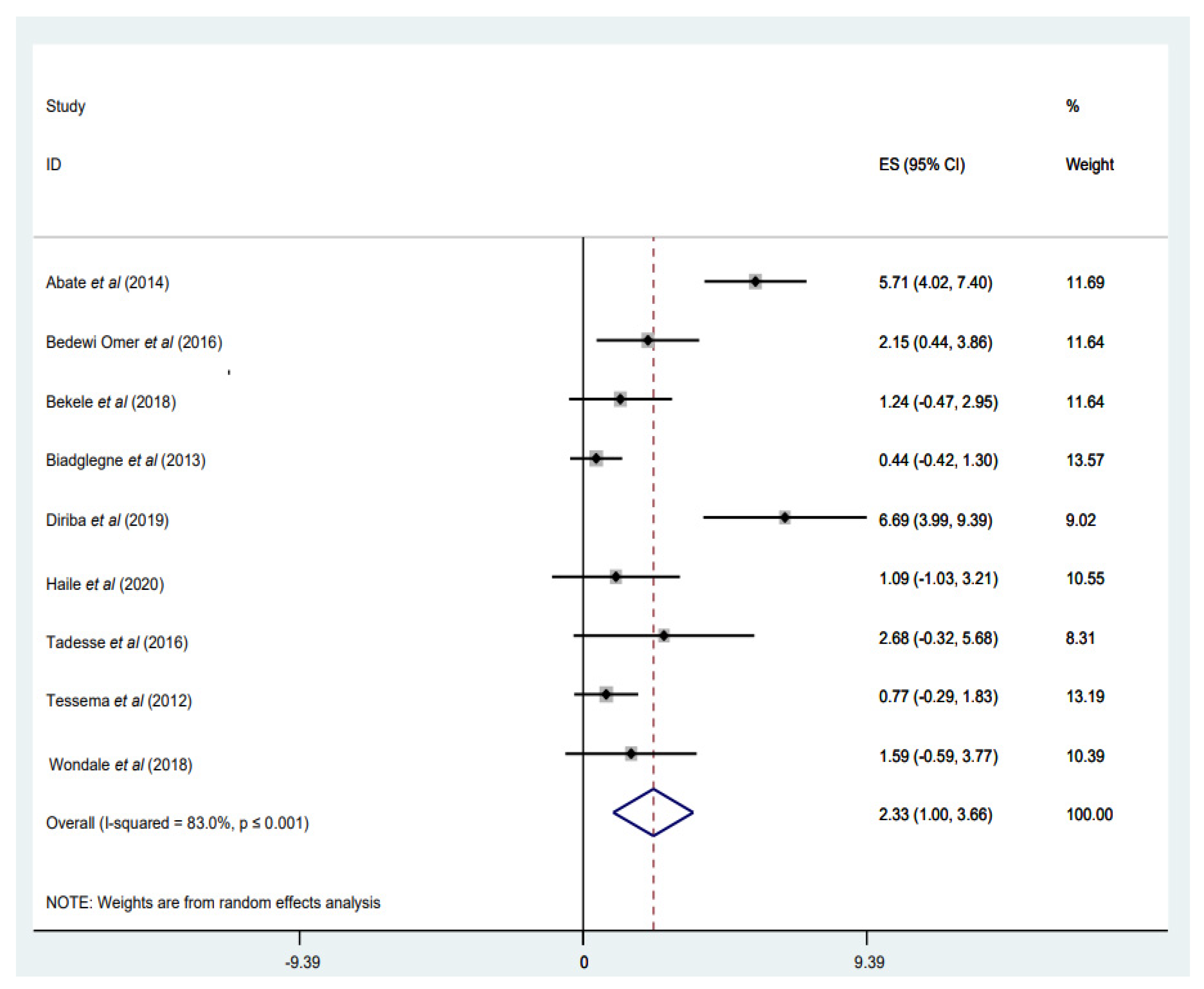
3.3.4. Prevalence of INH Mono-Resistance
3.3.5. Prevalence of RIF Mono-Resistance
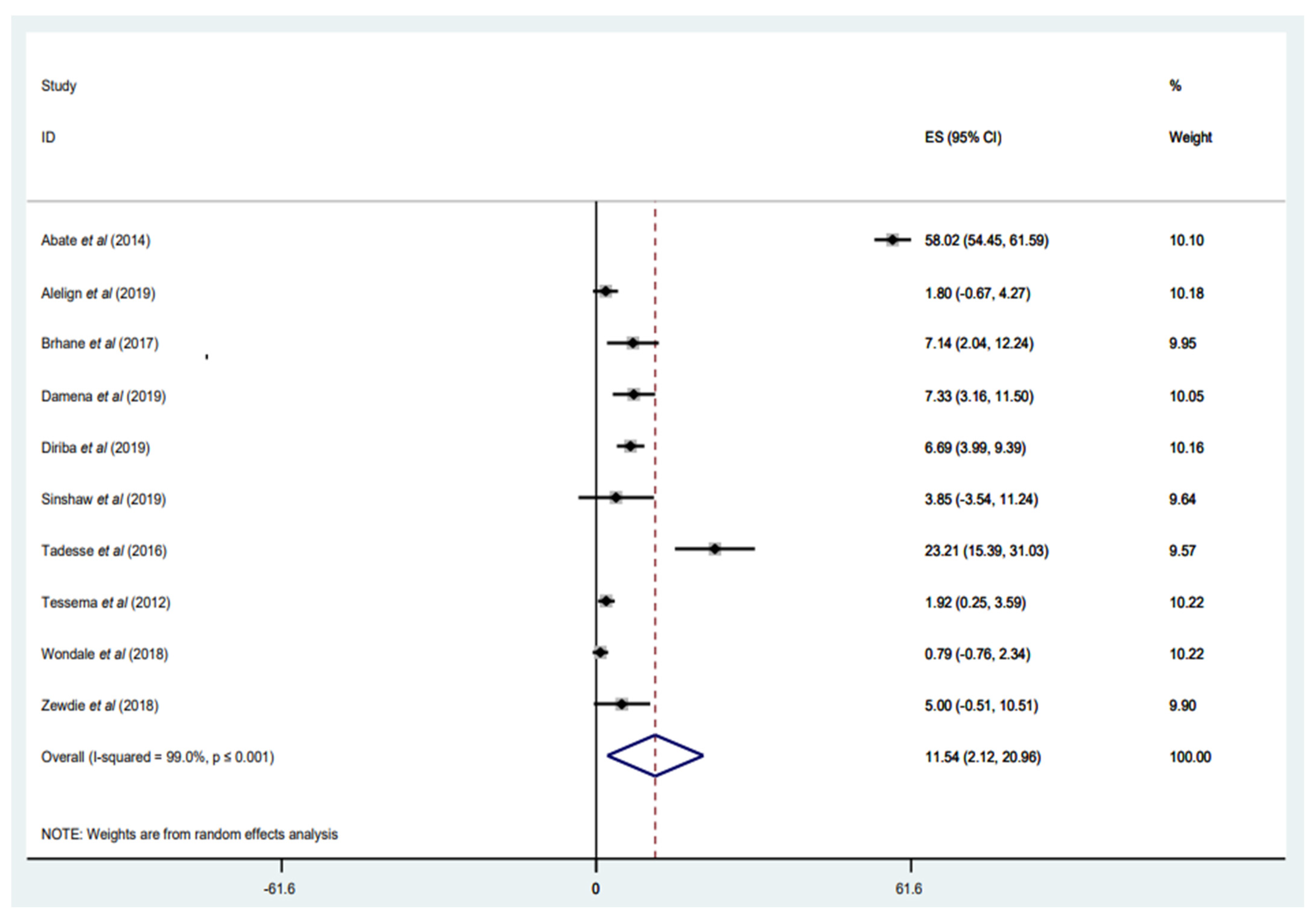
3.3.6. Prevalence of MDR-TB among Newly Diagnosed Cases
3.3.7. Prevalence of MDR-TB among Retreated Patients with TB
3.3.8. Prevalence of MDR-TB among Overall Patients with TB
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- WHO. Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. Global Tuberculosis Report 2016. 2016. Available online: http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf (accessed on 2 June 2021).
