Risk Factors for Hospitalisation amongst Leptospirosis Patients in New Zealand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Data Categorisation
2.2.1. Diagnostic Status, Travel, and Hospitalisation
2.2.2. Demographic Categories
2.2.3. Putative Risk Factors
2.2.4. Spatial and Temporal Categories
2.3. Statistical Methods
2.3.1. Descriptive Data Analysis
2.3.2. Logistic Regression Model Building
3. Results
3.1. Descriptive Analysis
3.2. Multivariable Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Report of the Second Meeting of the Leptospirosis Burden Epidemiology Reference Group; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl. Trop. Dis. 2015, 9, e0003898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreyfus, A.; Heuer, C.; Wilson, P.; Collins-Emerson, J.; Baker, M.G.; Benschop, J. Risk of infection and associated influenza-like disease among abattoir workers due to two Leptospira species. Epidemiol. Infect. 2015, 143, 2095–2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Notifiable and Other Diseases in New Zealand. Annual Report. 2019. Available online: https://surv.esr.cri.nz/PDF_surveillance/AnnualRpt/AnnualSurv/ (accessed on 20 July 2021).
- Marshall, R.; Chereshsky, A. Vaccination of Dairy Cattle Against Leptospirosis as a Means of Preventing Human Infections; Ministry for Primary Industries: Wellington, New Zealand, 1996; pp. 27–28.
- Thornley, C.N.; Baker, M.G.; Weinstein, P.; Maas, E.W. Changing epidemiology of human leptospirosis in New Zealand. Epidemiol. Infect. 2002, 128, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Nisa, S.; Wilkinson, D.; Angelin-Bonnet, O.; Paine, S.; Cullen, K.; Wright, J.; Baker, M.; Benschop, J. Diverse Epidemiology of Leptospira Serovars Notified in New Zealand, 1999–2017. Pathogens 2020, 9, 841. [Google Scholar] [CrossRef] [PubMed]
- Yupiana, Y.; Wilson, P.; Collins-Emerson, J.; Weston, J.; Benschop, J.; Vallée, E.; Heuer, C. Vaccination practices for Leptospira spp. on New Zealand dairy farms. New Zeal. Vet. J. 2021, 69, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.; Neumann, E.; Brangenberg, N. Diseases of backyard pigs in New Zealand. Surveillance 2018, 45, 5–14. [Google Scholar]
- Sanhueza, J.; Heuer, C.; Wilson, P.; Benschop, J.; Collins-Emerson, J. Seroprevalence and risk factors for leptospira seropositivity in beef cattle, sheep and deer farmers in New Zealand. Zoonoses Public Health 2017, 64, 370–380. [Google Scholar] [CrossRef]
- Adler, B.; Moctezuma, A.D.L.P. Leptospira and leptospirosis. Vet. Microbiol. 2010, 140, 287–296. [Google Scholar] [CrossRef]
- Haake, D.A.; Levett, P.N. Leptospirosis in humans. Curr. Top. Microbiol. Immunol. 2015, 387, 65–97. [Google Scholar]
- Benschop, J.; Collins-Emerson, J.; Maskill, A.; O’Connor, P.; Tunbridge, M.; Yupiana, Y.; Weston, J. Leptospirosis in three workers on a dairy farm with unvaccinated cattle. N. Z. Med. J. 2017, 130, 102–108. [Google Scholar]
- McLean, M.; Ruscoe, Q.; Kline, T.; King, C.; Nesdale, A. A cluster of three cases of leptospirosis in dairy farm workers in New Zealand. N. Z. Med. J. 2014, 127, 13–20. [Google Scholar]
- Vickery, B.; Flynn, S.A.; Calder, L.; Freebairn, R.C. Leptospirosis presenting to an intensive care unit in provincial New Zealand: A case series and review. Crit. Care Resusc. 2006, 8, 192–199. [Google Scholar]
- Earl, L.; Fang, F.; Janes, R.; Gedye, K.; French, N.; Collins-Emerson, J.; Benschop, J. An evaluation of diagnostic tests in a case series of suspected leptospirosis patients seen in primary care. N. Z. Med. J. 2021, 134, 33–43. [Google Scholar]
- Report on New Zealand Cost-of-Illness Studies on Long-Term Conditions. 2009. Available online: https://www.health.govt.nz/system/files/documents/publications/nz-cost-of-illness-jul09.pdf (accessed on 20 July 2021).
