Mediterranean Spotted Fever: Current Knowledge and Recent Advances
Abstract
1. Introduction
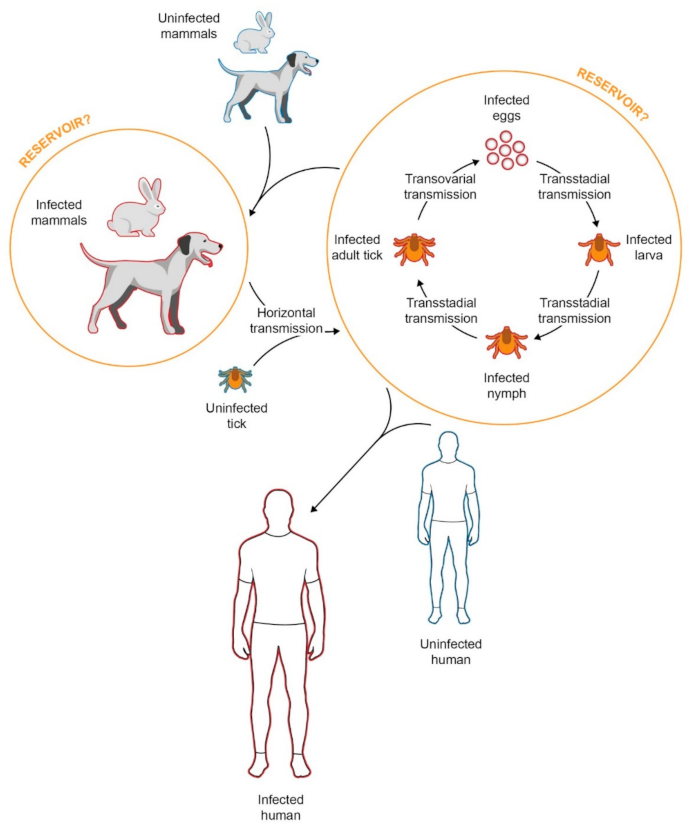
2. Epidemiology
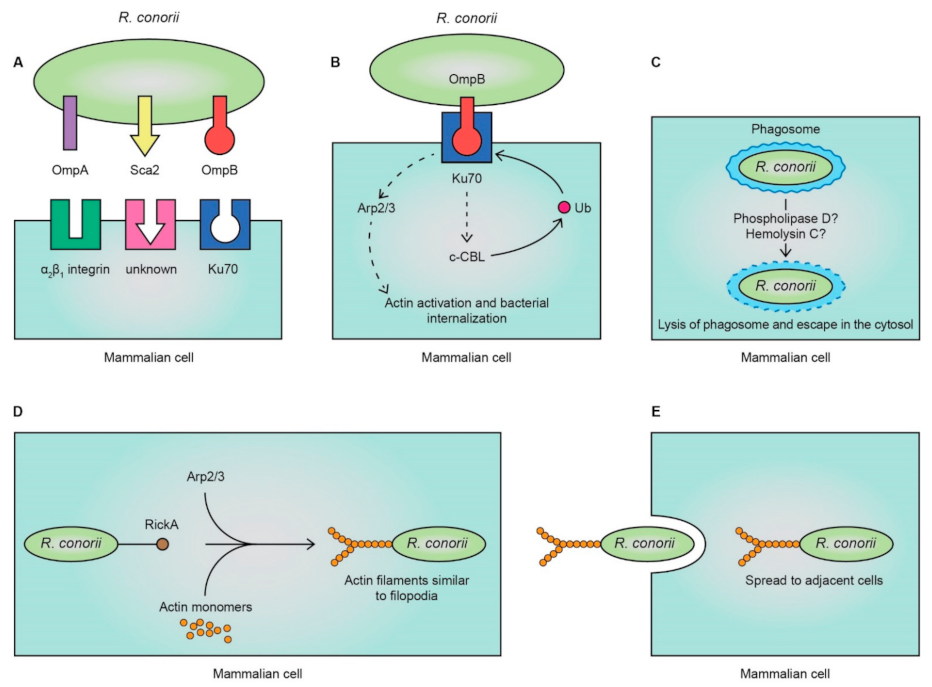
3. Pathogenesis
4. Clinical Features
5. Diagnosis
6. Treatment
7. Prevention
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Rovery, C.; Brouqui, P.; Raoult, D. Questions on Mediterranean spotted fever a century after its discovery. Emerg. Infect. Dis. 2008, 14, 1360–1367. [Google Scholar] [CrossRef]
- Paris, D.H.; Day, N.P.J. Tropical rickettsial infections. In Manson’s Tropical Diseases; Farrar, J., Hotez, P.J., Junghanss, T., Kang, G., Lalloo, D., White, N.J., Eds.; Elsevier Saunders: Edinburgh, UK, 2014; pp. 273–291. [Google Scholar]
- Delord, M.; Socolovschi, C.; Parola, P. Rickettsioses and Q fever in travelers (2004–2013). Travel Med. Infect. Dis. 2014, 12, 443–458. [Google Scholar] [CrossRef]
- Jensenius, M.; Fournier, P.E.; Raoult, D. Tick-borne rickettsioses in international travellers. Int. J. Infect. Dis. 2004, 8, 139–146. [Google Scholar] [CrossRef]
- Parola, P.; Raoult, D. Ticks and tickborne bacterial diseases in humans: An emerging infectious threat. Clin. Infect. Dis. 2001, 32, 897–928. [Google Scholar] [CrossRef]
- Raoult, D.; Roux, V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 1997, 10, 694–719. [Google Scholar] [CrossRef] [PubMed]
- Weinert, L.A.; Werren, J.H.; Aebi, A.; Stone, G.N.; Jiggins, F.M. Evolution and diversity of Rickettsia bacteria. BMC Biol. 2009, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.K.; Narra, H.P.; Sahni, A.; Walker, D.H. Recent molecular insights into rickettsial pathogenesis and immunity. Future Microbiol. 2013, 8, 1265–1288. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.; Beth-Din, A.; Cohen, R.; Lazar, S.; Glinert, I.; Zayyad, H.; Atiya-Nasagi, Y. New spotted fever group rickettsia isolate, identified by sequence analysis of conserved genomic regions. Pathogens 2019, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Audic, S.; Renesto-Audiffren, P.; Fournier, P.E.; Barbe, V.; Samson, D.; Roux, V.; Cossart, P.; Weissenbach, J.; Claverie, J.M.; et al. Mechanisms of evolution in Rickettsia conorii and R. prowazekii. Science 2001, 293, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Fournier, P.E.; Eremeeva, M.; Raoult, D. Proposal to create subspecies of Rickettsia conorii based on multi-locus sequence typing and an emended description of Rickettsia conorii. BMC Microbiol. 2005, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.; Kumar, D.; Budachetri, K. Recent advances in understanding tick and rickettsiae interactions. Parasite Immunol. 2021, 43, e12830. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.H.; Ismail, N. Emerging and re-emerging rickettsioses: Endothelial cell infection and early disease events. Nat. Rev. Microbiol. 2008, 6, 375–386. [Google Scholar] [CrossRef]
- Parola, P.; Socolovschi, C.; Raoult, D. Deciphering the relationships between Rickettsia conorii conorii and Rhipicephalus sanguineus in the ecology and epidemiology of Mediterranean spotted fever. Ann. N. Y. Acad. Sci. 2009, 1166, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Socolovschi, C.; Gaudart, J.; Bitam, I.; Huynh, T.P.; Raoult, D.; Parola, P. Why are there so few Rickettsia conorii conorii-infected Rhipicephalus sanguineus ticks in the wild? PLoS Negl. Trop. Dis. 2012, 6, e1697. [Google Scholar] [CrossRef]
- Raoult, D.; Dupont, H.T.; Chicheportiche, C.; Peter, O.; Gilot, B.; Drancourt, M. Mediterranean spotted fever in Marseille, France: Correlation between prevalence of hospitalized patients, seroepidemiology, and prevalence of infected ticks in three different areas. Am. J. Trop. Med. Hyg. 1993, 48, 249–256. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Pérez-Sánchez, R.; Alamo-Sanz, R.; Encinas-Grandes, A. Spotted fever group rickettsiae in ticks feeding on humans in northwestern Spain: Is Rickettsia conorii vanishing? Ann. N. Y. Acad. Sci. 2006, 1078, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Marquez, F.J.; Rodriguez-Liebana, J.J.; Soriguer, R.C.; Muniain, M.A.; Bernabeu-Wittel, M.; Caruz, A.; Contreras-Chova, F. Spotted fever group Rickettsia in brown dog ticks Rhipicephalus sanguineus in southwestern Spain. Parasitol. Res. 2008, 103, 119–122. [Google Scholar] [CrossRef]
