Double Trouble: Dengue Followed by COVID-19 Infection Acquired in Two Different Regions: A Doctor’s Case Report and Spatial Distribution of Cases in Presidente Prudente, São Paulo, Brazil
Abstract
:1. Introduction
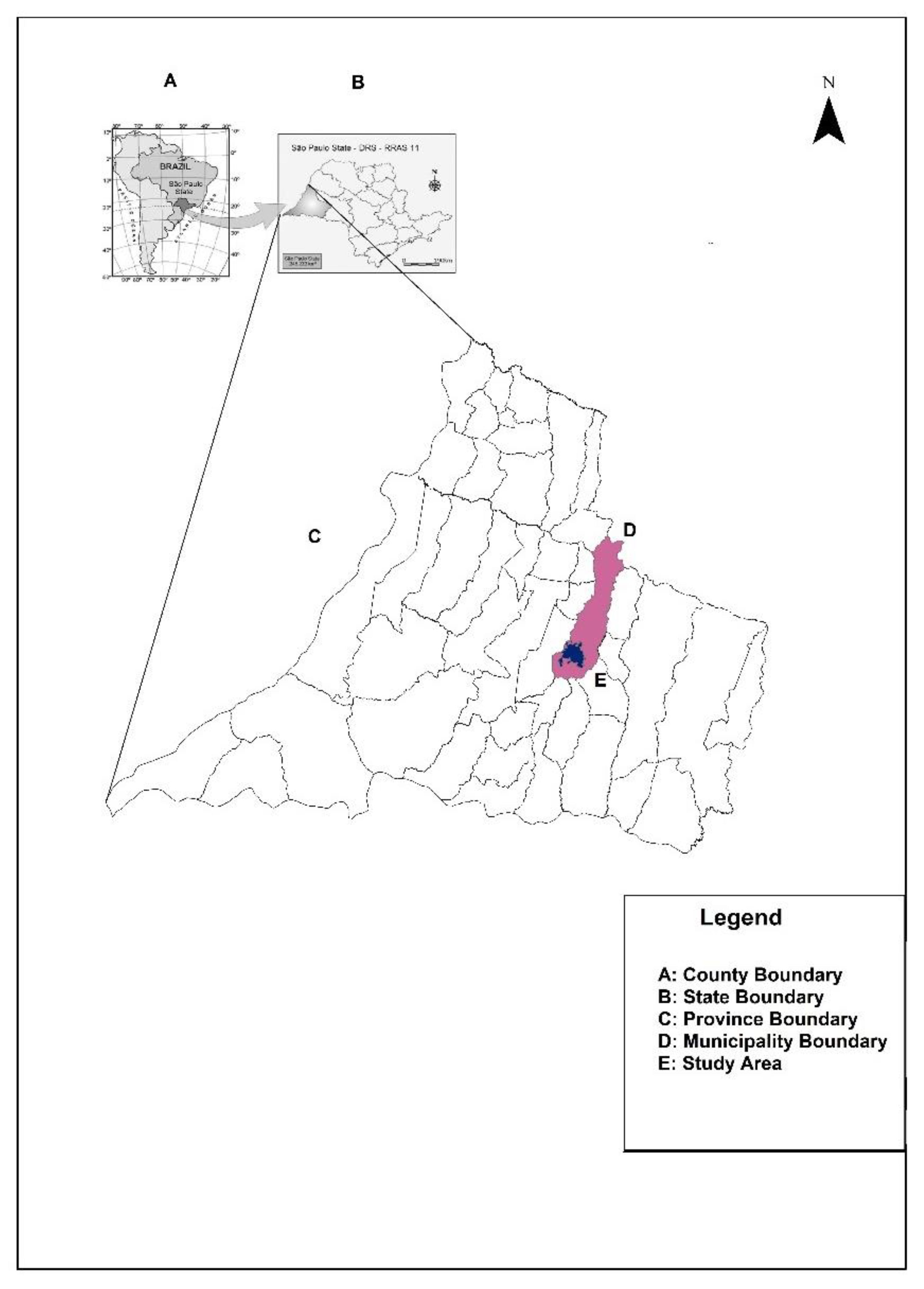