- Notifiable Disease Surveillance. 2020. Available online: https://surv.esr.cri.nz/public_health_surveillance/notifiable_disease_surveillance.php (accessed on 20 July 2021).
- Case Report Forms. Notifiable Disease Surveillance. 2020. Available online: https://surv.esr.cri.nz/episurv/crf.php (accessed on 20 July 2021).
- Atkinson, J.; Salmond, C.; Crampton, P. NZDep2013 Index of Deprivation; Department of Public Health, University of Otago: Wellington, New Zealand, 2014. [Google Scholar]
- Statistics New Zealand. Urban Accessibility—Methodology and Classification. 2020. Available online: https://www.stats.govt.nz/assets/Uploads/Methods/Urban-accessibility-methodology-and-classification/Download-document/Urban-accessibility-methodology-and-classification.pdf (accessed on 20 July 2021).
- Australian and New Zealand Standard Classification of Occupations: Australian Bureau of Statistics. 2013; Version 1.2. Available online: https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/1220.02013,%20Version%201.2?OpenDocument (accessed on 20 July 2021).
- District Health Board Boundaries. 2012. Available online: https://koordinates.com/layer/4324-nz-district-health-boards-2012/ (accessed on 20 July 2021).
- Somers, R.H. A new asymmetric measure of association for ordinal variables. Am. Sociol. Rev. 1962, 27, 799–811. [Google Scholar] [CrossRef]
- Dohoo, I.R.; Martin, W.; Stryhn, H.E. Veterinary Epidemiologic Research; Charlottetown, P.E.I., Ed.; University of Prince Edward Island: Charlottetown, PE, Canada, 2003. [Google Scholar]
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle. In Selected Papers of Hirotugu Akaike; Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer: New York, NY, USA, 1998; pp. 199–213. [Google Scholar]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Stevenson, M.; Nunes, T.; Sanchez, J.; Thornton, R.; Reiczigel, J.; Robison-Cox, J.; Sebastiani, P. EpiR: An R Package for the Analysis of Epidemiological Data. 2018. Available online: https://CRAN.R-project.org/package=epiR. (accessed on 20 July 2021).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, L.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Lele, S.; Keim, J.; Solymos, P. ResourceSelection: Resource Selection (Probability) Functions for Use-Availability Data, Version 0.3-0. 2017. Available online: https://cran.r-project.org/web/packages/ResourceSelection (accessed on 20 July 2021).
- Sing, T.; Sander, O.; Beerenwinkel, N.; Lengauer, T. ROCR: Visualizing classifier performance in R. Bioinformatics 2005, 21, 3940–3941. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2. WIREs Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Lüdecke, D. sjPlot: Data Visualization for Statistics in Social Science. 2021. Available online: https://cran.r-project.org/web/packages/sjPlot (accessed on 20 July 2021).
- Pearson, R. Goodman Kruskal: Association Analysis for Categorical Variables. R Package Version 00. 2016. Available online: https://cran.r-project.org/web/packages/GoodmanKruskal (accessed on 20 July 2021).
- Zeileis, A.; Hothorn, T. Diagnostic Checking in Regression Relationships; 2002; Available online: https://cran.r-project.org/web/packages/lmtest.