- Segura-Porta, F.; Diestre-Ortin, G.; Ortuno-Romero, A.; Sanfeliu-Sala, I.; Font-Creus, B.; Munoz-Espin, T.; de Antonio, E.M.; Casal-Fabrega, J. Prevalence of antibodies to spotted fever group rickettsiae in human beings and dogs from and endemic area of mediterranean spotted fever in Catalonia, Spain. Eur. J. Epidemiol. 1998, 14, 395–398. [Google Scholar] [CrossRef]
- Alexandre, N.; Santos, A.S.; Bacellar, F.; Boinas, F.J.; Núncio, M.S.; de Sousa, R. Detection of Rickettsia conorii strains in Portuguese dogs (Canis familiaris). Ticks Tick-Borne Dis. 2011, 2, 119–122. [Google Scholar] [CrossRef]
- Kelly, P.J.; Matthewman, L.A.; Mason, P.R.; Courtney, S.; Katsande, C.; Rukwava, J. Experimental infection of dogs with a Zimbabwean strain of Rickettsia conorii. J. Trop. Med. Hyg. 1992, 95, 322–326. [Google Scholar]
- Le Gac, P. Repercussions of myxomatosis on Mediterranean boutonneuse exanthematic fever. Bull. World Health Organ. 1966, 35, 143–147. [Google Scholar] [PubMed]
- De Sousa, R.; Nobrega, S.D.; Bacellar, F.; Torgal, J. Mediterranean spotted fever in Portugal: Risk factors for fatal outcome in 105 hospitalized patients. Ann. N. Y. Acad. Sci. 2003, 990, 285–294. [Google Scholar] [CrossRef]
- Vitale, G.; Mansuelo, S.; Rolain, J.M.; Raoult, D. Rickettsia massiliae human isolation. Emerg. Infect. Dis. 2006, 12, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Colomba, C.; Trizzino, M.; Giammanco, A.; Bonura, C.; Di Bona, D.; Tolomeo, M.; Cascio, A. Israeli Spotted Fever in Sicily. Description of two cases and minireview. Int. J. Infect. Dis. 2017, 61, 7–12. [Google Scholar] [CrossRef]
- Guccione, C.; Colomba, C.; Tolomeo, M.; Trizzino, M.; Iaria, C.; Cascio, A. Rickettsiales in Italy. Pathogens 2021, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Pascucci, I.; Di Domenico, M.; Curini, V.; Cocco, A.; Averaimo, D.; D’Alterio, N.; Cammà, C. Diversity of Rickettsia in ticks collected in Abruzzi and Molise regions (central Italy). Microorganisms 2019, 7, 696. [Google Scholar] [CrossRef]
- Gilot, B.; Laforge, M.L.; Pichot, J.; Raoult, D. Relationships between the Rhipicephalus sanguineus complex ecology and Mediterranean spotted fever epidemiology in France. Eur. J. Epidemiol. 1990, 6, 357–362. [Google Scholar] [CrossRef]
- Raoult, D.; Tissot Dupont, H.; Caraco, P.; Brouqui, P.; Drancourt, M.; Charrel, C. Mediterranean spotted fever in Marseille: Descriptive epidemiology and the influence of climatic factors. Eur. J. Epidemiol. 1992, 8, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.E.; et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef]
- Parola, P.; Socolovschi, C.; Jeanjean, L.; Bitam, I.; Fournier, P.E.; Sotto, A.; Labauge, P.; Raoult, D. Warmer weather linked to tick attack and emergence of severe rickettsioses. PLoS Negl. Trop. Dis. 2008, 2, e338. [Google Scholar] [CrossRef]
- Rovery, C.; Raoult, D. Mediterranean spotted fever. Infect. Dis. Clin. N. Am. 2008, 22, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, K.R.; Sonenshine, D.E.; Ceraul, S.M.; Azad, A.F. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J. Med. Entomol. 2002, 39, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.R.; Spencer, R.R. Rocky mountain spotted fever: A study of the relationship between the presence of Rickettsia-like organisms in tick smears and the infectiveness of the same ticks. In Public Health Reports (1896–1970); Sage Publications, Inc.: Thousand Oaks, CA, USA, 1926; Volume 41, pp. 461–469. [Google Scholar] [CrossRef]
- Chan, Y.G.; Riley, S.P.; Martinez, J.J. Adherence to and invasion of host cells by spotted fever group Rickettsia species. Front. Microbiol. 2010, 1, 139. [Google Scholar] [CrossRef]
- Cardwell, M.M.; Martinez, J.J. The Sca2 autotransporter protein from Rickettsia conorii is sufficient to mediate adherence to and invasion of cultured mammalian cells. Infect. Immun. 2009, 77, 5272–5280. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.J.; Seveau, S.; Veiga, E.; Matsuyama, S.; Cossart, P. Ku70, a component of DNA-dependent protein kinase, is a mammalian receptor for Rickettsia conorii. Cell 2005, 123, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Blanc, G.; Ngwamidiba, M.; Ogata, H.; Fournier, P.-E.; Claverie, J.-M.; Raoult, D. Molecular evolution of Rickettsia surface antigens: Evidence of positive selection. Mol. Biol. Evol. 2005, 22, 2073–2083. [Google Scholar] [CrossRef] [PubMed]
- Blanda, V.; D’Agostino, R.; Giudice, E.; Randazzo, K.; La Russa, F.; Villari, S.; Vullo, S.; Torina, A. New real-time PCRs to differentiate Rickettsia spp. and Rickettsia conorii. Molecules 2020, 25, 4431. [Google Scholar] [CrossRef]