- WHO. Global Tuberculosis Report 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Churchyard, G.; Mametja, L.; Mvusi, L.; Ndjeka, N.; Hesseling, A.; Reid, A.; Babatunde, S.; Pillay, Y. Tuberculosis control in South Africa: Successes, challenges, and recommendations. SAMJ S. Afr. Med. J. 2014, 104, 234–248. [Google Scholar] [CrossRef]
- WHO. Global Tuberculosis Control: A Short Update to the 2009 Report; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Onyedum, C.C.; Alobu, I.; Ukwaja, K.N. Prevalence of drug-resistant tuberculosis in Nigeria: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0180996. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Niguse, S.; Abdulkader, M.; Tsegay, E.; Hailekiros, H.; Gebrekidan, A.; Araya, T.; Pugazhendhi, A. Review on the emergence of drug-resistant tuberculosis (MDR & XDR-TB) and its molecular diagnosis in Ethiopia. Microb. Pathog. 2018, 117, 237–242. [Google Scholar] [PubMed]
- Deutschendorf, C.; Goldani, L.Z.; dos Santos, R.P. Previous use of quinolones: A surrogate marker for first-line anti-tuberculosis drugs resistance in HIV-infected patients? Braz. J. Infect. Dis. 2012, 16, 142–145. [Google Scholar] [PubMed]
- Van der Werf, M.J.; Langendam, M.W.; Huitric, E.; Manissero, D. Multidrug resistance after inappropriate tuberculosis treatment: A meta-analysis. Eur. Respir. J. 2012, 39, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, X.; Zhang, S.; Wang, X.; Liu, C. Social behaviour risk factors for drug-resistant tuberculosis in mainland China: A meta-analysis. J. Int. Med. Res. 2012, 40, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Bedewi, Z.; Mekonnen, Y.; Worku, A.; Medhin, G.; Zewde, A.; Yimer, G.; Pieper, R.; Ameni, G. Mycobacterium tuberculosis in central Ethiopia: Drug sensitivity patterns and association with genotype. New Microbes New Infect. 2017, 17, 69–74. [Google Scholar] [CrossRef]
- Mesfin, M.M.; Newell, J.N.; Walley, J.D.; Gessessew, A.; Madeley, R.J. Delayed consultation among pulmonary tuberculosis patients: A cross-sectional study of 10 DOTS districts of Ethiopia. BMC Public Health 2009, 9, 53. [Google Scholar] [CrossRef]
- Suchindran, S.; Brouwer, E.S.; Van Rie, A. Is HIV infection a risk factor for multi-drug-resistant tuberculosis? A systematic review. PLoS ONE 2009, 4, e5561. [Google Scholar] [CrossRef]
- Seifert, M.; Catanzaro, D.; Catanzaro, A.; Rodwell, T.C. Genetic mutations associated with isoniazid resistance in Mycobacterium tuberculosis: A systematic review. PLoS ONE 2015, 10, e0119628. [Google Scholar] [CrossRef] [PubMed]
- Abebe, G.; Paasch, F.; Apers, L.; Rigouts, L.; Colebunders, R. Tuberculosis drug resistance testing by molecular methods: Opportunities and challenges in resource-limited settings. J. Microbiol. Methods 2011, 84, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Drobniewski, F.; Rüsch-Gerdes, S.; Hoffner, S. Antimicrobial susceptibility testing of Mycobacterium tuberculosis (EUCAST document E. DEF 8.1)–report of the Subcommittee on Antimicrobial Susceptibility Testing of Mycobacterium tuberculosis of the European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Clin. Microbiol. Infect. 2007, 13, 1144–1156. [Google Scholar] [PubMed]
- WHO. The Use of Next-Generation Sequencing Technologies for the Detection of Mutations Associated with Drug Resistance in Mycobacterium tuberculosis Complex: Technical Guide; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Arora, D.; Dhanashree, B. Utility of smear microscopy and GeneXpert for the detection of Mycobacterium tuberculosis in clinical samples. Germs 2020, 10, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Pillay, S.; Steingart, K.R.; Davies, G.R.; Chaplin, M.; De Vos, M.; Schumacher, S.G.; Warren, R.; Theron, G. Xpert MTB/XDR for detection of pulmonary tuberculosis and resistance to isoniazid, fluoroquinolones, ethionamide, and amikacin. Cochrane Database Syst. Rev. 2022, 5, Cd014841. [Google Scholar] [PubMed]
