Feasibility Study for a Randomised Controlled Trial for the Topical Treatment of Impetigo in Australian General Practice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.3. Outcomes
2.4. Participants
2.5. Randomisation
2.6. Intervention
Procedure
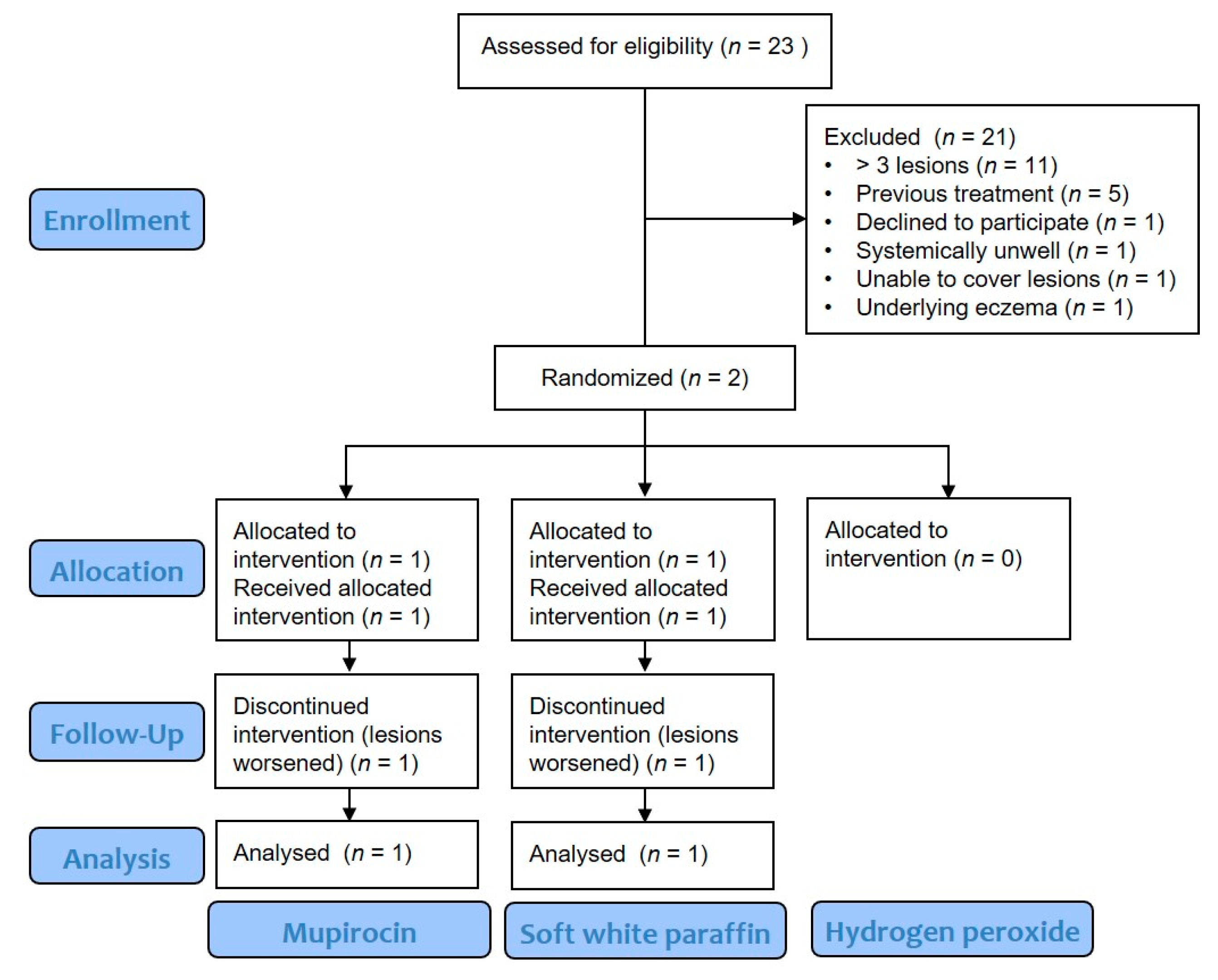
3. Results
3.1. Outcomes
3.1.1. Barriers to Recruitment
3.1.2. Improved Delivery
3.1.3. Protocol Acceptability
4. Discussion
4.1. Self-Initiated At-Home Treatments
4.2. Lesion Severity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Epidemiology and Management of Common Skin Diseases in Children in Developing Countries; Discussion Papers on Child Health 1–54; World Health Organization: Geneva, Switzerland, 2005; Available online: https://www.who.int/maternal_child_adolescent/documents/fch_cah_05_12/en/ (accessed on 5 February 2021).