- Monferran, S.; Muller, C.; Mourey, L.; Frit, P.; Salles, B. The membrane-associated form of the DNA repair protein Ku is involved in cell adhesion to fibronectin. J. Mol. Biol. 2004, 337, 503–511. [Google Scholar] [CrossRef]
- Lucero, H.; Gae, D.; Taccioli, G.E. Novel localization of the DNA-PK complex in lipid rafts: A putative role in the signal transduction pathway of the ionizing radiation response. J. Biol. Chem. 2003, 278, 22136–22143. [Google Scholar] [CrossRef]
- Chan, Y.G.; Cardwell, M.M.; Hermanas, T.M.; Uchiyama, T.; Martinez, J.J. Rickettsial outer-membrane protein B (rOmpB) mediates bacterial invasion through Ku70 in an actin, c-Cbl, clathrin and caveolin 2-dependent manner. Cell. Microbiol. 2009, 11, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.J.; Cossart, P. Early signaling events involved in the entry of Rickettsia conorii into mammalian cells. J. Cell Sci. 2004, 117, 5097–5106. [Google Scholar] [CrossRef]
- Riley, S.P.; Patterson, J.L.; Martinez, J.J. The rickettsial OmpB β-peptide of Rickettsia conorii is sufficient to facilitate factor H-mediated serum resistance. Infect. Immun. 2012, 80, 2735–2743. [Google Scholar] [CrossRef]
- Hillman, R.D., Jr.; Baktash, Y.M.; Martinez, J.J. OmpA-mediated rickettsial adherence to and invasion of human endothelial cells is dependent upon interaction with alpha2beta1 integrin. Cell. Microbiol. 2013, 15, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Sahni, A.; Patel, J.; Narra, H.P.; Schroeder, C.L.C.; Walker, D.H.; Sahni, S.K. Fibroblast growth factor receptor-1 mediates internalization of pathogenic spotted fever rickettsiae into host endothelium. PLoS ONE 2017, 12, e0183181. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, S.; Troyer, J.M.; Beier, M.S.; Lau, A.O.; Azad, A.F. Identification and molecular analysis of the gene encoding Rickettsia typhi hemolysin. Infect. Immun. 1999, 67, 6104–6108. [Google Scholar] [CrossRef]
- Renesto, P.; Dehoux, P.; Gouin, E.; Touqui, L.; Cossart, P.; Raoult, D. Identification and characterization of a phospholipase D-superfamily gene in Rickettsiae. J. Infect. Dis. 2003, 188, 1276–1283. [Google Scholar] [CrossRef]
- Whitworth, T.; Popov, V.L.; Yu, X.J.; Walker, D.H.; Bouyer, D.H. Expression of the Rickettsia prowazekii pld or tlyC gene in Salmonella enterica serovar typhimurium mediates phagosomal escape. Infect. Immun. 2005, 73, 6668–6673. [Google Scholar] [CrossRef]
- Gouin, E.; Egile, C.; Dehoux, P.; Villiers, V.; Adams, J.; Gertler, F.; Li, R.; Cossart, P. The RickA protein of Rickettsia conorii activates the Arp2/3 complex. Nature 2004, 427, 457–461. [Google Scholar] [CrossRef]
- Heinzen, R.A.; Grieshaber, S.S.; Van Kirk, L.S.; Devin, C.J. Dynamics of actin-based movement by Rickettsia rickettsii in vero cells. Infect. Immun. 1999, 67, 4201–4207. [Google Scholar] [CrossRef]
- Van Kirk, L.S.; Hayes, S.F.; Heinzen, R.A. Ultrastructure of Rickettsia rickettsii actin tails and localization of cytoskeletal proteins. Infect. Immun. 2000, 68, 4706–4713. [Google Scholar] [CrossRef]
- Walker, D.H.; Gear, J.H. Correlation of the distribution of Rickettsia conorii, microscopic lesions, and clinical features in South African tick bite fever. Am. J. Trop. Med. Hyg. 1985, 34, 361–371. [Google Scholar] [CrossRef]
- Osterloh, A. Immune response against rickettsiae: Lessons from murine infection models. Med. Microbiol. Immunol. 2017, 206, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Rydkina, E.; Sahni, A.; Baggs, R.B.; Silverman, D.J.; Sahni, S.K. Infection of human endothelial cells with spotted fever group rickettsiae stimulates cyclooxygenase 2 expression and release of vasoactive prostaglandins. Infect. Immun. 2006, 74, 5067–5074. [Google Scholar] [CrossRef]
- Mansueto, P.; Vitale, G.; Cascio, A.; Seidita, A.; Pepe, I.; Carroccio, A.; di Rosa, S.; Rini, G.B.; Cillari, E.; Walker, D.H. New insight into immunity and immunopathology of Rickettsial diseases. Clin. Dev. Immunol. 2012, 2012, 967852. [Google Scholar] [CrossRef]
- Sporn, L.A.; Sahni, S.K.; Lerner, N.B.; Marder, V.J.; Silverman, D.J.; Turpin, L.C.; Schwab, A.L. Rickettsia rickettsii infection of cultured human endothelial cells induces NF-kappaB activation. Infect. Immun. 1997, 65, 2786–2791. [Google Scholar] [CrossRef]