- Florea, D.; Oţelea, D.; Olaru, I.D.; Hristea, A. Broad-range PCR coupled with mass-spectrometry for the detection of Mycobacterium tuberculosis drug resistance. Germs 2016, 6, 10–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nasiri, M.J.; Dabiri, H.; Darban-Sarokhalil, D.; Rezadehbashi, M.; Zamani, S. Prevalence of drug-resistant tuberculosis in Iran: Systematic review and meta-analysis. Am. J. Infect. Control 2014, 42, 1212–1218. [Google Scholar] [CrossRef]
- Ethiopia-Ministry of Health. Guidelines for Clinical and Programmatic Management of TB, Leprosy and TB/HIV in Ethiopia; EFDR: Addis Ababa, Ethiopia, 2012; p. 149. [Google Scholar]
- Abayneh, M.; HaileMariam, S.; Asres, A. Low Tuberculosis (TB) Case Detection: A Health Facility-Based Study of Possible Obstacles in Kaffa Zone, Southwest District of Ethiopia. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 7029458. [Google Scholar] [CrossRef]
- Girum, T.; Muktar, E.; Lentiro, K.; Wondiye, H.; Shewangizaw, M. Epidemiology of multidrug-resistant tuberculosis (MDR-TB) in Ethiopia: A systematic review and meta-analysis of the prevalence, determinants and treatment outcome. Trop. Dis. Travel Med. Vaccines 2018, 4, 5. [Google Scholar] [CrossRef]
- Eshetie, S.; Moges, F.; Dagnew, M. Multidrug-resistant tuberculosis in Ethiopian settings and its association with previous antituberculosis treatment: A systematic review and meta-analysis. Int. J. Mycobacteriol. 2016, 5 (Suppl. S1.), S119–S120. [Google Scholar] [CrossRef]
- Berhan, A.; Berhan, Y.; Yizengaw, D. A meta-analysis of drug-resistant tuberculosis in Sub-Saharan Africa: How strongly associated with previous treatment and HIV co-infection? Ethiop. J. Health Sci. 2013, 23, 271–282. [Google Scholar] [CrossRef]
- Weldegebreal, S.; Mebrahtu, T. Anti-tuberculosis drug resistance in Ethiopia: Systematic review. Int. J. Tuberc. Lung Dis. 2017, 21, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Joanna Briggs Institute. The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews: Checklist for Prevalence Studies. Retreived Novemb. 2017, 15, 2018. [Google Scholar]
- Duan, Q.; Chen, Z.; Chen, C.; Zhang, Z.; Lu, Z.; Yang, Y.; Zhang, L. The prevalence of drug-resistant tuberculosis in mainland China: An updated systematic review and meta-analysis. PLoS ONE 2016, 11, e0148041. [Google Scholar] [CrossRef] [PubMed]
- Zewdie, O.; Mihret, A.; Abebe, T.; Kebede, A.; Desta, K.; Worku, A.; Ameni, G. Genotyping and molecular detection of multidrug-resistant Mycobacterium tuberculosis among tuberculosis lymphadenitis cases in Addis Ababa, Ethiopia. New Microbes New Infect. 2018, 21, 36–41. [Google Scholar] [CrossRef]
- Workalemahu, B.; Berg, S.; Tsegaye, W.; Abdissa, A.; Girma, T.; Abebe, M.; Aseffa, A. Genotype diversity of Mycobacterium isolates from children in Jimma, Ethiopia. BMC Res. Notes 2013, 6, 352. [Google Scholar] [CrossRef]
- Wondale, B.; Medhin, G.; Abebe, G.; Tolosa, S.; Mohammed, T.; Teklu, T.; Pieper, R.; Ameni, G. Phenotypic and genotypic drug sensitivity of Mycobacterium tuberculosis complex isolated from South Omo Zone, Southern Ethiopia. Infect. Drug Resist 2018, 11, 1581–1589. [Google Scholar] [CrossRef]
- Tessema, B.; Beer, J.; Emmrich, F.; Sack, U.; Rodloff, A.C. Analysis of gene mutations associated with isoniazid, rifampicin and ethambutol resistance among Mycobacterium tuberculosis isolates from Ethiopia. BMC Infect. Dis. 2012, 12, 37. [Google Scholar] [CrossRef]