- Bowen, A.C.; Mahé, A.; Hay, R.J.; Andrews, R.M.; Steer, A.C.; Tong, S.Y.C.; Carapetis, J.R. The Global Epidemiology of Impetigo: A Systematic Review of the Population Prevalence of Impetigo and Pyoderma. PLoS ONE 2015, 10, e0136789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowen, A.C.; Tong, S.Y.; Chatfield, M.D.; Carapetis, J.R. The microbiology of impetigo in indigenous children: Associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect. Dis. 2014, 14, 727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steer, A.C.; Carapetis, J.R. Acute rheumatic fever and rheumatic heart disease in indigenous populations. Pediatr. Clin. N. Am. 2009, 56, 1401–1419. [Google Scholar] [CrossRef]
- World Health Organization. Worldwide Country Situation: Response to Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015; Available online: https://apps.who.int/iris/bitstream/handle/10665/163473/WHO_HSE_PED_AIP_2015.1_eng.pdf;jsessionid=D5BFEB02EF1DA68E4799ED91D2D5BDE3?sequence=1 (accessed on 8 February 2021).
- World Health Organization. Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 8 January 2021).
- Antonov, N.K.; Garzon, M.C.; Morel, K.D.; Whittier, S.; Planet, P.J.; Lauren, C.T. High prevalence of mupirocin resistance in Staphylococcus aureus isolates from a pediatric population. Antimicrob. Agents Chemother. 2015, 59, 3350–3356. [Google Scholar] [CrossRef] [Green Version]
- Hajikhani, B.; Goudarzi, M.; Kakavandi, S.; Amini, S.; Zamani, S.; van Belkum, A.; Goudarzi, H.; Dadashi, M. The global prevalence of fusidic acid resistance in clinical isolates of Staphylococcus aureus: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control. 2021, 10, 75. [Google Scholar] [CrossRef]
- Therapeutic Guidelines. Impetigo—Antibiotic Therapy for Impetigo in Nonendemic Settings. eTG Complete. March 2021. Available online: https://tgldcdp.tg.org.au/viewTopic?topicfile=impetigo&guidelineName=Antibiotic&topicNavigation=navigateTopic#toc_d1e108 (accessed on 15 January 2021).
- Therapeutic Guidelines. Impetigo—Antibiotic Therapy for Impetigo in Endemic Settings. eTG Complete. March 2021. Available online: https://tgldcdp.tg.org.au/viewTopic?topicfile=impetigo&guidelineName=Antibiotic&topicNavigation=navigateTopic#toc_d1e108 (accessed on 15 January 2021).
- Hall, L.M.; Gorges, H.J.; Van Driel, M.; Magin, P.; Francis, N.; Heal, C.F. International comparison of guidelines for management of impetigo: A systematic review. Fam. Pract. 2021, cmab066. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, E.L.; Montoya, J.G.; Wade, J.C. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin. Infect. Dis. 2014, 59, 147–159. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Impetigo: Antimicrobial Prescribing (NICE Guideline NG153); National Institute for Health and Care Excellence NICE: London, UK, 2020. [Google Scholar]
- Koning, S.; Van Der Sande, R.; Verhagen, A.P.; van Suijlekom-Smit, L.W.; Morris, A.D.; Butler, C.C.; Berger, M.; van der Wouden, J.C. Interventions for impetigo. Cochrane Database Syst. Rev. 2012, 1, CD003261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruby, R.J.; Nelson, J.D. The influence of hexachlorophene scrubs on the response to placebo or penicillin therapy in impetigo. Pediatrics 1973, 52, 854–859. [Google Scholar] [PubMed]
- Christensen, O.B.; Anehus, S. Hydrogen peroxide cream: An alternative to topical antibiotics in the treatment of impetigo contagiosa. Acta Derm. Venereol. 1994, 74, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Population Australia. Mackay Population. 2021. Available online: https://www.population.net.au/mackay-population/ (accessed on 17 May 2021).
- Heal, C.; Buettner, P.; Raasch, B.; Browning, S.; Graham, D.; Bidgood, R.; Campbell, M.; Cruikshank, R. Can sutures get wet? Prospective randomised controlled trial of wound management in general practice. Br. Med. J. 2006, 332, 1053–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heal, C.F.; Buettner, P.; Cruikshank, R.; Graham, D.; Browning, S.; Pendergast, J.; Drobetz, H.; Gluer, R.; Lisec, C. Does single application of topical chloramphenicol to high risk sutured wounds reduce incidence of wound infection after minor surgery? Prospective randomised placebo controlled double blind trial. Br. Med. J. 2009, 338, a2812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Australian Healthy Skin Consortium. National Healthy Skin Guideline for the Prevention, Treatment and Public Health Control of Impetigo, Scabies, Crusted Scabies and Tinea for Indigenous Populations and Communities in Australia, 1st ed.; Telethon Kids Institute: Perth, Australia, 2018. [Google Scholar]
- Bpac. Antibiotics: Choices for Common Infections. 2017. Available online: https://bpac.org.nz/antibiotics/guide.aspx#impetigo (accessed on 21 December 2019).