- Sahni, S.K.; Van Antwerp, D.J.; Eremeeva, M.E.; Silverman, D.J.; Marder, V.J.; Sporn, L.A. Proteasome-independent activation of nuclear factor kappaB in cytoplasmic extracts from human endothelial cells by Rickettsia rickettsii. Infect. Immun. 1998, 66, 1827–1833. [Google Scholar] [CrossRef]
- Rydkina, E.; Silverman, D.J.; Sahni, S.K. Activation of p38 stress-activated protein kinase during Rickettsia rickettsii infection of human endothelial cells: Role in the induction of chemokine response. Cell. Microbiol. 2005, 7, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. The IL-1 family and inflammatory diseases. Clin. Exp. Rheumatol. 2002, 20, S1–S13. [Google Scholar] [PubMed]
- Kaplanski, G.; Teysseire, N.; Farnarier, C.; Kaplanski, S.; Lissitzky, J.C.; Durand, J.M.; Soubeyrand, J.; Dinarello, C.A.; Bongrand, P. IL-6 and IL-8 production from cultured human endothelial cells stimulated by infection with Rickettsia conorii via a cell-associated IL-1 alpha-dependent pathway. J. Clin. Investig. 1995, 96, 2839–2844. [Google Scholar] [CrossRef]
- Colonne, P.M.; Eremeeva, M.E.; Sahni, S.K. Beta interferon-mediated activation of signal transducer and activator of transcription protein 1 interferes with Rickettsia conorii replication in human endothelial cells. Infect. Immun. 2011, 79, 3733–3743. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Valbuena, G.; Walker, D.H.; Gazi, M.; Hidalgo, M.; DeSousa, R.; Oteo, J.A.; Goez, Y.; Brasier, A.R. Endothelial cell proteomic response to Rickettsia conorii infection reveals activation of the Janus Kinase (JAK)-Signal Transducer and Activator of Transcription (STAT)-Inferferon Stimulated Gene (ISG)15 pathway and reprogramming plasma membrane integrin/cadherin signaling. Mol. Cell. Proteom. MCP 2016, 15, 289–304. [Google Scholar]
- Feng, H.M.; Walker, D.H. Mechanisms of intracellular killing of Rickettsia conorii in infected human endothelial cells, hepatocytes, and macrophages. Infect. Immun. 2000, 68, 6729–6736. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.M.; Popov, V.L.; Walker, D.H. Depletion of gamma interferon and tumor necrosis factor alpha in mice with Rickettsia conorii-infected endothelium: Impairment of rickettsicidal nitric oxide production resulting in fatal, overwhelming rickettsial disease. Infect. Immun. 1994, 62, 1952–1960. [Google Scholar] [CrossRef] [PubMed]
- Narra, H.P.; Sahni, A.; Khanipov, K.; Fofanov, Y.; Sahni, S.K. Global transcriptomic profiling of pulmonary gene expression in an experimental murine model of Rickettsia conorii infection. Genes 2019, 10, 204. [Google Scholar] [CrossRef]
- Jordan, J.M.; Woods, M.E.; Feng, H.M.; Soong, L.; Walker, D.H. Rickettsiae-stimulated dendritic cells mediate protection against lethal rickettsial challenge in an animal model of spotted fever rickettsiosis. J. Infect. Dis. 2007, 196, 629–638. [Google Scholar] [CrossRef]
- Curto, P.; Santa, C.; Allen, P.; Manadas, B.; Simões, I.; Martinez, J.J. A pathogen and a non-pathogen spotted fever group rickettsia trigger differential proteome signatures in macrophages. Front. Cell. Infect. Microbiol. 2019, 9, 43. [Google Scholar] [CrossRef]
- Allen, P.E.; Noland, R.C.; Martinez, J.J. Rickettsia conorii survival in THP-1 macrophages involves host lipid droplet alterations and active rickettsial protein production. Cell. Microbiol. 2021, e13390. [Google Scholar] [CrossRef]
- Curto, P.; Riley, S.P.; Simões, I.; Martinez, J.J. Macrophages infected by a pathogen and a non-pathogen spotted fever group Rickettsia reveal differential reprogramming signatures early in infection. Front. Cell. Infect. Microbiol. 2019, 9, 97. [Google Scholar] [CrossRef]
- Sahni, A.; Narra, H.P.; Sahni, S.K. Activation of mechanistic target of rapamycin (mTOR) in human endothelial cells infected with pathogenic spotted fever group rickettsiae. Int. J. Mol. Sci. 2020, 21, 7179. [Google Scholar] [CrossRef]
- Chowdhury, I.H.; Narra, H.P.; Sahni, A.; Khanipov, K.; Fofanov, Y.; Sahni, S.K. Enhancer associated long non-coding RNA transcription and gene regulation in experimental models of rickettsial infection. Front. Immunol. 2019, 9, 3014. [Google Scholar] [CrossRef]
- Patel, J.G.; Narra, H.P.; Sepuru, K.M.; Sahni, A.; Golla, S.R.; Sahni, A.; Singh, A.; Schroeder, C.L.C.; Chowdhury, I.H.; Popov, V.L.; et al. Evolution, purification, and characterization of RC0497: A peptidoglycan amidase from the prototypical spotted fever species Rickettsia conorii. Biol. Chem. 2020, 401, 249–262. [Google Scholar] [CrossRef]