- Mauri, M.; Elli, T.; Caviglia, G.; Uboldi, G.; Azzi, M. (Eds.) RAWGraphs: A visualisation platform to create open outputs. In Proceedings of the 12th Biannual Conference on Italian SIGCHI Chapter, Cagliari, Italy, 18–20 September 2017; ACM: New York, NY, USA. [Google Scholar]
- Tubiana, S.; Mikulski, M.; Becam, J.; Lacassin, F.; Lefevre, P.; Gourinat, A.C.; Goarant, C.; D’Ortenzio, E. Risk factors and predictors of severe leptospirosis in New Caledonia. PLoS Negl. Trop. Dis. 2013, 7, e1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picardeau, M. Virulence of the zoonotic agent of leptospirosis: Still terra incognita? Nat. Rev. Microbiol. 2017, 15, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Levett, P.N. Leptospirosis. Clin. Microbiol. Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benschop, J.; Nisa, S.; Spencer, S.E.F. Still ‘dairy farm fever’? A Bayesian model for leptospirosis notification data in New Zealand. J. R. Soc. Interface 2021, 18, 20200964. [Google Scholar] [CrossRef] [PubMed]
- Vallée, E.; Ridler, A.L.; Heuer, C.; Collins-Emerson, J.M.; Benschop, J.; Wilson, P.R. Effectiveness of a commercial leptospiral vaccine on urinary shedding in naturally exposed sheep in New Zealand. Vaccine 2017, 35, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Sanhueza, J.M.; Wilson, P.R.; Benschop, J.; Collins-Emerson, J.M.; Heuer, C. Meta-analysis of the efficacy of Leptospira serovar Hardjo vaccines to prevent urinary shedding in cattle. Prev. Vet. Med. 2018, 153, 71–76. [Google Scholar] [CrossRef]
- Sanhueza, J.M.; Baker, M.G.; Benschop, J.; Collins-Emerson, J.M.; Wilson, P.R.; Heuer, C. Estimation of the burden of leptospirosis in New Zealand. Zoonoses Public Health 2020, 67, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Subharat, S.; Wilson, P.; Heuer, C.; Collins-Emerson, J. Longitudinal serological survey and herd-level risk factors for Leptospira spp. serovars Hardjo-bovis and Pomona on deer farms with sheep and/or beef cattle. N. Z. Vet. J. 2012, 60, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Dreyfus, A.; Wilson, P.; Benschop, J.; Collins-Emerson, J.; Verdugo, C.; Heuer, C. Seroprevalence and herd-level risk factors for seroprevalence of Leptospira spp. in sheep, beef cattle and deer in New Zealand. N. Z. Vet. J. 2018, 66, 302–311. [Google Scholar] [CrossRef]
- Wilson, P.R.; Mannewald, A.; Collins-Emerson, J.M.; Dreyfus, A.; Sanhueza, J.M.; Benschop, J.; Verdugo, C.; Emanuelson, U.; Boqvist, S.; Heuer, C. Serological study of Leptospira interrogans serovar Copenhageni and L. borgpetersenii serovars Tarassovi and Ballum in beef cattle, sheep and deer in New Zealand. N. Z. Vet. J. 2021, 69, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Boey, K.; Shiokawa, K.; Rajeev, S. Leptospira infection in rats: A literature review of global prevalence and distribution. PLoS Negl. Trop. Dis. 2019, 13, e0007499. [Google Scholar] [CrossRef]
- Hathaway, S.; Blackmore, D.; Marshall, R. Leptospirosis in free-living species in New Zealand. J. Wildl. Dis. 1981, 17, 489–496. [Google Scholar] [CrossRef]
- Lau, C.L.; Townell, N.; Stephenson, E.; Craig, S.B. Leptospirosis: An important zoonosis acquired through work, play and travel. Aust. J. Gen. Pract. 2018, 47, 105. [Google Scholar] [CrossRef] [Green Version]
- Sakundarno, M.; Bertolatti, D.; Maycock, B.; Spickett, J.; Dhaliwal, S. Risk Factors for Leptospirosis Infection in Humans and Implications for Public Health Intervention in Indonesia and the Asia-Pacific Region. Asia Pac. J. Public Health 2013, 26, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Brew, B.; Inder, K.; Allen, J.; Thomas, M.; Kelly, B. The health and wellbeing of Australian farmers: A longitudinal cohort study. BMC Public Health 2016, 16, 988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarlatti. Health and Wellness of New Zealand Farmers. Growing NZ. 2017. Available online: https://www.growingnz.org.nz/health-and-wellness-of-new-zealand-farmers_Initial-findings-from-research_Into-the-wellness-of-and-wellness-behaviour-patterns-of-new-zealand-farmers-research_paper-27.html (accessed on 20 July 2021).