- Tadesse, M.; Aragaw, D.; Dimah, B.; Efa, F.; Abdella, K.; Kebede, W.; Abdissa, K.; Abebe, G. Drug resistance-conferring mutations in Mycobacterium tuberculosis from pulmonary tuberculosis patients in Southwest Ethiopia. Int. J. Mycobacteriol. 2016, 5, 185–191. [Google Scholar] [CrossRef]
- Tadesse, M.; Abebe, G.; Bekele, A.; Bezabih, M.; de Rijk, P.; Meehan, C.J.; de Jong, B.C.; Rigouts, L. The predominance of Ethiopian-specific Mycobacterium tuberculosis families and minimal contribution of Mycobacterium bovis in tuberculous lymphadenitis patients in Southwest Ethiopia. Infect. Genet. Evol. 2017, 55, 251–259. [Google Scholar] [CrossRef]
- Sinshaw, W.; Kebede, A.; Bitew, A.; Tesfaye, E.; Tadesse, M.; Mehamed, Z.; Yenew, B.; Amare, M.; Dagne, B.; Diriba, G.; et al. Prevalence of tuberculosis, multidrug-resistant tuberculosis and associated risk factors among smear-negative presumptive pulmonary tuberculosis patients in Addis Ababa, Ethiopia. BMC Infect. Dis. 2019, 19, 641. [Google Scholar] [CrossRef]
- Mulu, W.; Abera, B.; Yimer, M.; Hailu, T.; Ayele, H.; Abate, D. Rifampicin-resistance pattern of Mycobacterium tuberculosis and associated factors among presumptive tuberculosis patients referred to Debre Markos Referral Hospital, Ethiopia: A cross-sectional study. BMC Res. Notes 2017, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Jaleta, K.N.; Gizachew, M.; Gelaw, B.; Tesfa, H.; Getaneh, A.; Biadgo, B. Rifampicin-resistant Mycobacterium tuberculosis among tuberculosis-presumptive cases at University of Gondar Hospital, northwest Ethiopia. Infect. Drug Resist. 2017, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Haile, B.; Tafess, K.; Zewude, A.; Yenew, B.; Siu, G.; Ameni, G. Spoligotyping and drug sensitivity of Mycobacterium tuberculosis isolated from pulmonary tuberculosis patients in the Arsi Zone of southeastern Ethiopia. New Microbes New Infect 2020, 33, 100620. [Google Scholar] [CrossRef] [PubMed]
- Habte, D.; Melese, M.; Hiruy, N.; Gashu, Z.; Jerene, D.; Moges, F.; Yifru, S.; Girma, B.; Kassie, Y.; Haile, Y.K.; et al. The additional yield of GeneXpert MTB/RIF test in the diagnosis of pulmonary tuberculosis among household contacts of smear-positive TB cases. Int. J. Infect. Dis. 2016, 49, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Gizachew Beza, M.; Hunegnaw, E.; Tiruneh, M. Prevalence and Associated Factors of Tuberculosis in Prisons Settings of East Gojjam Zone, Northwest Ethiopia. Int. J. Bacteriol. 2017, 2017, 3826980. [Google Scholar] [CrossRef]
- Gebrehiwet, G.B.; Kahsay, A.G.; Welekidan, L.N.; Hagos, A.K.; Abay, G.K.; Hagos, D.G. Rifampicin resistant tuberculosis in presumptive pulmonary tuberculosis cases in Dubti Hospital, Afar, Ethiopia. J. Infect. Dev. Ctries 2019, 13, 21–27. [Google Scholar] [CrossRef]
- Fanosie, A.; Gelaw, B.; Tessema, B.; Tesfay, W.; Admasu, A.; Yitayew, G. Mycobacterium tuberculosis Complex, and HIV Co-Infection among Extrapulmonary Tuberculosis Suspected Cases at the University of Gondar Hospital, Northwestern Ethiopia. PLoS ONE 2016, 11, e0150646. [Google Scholar] [CrossRef]
- Ejeta, E.; Beyene, G.; Bonsa, Z.; Abebe, G. Xpert MTB/RIF assay for the diagnosis of Mycobacterium tuberculosis and Rifampicin resistance in high Human Immunodeficiency Virus setting in Gambella regional state, southwest Ethiopia. J. Clin. Tuberc. Other Mycobact. Dis. 2018, 12, 14–20. [Google Scholar] [CrossRef]
- Diriba, G.; Kebede, A.; Tola, H.H.; Alemu, A.; Tadesse, M.; Tesfaye, E.; Mehamed, Z.; Meaza, A.; Yenew, B.; Molalign, H.; et al. Surveillance of drug resistance tuberculosis based on reference laboratory data in Ethiopia. Infect. Dis. Poverty 2019, 8, 54. [Google Scholar] [CrossRef]