- Zhao, Y.; Fang, R.; Zhang, J.; Zhang, Y.; Bechelli, J.; Smalley, C.; Valbuena, G.; Walker, D.H.; Oteo, J.A.; Brasier, A.R. Quantitative proteomics of the endothelial secretome identifies RC0497 as diagnostic of acute rickettsial spotted fever infections. Am. J. Pathol. 2020, 190, 306–322. [Google Scholar] [CrossRef]
- Martín Farfán, A.; Juárez Fernández, C.; Calbo Torrecillas, F.; Porras Ballesteros, J.; Díaz Recio, M.; Bermúndez Recio, F. Clinico-epidemiological study of 164 cases of boutonneuse fever. Rev. Clin. Esp. 1985, 176, 333–339. [Google Scholar]
- Crespo, P.; Seixas, D.; Marques, N.; Oliveira, J.; da Cunha, S.; Melico-Silvestre, A. Mediterranean spotted fever: Case series of 24 years (1989–2012). SpringerPlus 2015, 4, 272. [Google Scholar] [CrossRef] [PubMed]
- Anton, E.; Font, B.; Munoz, T.; Sanfeliu, I.; Segura, F. Clinical and laboratory characteristics of 144 patients with Mediterranean spotted fever. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Colomba, C.; Saporito, L.; Polara, V.F.; Rubino, R.; Titone, L. Mediterranean spotted fever: Clinical and laboratory characteristics of 415 Sicilian children. BMC Infect. Dis. 2006, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Weiller, P.J.; Chagnon, A.; Chaudet, H.; Gallais, H.; Casanova, P. Mediterranean spotted fever: Clinical, laboratory and epidemiological features of 199 cases. Am. J. Trop. Med. Hyg. 1986, 35, 845–850. [Google Scholar] [CrossRef] [PubMed]
- López Parés, P.; Muñoz Espín, T.; Espejo Arenas, E.; Font Creus, B.; Segura Porta, F.; Martínez Vila, I.; Travería Casanova, J.; Bella Cueto, F. Mediterranean spotted fever in childhood. Prospective study of 130 cases. An. Esp. Pediatr. 1988, 28, 293–296. [Google Scholar]
- Vitaliti, G.; Falsaperla, R.; Lubrano, R.; Rapisarda, V.; Cocuzza, S.; Nunnari, G.; Pavone, P. Incidence of Mediterranean spotted fever in Sicilian children: A clinical-epidemiological observational retrospective study from 1987 to 2010. Int. J. Infect. Dis. 2015, 31, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, S.; Ferreira, J.; Carvalho, J.; Martins, V. Mediterranean spotted fever in children: Study of a Portuguese endemic region. Acta Med. Port. 2018, 31, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Cascio, A.; Dones, P.; Romano, A.; Titone, L. Clinical and laboratory findings of boutonneuse fever in Sicilian children. Eur. J. Pediatr. 1998, 157, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Baltadzhiev, I.; Kevorkyan, A.; Popivanova, N. Mediterranean spotted fever in child and adult patients: Investigation from an endemic region in Bulgaria. Cent. Eur. J. Public Health 2020, 28, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Herrador, Z.; Fernandez-Martinez, A.; Gomez-Barroso, D.; Leon, I.; Vieira, C.; Muro, A.; Benito, A. Mediterranean spotted fever in Spain, 1997–2014: Epidemiological situation based on hospitalization records. PLoS ONE 2017, 12, e0174745. [Google Scholar] [CrossRef]
- Mansueto, S.; Vitale, G.; Miceli, M.D.; Tringali, G.; Quartararo, P.; Picone, D.M.; Occhino, C. A sero-epidemiological survey of asymptomatic cases of Boutonneuse fever in western Sicily. Trans. R. Soc. Trop. Med. Hyg. 1984, 78, 16–18. [Google Scholar] [CrossRef]
- Raoult, D.; Nicolas, D.; De Micco, P.; Gallais, H.; Casanova, P. Epidemiologic aspects of Mediterranean Boutonneuse fever in the south of Corsica. Bull. Soc. Pathol. Exot. Fil. 1985, 78, 446–451. [Google Scholar]
- Demeester, R.; Claus, M.; Hildebrand, M.; Vlieghe, E.; Bottieau, E. Diversity of life-threatening complications due to Mediterranean spotted fever in returning travelers. J. Travel Med. 2010, 17, 100–104. [Google Scholar] [CrossRef]
- Raoult, D.; Zuchelli, P.; Weiller, P.J.; Charrel, C.; San Marco, J.L.; Gallais, H.; Casanova, P. Incidence, clinical observations and risk factors in the severe form of Mediterranean spotted fever among patients admitted to hospital in Marseilles 1983–1984. J. Infect. 1986, 12, 111–116. [Google Scholar] [CrossRef]
- Botelho-Nevers, E.; Rovery, C.; Richet, H.; Raoult, D. Analysis of risk factors for malignant Mediterranean spotted fever indicates that fluoroquinolone treatment has a deleterious effect. J. Antimicrob. Chemother. 2011, 66, 1821–1830. [Google Scholar] [CrossRef]
- Piras, M.A.; Calia, G.; Saba, F.; Gakis, C.; Andreoni, G. Glucose-6-phosphate dehydrogenase deficiency in male patients with Mediterranean spotted fever in Sardinia. J. Infect. Dis. 1983, 147, 607–608. [Google Scholar] [CrossRef]