- Gostic, K.M.; Wunder, E.A., Jr.; Bisht, V.; Hamond, C.; Julian, T.R.; Ko, A.I.; Lloyd-Smith, J.O. Mechanistic dose–response modelling of animal challenge data shows that intact skin is a crucial barrier to leptospiral infection. Philos. Trans. R. Soc. B 2019, 374, 20190367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barragan, V.; Nieto, N.; Keim, P.; Pearson, T. Meta-analysis to estimate the load of Leptospira excreted in urine: Beyond rats as important sources of transmission in low-income rural communities. BMC Res. Notes 2017, 10, 71. [Google Scholar] [CrossRef] [Green Version]
- Edwards, C.N.; Nicholson, G.D.; Hassell, T.A.; Everard, C.O.; Callender, J. Penicillin therapy in icteric leptospirosis. Am. J. Trop. Med. Hyg. 1988, 39, 388–390. [Google Scholar] [CrossRef]
- Watt, G.; Tuazon, M.L.; Santiago, E.; Padre, L.; Calubaquib, C.; Ranoa, C.; Laughlin, L. Placebo-controlled trial of intravenous penicillin for severe and late leptospirosis. Lancet 1988, 331, 433–435. [Google Scholar] [CrossRef]
- Benschop, J. Emerging Sources and Pathways for Leptospirosis: A Paradigm Shift. Unpublished Raw Data. 2020. [Google Scholar]
- Buswell, K. Leptospirosis: A General Practitioner Perspective [Power Point Slides]; Massey University: Wellington, New Zealand, 2017; Available online: https://leptospirosis.org.nz/Portals/0/Images/News/Leptoforum_2017_Keith%20Buswell.pdf (accessed on 20 July 2021).
- Newall, A.T.; MacIntyre, R.; Wang, H.; Hull, B.; Macartney, K. Burden of severe rotavirus disease in Australia. J. Paediatr. Child Health 2006, 42, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Goris, M.G.; Boer, K.R.; Duarte, T.A.; Kliffen, S.J.; Hartskeerl, R.A. Human leptospirosis trends, the Netherlands, 1925–2008. Emerg. Infect. Dis. 2013, 19, 371. [Google Scholar] [CrossRef]