- Starship. Cellulitis. 2019. Available online: https://www.starship.org.nz/guidelines/cellulitis/ (accessed on 21 December 2019).
- Gorges, H.; Heal, C.; van Driel, M.; Tapley, A.; Davis, J.; Davey, A.; Holiday, E.; Ball, J.; Nashwa, N.; Spike, N.; et al. Prevalence and associations of general practice registrars’ management of impetigo: A cross-sectional analysis from the Registrar Clinical Encounters in Training (ReCEnT) study. Dermatol. Pract. Concept. 2020, 10, e2020043. [Google Scholar] [CrossRef] [PubMed]
- Heal, C.; Gorges, H.; Van Driel, M.L.; Tapley, A.; Davis, J.; Davey, A.; Holliday, L.; Ball, J.; Najib, N.; FitzGerald, K.; et al. Antibiotic stewardship in skin infections: A cross-sectional analysis of early-career GP’s management of impetigo. Br. Med. J. Open 2019, 9, e031527. [Google Scholar] [CrossRef] [PubMed]
- Médecins Sans Frontières. Clinical Guidelines—Diagnosis and Treatment Manual: Impetigo; Médecins Sans Frontières: Geneva, Switzerland, 2019; Available online: https://medicalguidelines.msf.org/viewport/CG/english/impetigo-16689666.html (accessed on 8 January 2021).
- French Infectious Disease Society. Treatment of Common Bacterial Skin Infections; French National Authority for Health; French Society of Dermatology: St-Denis, France, 2019. [Google Scholar]
| Patient 1 | Patient 2 | |
|---|---|---|
| Initial visit (Day 1) | ||
| Intervention | Mupirocin | Soft white paraffin |
| Age (years) | 5 | 4 |
| Gender | Male | Female |
| Occupation | Student | Student |
| Current medications | Nil | Nil |
| Co-morbidities | Nil | Nil |
| Number of lesions | 3 | 3 |
| Size of lesions | Not measured (photograph not taken) | 1 mm × 1 mm |
| Wound swab | Not taken | No growth after 48 h |
| Follow-up visit (Day 6) | ||
| Adherence | 100% (reported verbally) | 100% |
| Number of lesions | 7 | 3 |
| Size of lesions | Larger (not measured, no photograph taken) | 2 mm × 2 mm |
| Adverse outcomes | Nil | Nil |
| Treatment diary | Not completed. Verbally conferred 100% adherence | Completed. 100% adherence |
| Outcome | Lesions worsened. Cephalexin commenced | Lesions ‘more crusty and slightly bigger’. Oral flucloxacillin commenced |
| Theme | Selected Verbal Narrative | |
|---|---|---|
| Barriers to Recruitment | Prior treatment | “parents have Bactroban from older siblings having impetigo and know what to do” (GP 2) |
| GP workload, including impact of COVID-19 | “Sometimes you just forget” (GP 6) “It’s hard to remember when you get busy” (GP 9) | |
| Impact of COVID-19 on infection control and impetigo incidence Impact of COVID-19 on GP attendence | “hand hygiene is being done with kids more” (GP 4) “most people have tried other things and just want definitive treatment” (GP 4) | |
| Improving Trial Delivery | Involving other health practitioners | “child health nurses could see kids sooner” (GP 2) “Pharmacists might be better positioned to see people before the initial treatment” (GP 3) |
| Practice demographics | “practices that aren’t booked out a week in advance” (GP 1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorges, H.; Hall, L.; Heal, C. Feasibility Study for a Randomised Controlled Trial for the Topical Treatment of Impetigo in Australian General Practice. Trop. Med. Infect. Dis. 2021, 6, 197. https://doi.org/10.3390/tropicalmed6040197
Gorges H, Hall L, Heal C. Feasibility Study for a Randomised Controlled Trial for the Topical Treatment of Impetigo in Australian General Practice. Tropical Medicine and Infectious Disease. 2021; 6(4):197. https://doi.org/10.3390/tropicalmed6040197
Chicago/Turabian StyleGorges, Hilary, Leanne Hall, and Clare Heal. 2021. "Feasibility Study for a Randomised Controlled Trial for the Topical Treatment of Impetigo in Australian General Practice" Tropical Medicine and Infectious Disease 6, no. 4: 197. https://doi.org/10.3390/tropicalmed6040197
APA StyleGorges, H., Hall, L., & Heal, C. (2021). Feasibility Study for a Randomised Controlled Trial for the Topical Treatment of Impetigo in Australian General Practice. Tropical Medicine and Infectious Disease, 6(4), 197. https://doi.org/10.3390/tropicalmed6040197