- Sousa, R.; Franca, A.; Doria Nobrega, S.; Belo, A.; Amaro, M.; Abreu, T.; Pocas, J.; Proenca, P.; Vaz, J.; Torgal, J.; et al. Host- and microbe-related risk factors for and pathophysiology of fatal Rickettsia conorii infection in Portuguese patients. J. Infect. Dis. 2008, 198, 576–585. [Google Scholar] [CrossRef]
- Baltadzhiev, I.G.; Popivanova, N.I.; Stoilova, Y.M.; Kevorkian, A.K. Mediterranean spotted fever–classification by disease course and criteria for determining the disease severity. Folia Med. (Plovdiv) 2012, 54, 53–61. [Google Scholar] [CrossRef]
- Walker, D.H.; Herrero-Herrero, J.I.; Ruiz-Beltran, R.; Bullon-Sopelana, A.; Ramos-Hidalgo, A. The pathology of fatal Mediterranean spotted fever. Am. J. Clin. Pathol. 1987, 87, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Cascio, A.; Maggio, M.C.; Cardella, F.; Zangara, V.; Accomando, S.; Costa, A.; Iaria, C.; Mansueto, P.; Giordano, S. Coronary involvement in Mediterranean spotted fever. New Microbiol. 2011, 34, 421–424. [Google Scholar] [PubMed]
- Colomba, C.; Saporito, L.; Colletti, P.; Mazzola, G.; Rubino, R.; Pampinella, D.; Titone, L. Atrial fibrillation in Mediterranean spotted fever. J. Med. Microbiol. 2008, 57, 1424–1426. [Google Scholar] [CrossRef] [PubMed]
- Botelho-Nevers, E.; Foucault, C.; Lepidi, H.; Brouqui, P. Cerebral infarction: An unusual complication of Mediterranean spotted fever. Eur. J. Intern. Med. 2005, 16, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Bougteba, A.; Basir, A.; Charradi, N. Meningoencephalitis caused by Rickettsia conorii in a young infant. Rev. Neurol. 2011, 167, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Tsiachris, D.; Deutsch, M.; Vassilopoulos, D.; Zafiropoulou, R.; Archimandritis, A.J. Sensorineural hearing loss complicating severe rickettsial diseases: Report of two cases. J. Infect. 2008, 56, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Montasser, D.I.; Zajjari, Y.; Alayoud, A.; Bahadi, A.; Aatif, T.; Hassani, K.; Hamzi, A.; Allam, M.; Benyahia, M.; Oualim, Z. Acute renal failure as a complication of Mediterranean spotted fever. Nephrol. Ther. 2011, 7, 245–247. [Google Scholar] [CrossRef]
- Agahan, A.L.; Torres, J.; Fuentes-Paez, G.; Martinez-Osorio, H.; Orduna, A.; Calonge, M. Intraocular inflammation as the main manifestation of Rickettsia conorii infection. Clin. Ophthalmol. 2011, 5, 1401–1407. [Google Scholar] [CrossRef]
- Rombola, F. Mediterranean spotted fever presenting as an acute pancreatitis. Acta Gastroenterol. Belg. 2011, 74, 91–92. [Google Scholar]
- Cascio, A.; Giordano, S.; Dones, P.; Venezia, S.; Iaria, C.; Ziino, O. Haemophagocytic syndrome and rickettsial diseases. J. Med. Microbiol. 2011, 60, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Letaief, A.; Souissi, J.; Trabelsi, H.; Ghannem, H.; Jemni, L. Evaluation of clinical diagnosis scores for Boutonneuse fever. Ann. N. Y. Acad. Sci. 2003, 990, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Blanton, L.S.; Walker, D.H. Rickettsiae as emerging infectious agents. Clin. Lab. Med. 2017, 37, 383–400. [Google Scholar] [CrossRef]
- La Scola, B.; Raoult, D. Laboratory diagnosis of rickettsioses: Current approaches to diagnosis of old and new rickettsial diseases. J. Clin. Microbiol. 1997, 35, 2715–2727. [Google Scholar] [CrossRef] [PubMed]
- Portillo, A.; de Sousa, R.; Santibanez, S.; Duarte, A.; Edouard, S.; Fonseca, I.P.; Marques, C.; Novakova, M.; Palomar, A.M.; Santos, M.; et al. Guidelines for the detection of Rickettsia spp. Vector-Borne Zoonotic Dis. 2017, 17, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Brouqui, P.; Bacellar, F.; Baranton, G.; Birtles, R.J.; Bjoersdorff, A.; Blanco, J.R.; Caruso, G.; Cinco, M.; Fournier, P.E.; Francavilla, E.; et al. Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin. Microbiol. Infect. 2004, 10, 1108–1132. [Google Scholar] [CrossRef] [PubMed]
- Biggs, H.M.; Behravesh, C.B.; Bradley, K.K.; Dahlgren, F.S.; Drexler, N.A.; Dumler, J.S.; Folk, S.M.; Kato, C.Y.; Lash, R.R.; Levin, M.L.; et al. Diagnosis and management of tickborne rickettsial diseases: Rocky mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm. Rep. 2016, 65, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Teysseire, N.; Raoult, D. Comparison of Western immunoblotting and microimmunofluorescence for diagnosis of Mediterranean spotted fever. J. Clin. Microbiol. 1992, 30, 455–460. [Google Scholar] [CrossRef]