- Levett, P.N. Usefulness of serologic analysis as a predictor of the infecting serovar in patients with severe leptospirosis. Clin. Infect. Dis. 2003, 36, 447–452. [Google Scholar] [CrossRef]
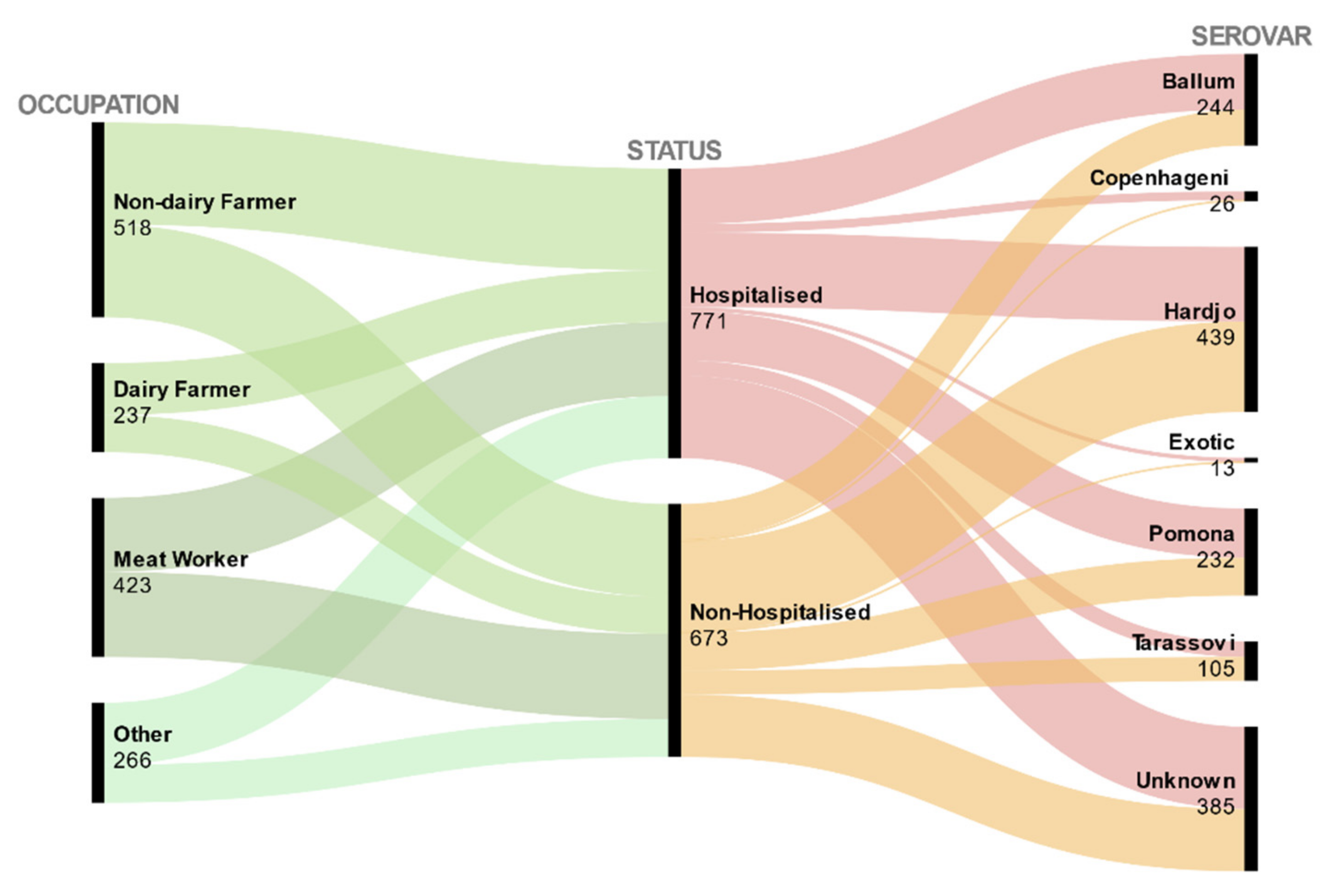
| Hospitalised (N = 771) | Non-Hospitalised (N 1 = 673) | Hospitalisation Rate | Bivariate Analysis Results | |||||
|---|---|---|---|---|---|---|---|---|
| Demographic Variables | Number (n) | % (n/N) | Number (n 1) | % (n 1/N 1) | n/(n + n 1) | β-Coefficient | Crude OR (95% CI) | p |
| Age | ||||||||
| Young (0–36) | 255 | 33 | 237 | 35 | 0.52 | Baseline | ||
| Middle Age (37–49) | 247 | 32 | 238 | 35 | 0.51 | −0.04 | 0.96 (0.75, 1.24) | 0.78 |
| Senior (>50) | 269 | 35 | 198 | 29 | 0.58 | 0.23 | 1.26 (0.98, 1.62) | 0.07 |
| Sex | ||||||||
| Female | 75 | 10 | 62 | 9 | 0.547 | Baseline | ||
| Male | 696 | 90 | 611 | 91 | 0.533 | −0.06 | 0.94 (0.66, 1.34) | 0.73 |
| Ethnicity | ||||||||
| European and Other | 590 | 77 | 509 | 76 | 0.54 | Baseline | ||
| Māori and Pacific Peoples | 145 | 19 | 111 | 16 | 0.57 | 0.12 | 1.13 (0.85, 1.48) | 0.39 |
| Unknown | 36 | 5 | 53 | 8 | 0.40 | −0.53 | 0.59 (0.38, 0.91) | 0.02 * |
| Deprivation Status | ||||||||
| Most Deprived | 241 | 31 | 233 | 35 | 0.51 | Baseline | ||
| Least Deprived | 167 | 22 | 105 | 16 | 0.61 | 0.43 | 1.54 (1.14, 2.08) | 0.01 * |
| Moderately Deprived | 222 | 29 | 174 | 26 | 0.56 | 0.21 | 1.23 (0.94, 1.62) | 0.12 |
| Unknown | 141 | 18 | 161 | 24 | 0.47 | −0.17 | 0.85 (0.63, 1.13) | 0.26 |
| Rurality | ||||||||
| Rural | 396 | 51 | 322 | 48 | 0.55 | Baseline | ||
| Unknown | 68 | 9 | 68 | 10 | 0.50 | −0.29 | 0.75 (0.52, 1.07) | 0.11 |
| Urban | 307 | 40 | 277 | 41 | 0.53 | −0.1 | 0.90 (0.72, 1.12) | 0.35 |
| Hospitalised (N = 771) | Non-Hospitalised (N = 673 1) | Hospitalisation Rate | Bivariate Analysis Results | |||||