- Damena, D.; Tolosa, S.; Hailemariam, M.; Zewude, A.; Worku, A.; Mekonnen, B.; Mohammed, T.; Admasu, A.; Chimusa, E.R.; Mihret, A.; et al. Genetic diversity and drug susceptibility profiles of Mycobacterium tuberculosis obtained from Saint Peter’s TB Specialized Hospital, Ethiopia. PLoS ONE 2019, 14, e0218545. [Google Scholar] [CrossRef] [PubMed]
- Brhane, M.; Kebede, A.; Petros, Y. Molecular detection of multidrug-resistant tuberculosis among smear-positive pulmonary tuberculosis patients in Jigjiga town, Ethiopia. Infect. Drug Resist. 2017, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Biadglegne, F.; Tessema, B.; Rodloff, A.C.; Sack, U. Magnitude of gene mutations conferring drug resistance in Mycobacterium tuberculosis isolates from lymph node aspirates in Ethiopia. Int. J. Med. Sci. 2013, 10, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Biadglegne, F.; Mulu, A.; Rodloff, A.C.; Sack, U. Diagnostic performance of the Xpert MTB/RIF assay for tuberculous lymphadenitis on fine needle aspirates from Ethiopia. Tuberculosis 2014, 94, 502–505. [Google Scholar] [CrossRef]
- Bekele, S.; Derese, Y.; Hailu, E.; Mihret, A.; Dagne, K.; Yamuah, L.; Hailu, T.; Ayele, S.; Beyene, D.; Berg, S.; et al. Line-probe assay and molecular typing reveal a potential drug-resistant clone of Mycobacterium tuberculosis in Ethiopia. Trop. Dis. Travel Med. Vaccines 2018, 4, 15. [Google Scholar] [CrossRef]
- Bedewi Omer, Z.; Mekonnen, Y.; Worku, A.; Zewde, A.; Medhin, G.; Mohammed, T.; Pieper, R.; Ameni, G. Evaluation of the GenoType MTBDRplus assay for detection of rifampicin- and isoniazid-resistant Mycobacterium tuberculosis isolates in central Ethiopia. Int. J. Mycobacteriol. 2016, 5, 475–481. [Google Scholar] [CrossRef]
- Amir Alelign; Aboma Zewude; Temesgen Mohammed; Samuel Tolosa; Gobena Ameni; Beyene Petros. Molecular detection of Mycobacterium tuberculosis sensitivity to rifampicin and isoniazid in South Gondar Zone, northwest Ethiopia. BMC Infect. Dis. 2019, 19, 343. [Google Scholar]
- Abate, D.; Tedla, Y.; Meressa, D.; Ameni, G. Isoniazid and rifampicin resistance mutations and their effect on second-line anti-tuberculosis treatment. Int. J. Tuberc. Lung Dis. 2014, 18, 946–951. [Google Scholar] [CrossRef]
- Chisompola, N.K.; Streicher, E.M.; Muchemwa, C.M.K.; Warren, R.M.; Sampson, S.L. Molecular epidemiology of drug-resistant Mycobacterium tuberculosis in Africa: A systematic review. BMC Infect. Dis. 2020, 20, 344. [Google Scholar] [CrossRef]
- Affolabi, D.; Adjagba, O.; Tanimomo-Kledjo, B.; Gninafon, M.; Anagonou, S.; Portaels, F. Anti-tuberculosis drug resistance among new and previously treated pulmonary tuberculosis patients in Cotonou, Benin. Int. J. Tuberc. Lung Dis. 2007, 11, 1221–1224. [Google Scholar]
- Sanders, M.; Van Deun, A.; Ntakirutimana, D.; Masabo, J.P.; Rukundo, J.; Rigouts, L.; Fissette, K.; Portaelst, F. Rifampicin mono-resistant Mycobacterium tuberculosis in Bujumbura, Burundi: Results of a drug resistance survey. Int. J. Tuberc. Lung Dis. 2006, 10, 178–183. [Google Scholar]
- Gudo, P.S.; Cuna, Z.; Coelho, E.; Maungate, S.; Borroni, E.; Miotto, P.; Ahmadova, S.; Brouwer, M.; Migliori, G.; Zignol, M. Is multidrug-resistant tuberculosis on the rise in Mozambique? Results of a national drug resistance survey. Eur. Respir. J. 2011, 38, 222–224. [Google Scholar] [CrossRef]
- Umubyeyi, A.N.; Vandebriel, G.; Gasana, M.; Basinga, P.; Zawadi, J.; Gatabazi, J.; Pauwels, P.; Nzabintwali, F.; Nyiramasarabwe, L.; Fissette, K. Results of a national survey on drug resistance among pulmonary tuberculosis patients in Rwanda. Int. J. Tuberc. Lung Dis. 2007, 11, 189–194. [Google Scholar]