- Robinson, M.T.; Satjanadumrong, J.; Hughes, T.; Stenos, J.; Blacksell, S.D. Diagnosis of spotted fever group Rickettsia infections: The Asian perspective. Epidemiol. Infect. 2019, 147, e286. [Google Scholar] [CrossRef]
- Marrero, M.; Raoult, D. Centrifugation-shell vial technique for rapid detection of Mediterranean spotted fever rickettsia in blood culture. Am. J. Trop. Med. Hyg. 1989, 40, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Gouriet, F.; Fenollar, F.; Patrice, J.Y.; Drancourt, M.; Raoult, D. Use of shell-vial cell culture assay for isolation of bacteria from clinical specimens: 13 years of experience. J. Clin. Microbiol. 2005, 43, 4993–5002. [Google Scholar] [CrossRef][Green Version]
- La Scola, B.; Raoult, D. Diagnosis of Mediterranean spotted fever by cultivation of Rickettsia conorii from blood and skin samples using the centrifugation-shell vial technique and by detection of R. conorii in circulating endothelial cells: A 6-year follow-up. J. Clin. Microbiol. 1996, 34, 2722–2727. [Google Scholar] [CrossRef] [PubMed]
- Bechah, Y.; Socolovschi, C.; Raoult, D. Identification of rickettsial infections by using cutaneous swab specimens and PCR. Emerg. Infect. Dis. 2011, 17, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Mouffok, N.; Socolovschi, C.; Renvoise, A.; Parola, P.; Raoult, D. Diagnosis of rickettsioses from eschar swab samples, Algeria. Emerg. Infect. Dis. 2011, 17, 1968–1969. [Google Scholar] [CrossRef]
- Angelakis, E.; Richet, H.; Rolain, J.M.; La Scola, B.; Raoult, D. Comparison of real-time quantitative PCR and culture for the diagnosis of emerging Rickettsioses. PLoS Negl. Trop. Dis. 2012, 6, e1540. [Google Scholar] [CrossRef] [PubMed]
- Roux, V.; Raoult, D. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int. J. Syst. Evol. Microbiol. 2000, 50 Pt 4, 1449–1455. [Google Scholar] [CrossRef]
- Ishikura, M.; Ando, S.; Shinagawa, Y.; Matsuura, K.; Hasegawa, S.; Nakayama, T.; Fujita, H.; Watanabe, M. Phylogenetic analysis of spotted fever group rickettsiae based on gltA, 17-kDa, and rOmpA genes amplified by nested PCR from ticks in Japan. Microbiol. Immunol. 2003, 47, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Roux, V.; Fournier, P.E.; Raoult, D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J. Clin. Microbiol. 1996, 34, 2058–2065. [Google Scholar] [CrossRef]
- Botelho-Nevers, E.; Socolovschi, C.; Raoult, D.; Parola, P. Treatment of Rickettsia spp. infections: A review. Expert Rev. Anti-Infect. Ther. 2012, 10, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Roussellier, P.; Vestris, G.; Tamalet, J. In vitro antibiotic susceptibility of Rickettsia rickettsii and Rickettsia conorii: Plaque assay and microplaque colorimetric assay. J. Infect. Dis. 1987, 155, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Rolain, J.M.; Maurin, M.; Vestris, G.; Raoult, D. In vitro susceptibilities of 27 rickettsiae to 13 antimicrobials. Antimicrob. Agents Chemother. 1998, 42, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Cascio, A.; Colomba, C.; Antinori, S.; Paterson, D.L.; Titone, L. Clarithromycin versus azithromycin in the treatment of Mediterranean spotted fever in children: A randomized controlled trial. Clin. Infect. Dis. 2002, 34, 154–158. [Google Scholar] [CrossRef]
- Anton, E.; Munoz, T.; Traveria, F.J.; Navarro, G.; Font, B.; Sanfeliu, I.; Segura, F. Randomized trial of clarithromycin for Mediterranean spotted fever. Antimicrob. Agents Chemother. 2015, 60, 1642–1645. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Botelho-Nevers, E.; Edouard, S.; Leroy, Q.; Raoult, D. Deleterious effect of ciprofloxacin on Rickettsia conorii-infected cells is linked to toxin-antitoxin module up-regulation. J. Antimicrob. Chemother. 2012, 67, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Blanton, L.S. The Rickettsioses: A practical update. Infect. Dis. Clin. N. Am. 2019, 33, 213–229. [Google Scholar] [CrossRef]
- Cross, R.; Ling, C.; Day, N.P.; McGready, R.; Paris, D.H. Revisiting doxycycline in pregnancy and early childhood—Time to rebuild its reputation? Expert Opin. Drug Saf. 2016, 15, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Nahum, G.G.; Uhl, K.; Kennedy, D.L. Antibiotic use in pregnancy and lactation: What is and is not known about teratogenic and toxic risks. Obstet. Gynecol. 2006, 107, 1120–1138. [Google Scholar] [CrossRef]
- Doryx Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050582s029lbl.pdf (accessed on 25 July 2021).