|---|---|---|---|---|---|---|---|---|
| Putative Risk Factors | Number (n) | % (n/N) | Number (n 1) | % (n 1/N 1) | n/(n + n 1) | β-Coefficient | Crude OR (95% CI) | p |
| Serovar | ||||||||
| Ballum | 148 | 19 | 96 | 14 | 0.61 | Baseline | ||
| Exotic | 11 | 1 | 2 | 0.3 | 0.85 | 1.27 | 3.57 (0.77, 16.44) | 0.1 |
| Copenhageni | 23 | 3 | 3 | 0.4 | 0.88 | 1.6 | 4.98 (1.45, 17.02) | 0.01 * |
| Hardjo | 198 | 26 | 241 | 36 | 0.45 | −0.63 | 0.53 (0.39, 0.73) | <0.01 * |
| Pomona | 131 | 17 | 101 | 15 | 0.56 | −0.17 | 0.84 (0.58, 1.21) | 0.35 |
| Tarassovi | 41 | 5 | 64 | 10 | 0.39 | −0.87 | 0.42 (0.26, 0.66) | <0.01 * |
| Unknown | 219 | 28 | 166 | 25 | 0.57 | −0.16 | 0.85 (0.61, 1.19) | 0.35 |
| Occupation | ||||||||
| Non-Dairy Farmer | 271 | 35 | 247 | 37 | 0.52 | Baseline | ||
| Dairy Farmer | 138 | 18 | 99 | 14 | 0.58 | 0.24 | 1.27 (0.93, 1.73) | 0.13 |
| Meat worker | 197 | 25 | 226 | 34 | 0.47 | −0.23 | 0.79 (0.61, 1.02) | 0.08 |
| Other | 165 | 21 | 101 | 15 | 0.62 | 0.4 | 1.49 (1.10, 2.01) | <0.01 * |
| ROE | ||||||||
| No | 126 | 16 | 74 | 11 | 0.63 | Baseline | ||
| Unknown | 89 | 12 | 73 | 11 | 0.55 | −0.33 | 0.72 (0.47, 1.09) | 0.12 |
| Yes | 556 | 72 | 526 | 78 | 0.51 | −0.48 | 0.62 (0.45, 0.85) | <0.01 * |
| Exposure to Animals | ||||||||
| No | 40 | 5 | 30 | 4 | 0.57 | Baseline | ||
| Unknown | 51 | 7 | 36 | 5 | 0.59 | 0.06 | 1.06 (0.56, 2.01) | 0.85 |
| Yes | 680 | 88 | 607 | 90 | 0.53 | −0.17 | 0.84 (0.52, 1.37) | 0.48 |
| Water Exposure | ||||||||
| No | 616 | 80 | 535 | 79 | 0.54 | Baseline | ||
| Unknown | 155 | 20 | 138 | 21 | 0.53 | −0.02 | 0.98 (0.75, 1.26) | 0.85 |
| Hospitalised (N = 771) | Non-Hospitalised (N = 673 1) | Hospitalisation Rate | Bivariate Analysis Results | |||||
|---|---|---|---|---|---|---|---|---|