- Jenkins, H.E.; Zignol, M.; Cohen, T. Quantifying the burden and trends of isoniazid-resistant tuberculosis, 1994–2009. PLoS ONE 2011, 6, e22927. [Google Scholar] [CrossRef]
- O’Donnell, M. Isoniazid Monoresistance: A Precursor to Multidrug-Resistant Tuberculosis? American Thoracic Society: New York, NY, USA, 2018. [Google Scholar]
- World Health Organization. WHO Treatment Guidelines for Isoniazid-Resistant Tuberculosis: Supplement to the WHO Treatment Guidelines for Drug-Resistant Tuberculosis; Report No.: 9241550074; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Dramowski, A.; Morsheimer, M.M.; Jordaan, A.M.; Victor, T.C.; Donald, P.R.; Schaaf, H.S. Rifampicin-monoresistant Mycobacterium tuberculosis disease among children in Cape Town, South Africa. Int. J. Tuberc. Lung Dis. 2012, 16, 76–81. [Google Scholar] [CrossRef]
- Kazemian, H.; Haeili, M.; Kardan, Y.J.; Rezaei, F.; Gizaw, F.S.; Zahednamazi, F.; Mohajeri, P.; Zaker, B.S.; Hashemi, S.A.; Imani, F.A. Antimycobacterial activity of linezolid against multidrug-resistant and extensively drug-resistant strains of Mycobacterium tuberculosis in Iran. Int. J. Antimicrob. Agents 2015, 45, 668. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, M.; Wang, Y.; Pang, Y.; Kam, K.; Zhao, Y. Molecular and phenotypic characterization of multidrug-resistant Mycobacterium tuberculosis isolates resistant to kanamycin, amikacin, and capreomycin in China. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Feyisa, S.G.; Abdurahman, A.A.; Jimma, W.; Chaka, E.E.; Kardan-Yamchi, J.; Kazemian, H. Resistance of Mycobacterium tuberculosis strains to Rifampicin: A systematic review and meta-analysis. Heliyon 2019, 5, e01081. [Google Scholar] [CrossRef]
- Malenfant, J.H.; Brewer, T.F. Rifampicin Mono-Resistant Tuberculosis-A Review of an Uncommon But Growing Challenge for Global Tuberculosis Control. Open Forum Infect. Dis. 2021, 8, ofab018. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2018; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/publications/i/item/9789241565646 (accessed on 15 June 2021).
- Kebede, A.H.; Alebachew, Z.; Tsegaye, F.; Lemma, E.; Abebe, A.; Agonafir, M.; Kebede, A.J.; Demissie, D.; Girmachew, F.; Yaregal, Z.; et al. The first population-based national tuberculosis prevalence survey in Ethiopia, 2010–2011. Int. J. Tuberc. Lung Dis. 2014, 18, 639. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reta, M.A.; Tamene, B.A.; Abate, B.B.; Mensah, E.; Maningi, N.E.; Fourie, P.B. Mycobacterium tuberculosis Drug Resistance in Ethiopia: An Updated Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2022, 7, 300. https://doi.org/10.3390/tropicalmed7100300
Reta MA, Tamene BA, Abate BB, Mensah E, Maningi NE, Fourie PB. Mycobacterium tuberculosis Drug Resistance in Ethiopia: An Updated Systematic Review and Meta-Analysis. Tropical Medicine and Infectious Disease. 2022; 7(10):300. https://doi.org/10.3390/tropicalmed7100300
Chicago/Turabian StyleReta, Melese Abate, Birhan Alemnew Tamene, Biruk Beletew Abate, Eric Mensah, Nontuthuko Excellent Maningi, and P. Bernard Fourie. 2022. "Mycobacterium tuberculosis Drug Resistance in Ethiopia: An Updated Systematic Review and Meta-Analysis" Tropical Medicine and Infectious Disease 7, no. 10: 300. https://doi.org/10.3390/tropicalmed7100300
APA StyleReta, M. A., Tamene, B. A., Abate, B. B., Mensah, E., Maningi, N. E., & Fourie, P. B. (2022). Mycobacterium tuberculosis Drug Resistance in Ethiopia: An Updated Systematic Review and Meta-Analysis. Tropical Medicine and Infectious Disease, 7(10), 300. https://doi.org/10.3390/tropicalmed7100300