- Todd, S.R.; Dahlgren, F.S.; Traeger, M.S.; Beltran-Aguilar, E.D.; Marianos, D.W.; Hamilton, C.; McQuiston, J.H.; Regan, J.J. No visible dental staining in children treated with doxycycline for suspected Rocky Mountain Spotted Fever. J. Pediatr. 2015, 166, 1246–1251. [Google Scholar] [CrossRef]
- Pöyhönen, H.; Nurmi, M.; Peltola, V.; Alaluusua, S.; Ruuskanen, O.; Lähdesmäki, T. Dental staining after doxycycline use in children. J. Antimicrob. Chemother. 2017, 72, 2887–2890. [Google Scholar] [CrossRef]
- Stultz, J.S.; Eiland, L.S. Doxycycline and tooth discoloration in children: Changing of recommendations based on evidence of safety. Ann. Pharmacother. 2019, 53, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Bella-Cueto, F.; Font-Creus, B.; Segura-Porta, F.; Espejo-Arenas, E.; Lopez-Pares, P.; Munoz-Espin, T. Comparative, randomized trial of one-day doxycycline versus 10-day tetracycline therapy for Mediterranean spotted fever. J. Infect. Dis. 1987, 155, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Shaked, Y.; Samra, Y.; Maier, M.K.; Rubinstein, E. Relapse of rickettsial Mediterranean spotted fever and murine typhus after treatment with chloramphenicol. J. Infect. 1989, 18, 35–37. [Google Scholar] [CrossRef]
- Chan, Y.G.; Riley, S.P.; Chen, E.; Martinez, J.J. Molecular basis of immunity to rickettsial infection conferred through outer membrane protein B. Infect. Immun. 2011, 79, 2303–2313. [Google Scholar] [CrossRef] [PubMed]
- Kazar, J.; Brezina, R. Control of rickettsial diseases. Eur. J. Epidemiol. 1991, 7, 282–286. [Google Scholar] [PubMed]
- Faulde, M.K.; Rutenfranz, M.; Keth, A.; Hepke, J.; Rogge, M.; Gorner, A. Pilot study assessing the effectiveness of factory-treated, long-lasting permethrin-impregnated clothing for the prevention of tick bites during occupational tick exposure in highly infested military training areas, Germany. Parasitol. Res. 2015, 114, 671–678. [Google Scholar] [CrossRef]
- Vaughn, M.F.; Funkhouser, S.W.; Lin, F.C.; Fine, J.; Juliano, J.J.; Apperson, C.S.; Meshnick, S.R. Long-lasting permethrin impregnated uniforms: A randomized-controlled trial for tick bite prevention. Am. J. Prev. Med. 2014, 46, 473–480. [Google Scholar] [CrossRef]
- Walker, A.R. Eradication and control of livestock ticks: Biological, economic and social perspectives. Parasitology 2011, 138, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Osterloh, A. The neglected challenge: Vaccination against rickettsiae. PLoS Negl. Trop. Dis. 2020, 14, e0008704. [Google Scholar] [CrossRef]
- Rego, R.O.M.; Trentelman, J.J.A.; Anguita, J.; Nijhof, A.M.; Sprong, H.; Klempa, B.; Hajdusek, O.; Tomas-Cortazar, J.; Azagi, T.; Strnad, M.; et al. Counterattacking the tick bite: Towards a rational design of anti-tick vaccines targeting pathogen transmission. Parasites Vectors 2019, 12, 229. [Google Scholar] [CrossRef]
- Petchampai, N.; Sunyakumthorn, P.; Banajee, K.H.; Verhoeve, V.I.; Kearney, M.T.; Macaluso, K.R. Identification of host proteins involved in rickettsial invasion of tick cells. Infect. Immun. 2015, 83, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spernovasilis, N.; Markaki, I.; Papadakis, M.; Mazonakis, N.; Ierodiakonou, D. Mediterranean Spotted Fever: Current Knowledge and Recent Advances. Trop. Med. Infect. Dis. 2021, 6, 172. https://doi.org/10.3390/tropicalmed6040172
Spernovasilis N, Markaki I, Papadakis M, Mazonakis N, Ierodiakonou D. Mediterranean Spotted Fever: Current Knowledge and Recent Advances. Tropical Medicine and Infectious Disease. 2021; 6(4):172. https://doi.org/10.3390/tropicalmed6040172
Chicago/Turabian StyleSpernovasilis, Nikolaos, Ioulia Markaki, Michail Papadakis, Nikolaos Mazonakis, and Despo Ierodiakonou. 2021. "Mediterranean Spotted Fever: Current Knowledge and Recent Advances" Tropical Medicine and Infectious Disease 6, no. 4: 172. https://doi.org/10.3390/tropicalmed6040172
APA StyleSpernovasilis, N., Markaki, I., Papadakis, M., Mazonakis, N., & Ierodiakonou, D. (2021). Mediterranean Spotted Fever: Current Knowledge and Recent Advances. Tropical Medicine and Infectious Disease, 6(4), 172. https://doi.org/10.3390/tropicalmed6040172