| Spatial and Temporal Variables | Number (n) | % (n/N) | Number (n 1) | % (n 1/N 1) | n/(n + n 1) | β-Coefficient | Crude OR (95% CI) | p |
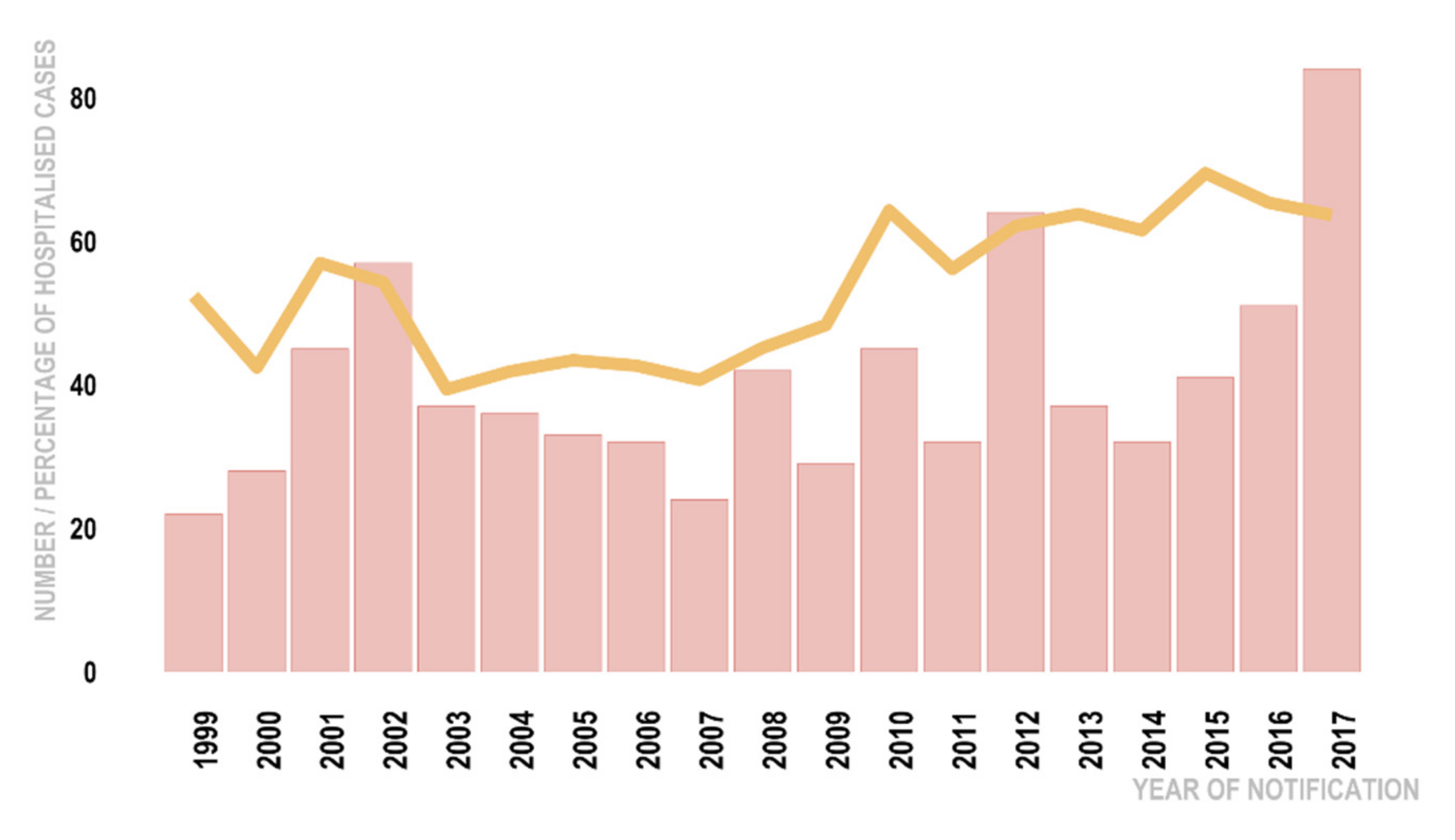
| Report Year | 0.06 | 1.06 (1.03, 1.08) | <0.01 * | |||||
| Season | ||||||||
| Autumn | 215 | 28 | 174 | 26 | 0.55 | Baseline | ||
| Spring | 160 | 21 | 196 | 29 | 0.45 | −0.41 | 0.66 (0.49, 0.88) | <0.01 * |
| Summer | 203 | 26 | 152 | 23 | 0.57 | 0.08 | 1.08 (0.81, 1.44) | 0.6 |
| Winter | 193 | 25 | 151 | 22 | 0.56 | 0.03 | 1.03 (0.77, 1.38) | 0.82 |
| Geographical Location | ||||||||
| Lower North Island | 321 | 42 | 284 | 42 | 0.53 | Baseline | ||
| South Island | 162 | 21 | 189 | 28 | 0.46 | −0.28 | 0.76 (0.58, 0.99) | 0.04 * |
| Upper North Island | 288 | 37 | 200 | 30 | 0.59 | 0.24 | 1.27 (1.00, 1.62) | 0.05 * |
| Variable | β-Coefficient | SE | Adj. OR (95% CI) | p |
|---|---|---|---|---|
| Age (Years) | ||||
| Young (0–36) | Baseline | |||
| Middle Age (37–49) | −0.01 | 0.14 | 0.99 (0.76,1.29) | 0.96 |
| Senior (>50) | 0.13 | 0.14 | 1.14 (0.86,1.51) | 0.36 |
| Ethnicity | ||||
| European and Other | Baseline | |||
| Māori and Pacific Peoples | 0.33 | 0.18 | 1.39 (0.99,1.96) | 0.06 * |
| Unknown | −0.36 | 0.24 | 0.70 (0.43,1.12) | 0.13 |
| Deprivation Status | ||||
| Most Deprived | Baseline | |||
| Least Deprived | 0.49 | 0.18 | 1.64 (1.15,2.33) | 0.01 * |
| Moderately Deprived | 0.26 | 0.16 | 1.30 (0.95,1.78) | 0.1 |
| Unknown | −0.03 | 0.18 | 0.97 (0.69,1.38) | 0.88 |
| Serovar | ||||
| Ballum | Baseline | |||
| Exotic | 1.21 | 0.79 | 3.37 (0.71,15.97) | 0.13 |
| Copenhageni | 1.78 | 0.65 | 5.96 (1.68,21.17) | 0.01 * |
| Hardjo | −0.35 | 0.18 | 0.71 (0.49,1.01) | 0.05 * |
| Pomona | 0.13 | 0.22 | 1.14 (0.74,1.74) | 0.56 |
| Tarassovi | −0.94 | 0.27 | 0.39 (0.23,0.66) | <0.01 * |
| Unknown | −0.03 | 0.18 | 0.97 (0.68,1.39) | 0.88 |
| Occupation | ||||
| Non-Dairy Farmer | Baseline | |||
| Dairy Farmer | 0.36 | 0.17 | 1.44 (1.02,2.02) | 0.04 * |
| Meat Worker | −0.15 | 0.17 | 0.86 (0.61,1.21) | 0.38 |
| Other | 0.16 | 0.19 | 1.17 (0.82,1.69) | 0.39 |
| ROE | ||||
| No | Baseline | |||
| Unknown | 1.14 | 0.24 | 0.87 (0.54,1.39) | 0.55 |
| Yes | −0.06 | 0.19 | 0.94 (0.64,1.39) | 0.76 |
| Geographical Location | ||||
| Lower North Island | Baseline | |||
| South Island | −0.63 | 0.27 | 0.53 (0.31,0.91) | 0.02 * |
| Upper North Island | −0.23 | 0.26 | 0.79 (0.64,1.39) | 0.38 |
| Report Year | 0.03 | 0.01 | 1.03 (1.01,1.05) | 0.01 * |
| Season | ||||
| Autumn | Baseline | |||
| Winter | −0.38 | 0.24 | 0.68 (0.42,1.10) | 0.12 |
| Spring | −0.75 | 0.24 | 0.47 (0.29,0.76) | <0.01 * |
| Summer | 0.01 | 0.25 | 1.01 (0.63,1.64) | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolova, M.; Marshall, J.C.; Benschop, J. Risk Factors for Hospitalisation amongst Leptospirosis Patients in New Zealand. Trop. Med. Infect. Dis. 2021, 6, 188. https://doi.org/10.3390/tropicalmed6040188
Sokolova M, Marshall JC, Benschop J. Risk Factors for Hospitalisation amongst Leptospirosis Patients in New Zealand. Tropical Medicine and Infectious Disease. 2021; 6(4):188. https://doi.org/10.3390/tropicalmed6040188
Chicago/Turabian StyleSokolova, Maryna, Jonathan C. Marshall, and Jackie Benschop. 2021. "Risk Factors for Hospitalisation amongst Leptospirosis Patients in New Zealand" Tropical Medicine and Infectious Disease 6, no. 4: 188. https://doi.org/10.3390/tropicalmed6040188
APA StyleSokolova, M., Marshall, J. C., & Benschop, J. (2021). Risk Factors for Hospitalisation amongst Leptospirosis Patients in New Zealand. Tropical Medicine and Infectious Disease, 6(4), 188. https://doi.org/10.3390/tropicalmed6040188