2. Settings, Materials, and Methods
3. Results
3.1. Case Report
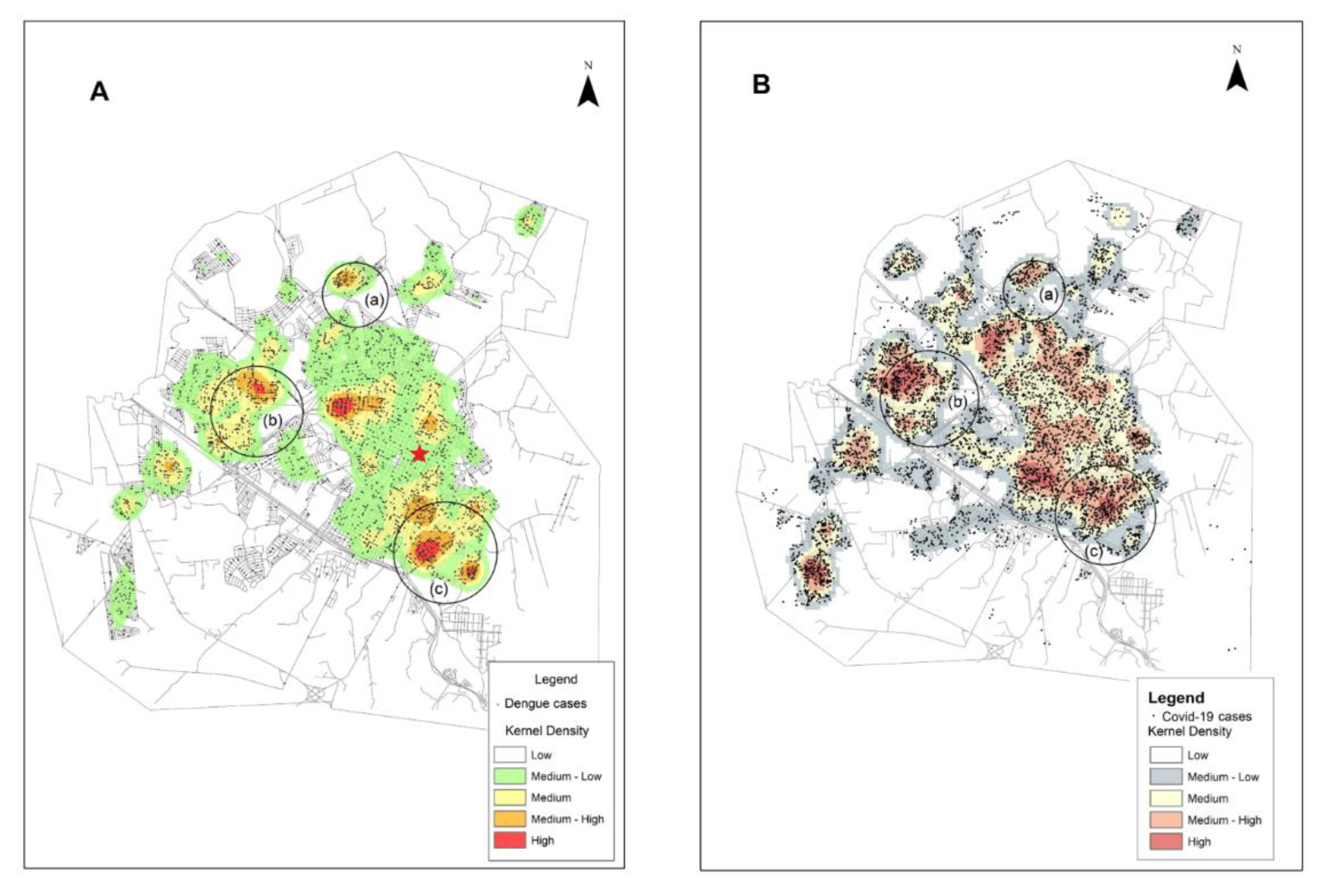
3.2. Dengue in the Context of COVID-19: Distribution of Cases of Dengue and COVID-19 Infection in the Urban Area of Presidente Prudente
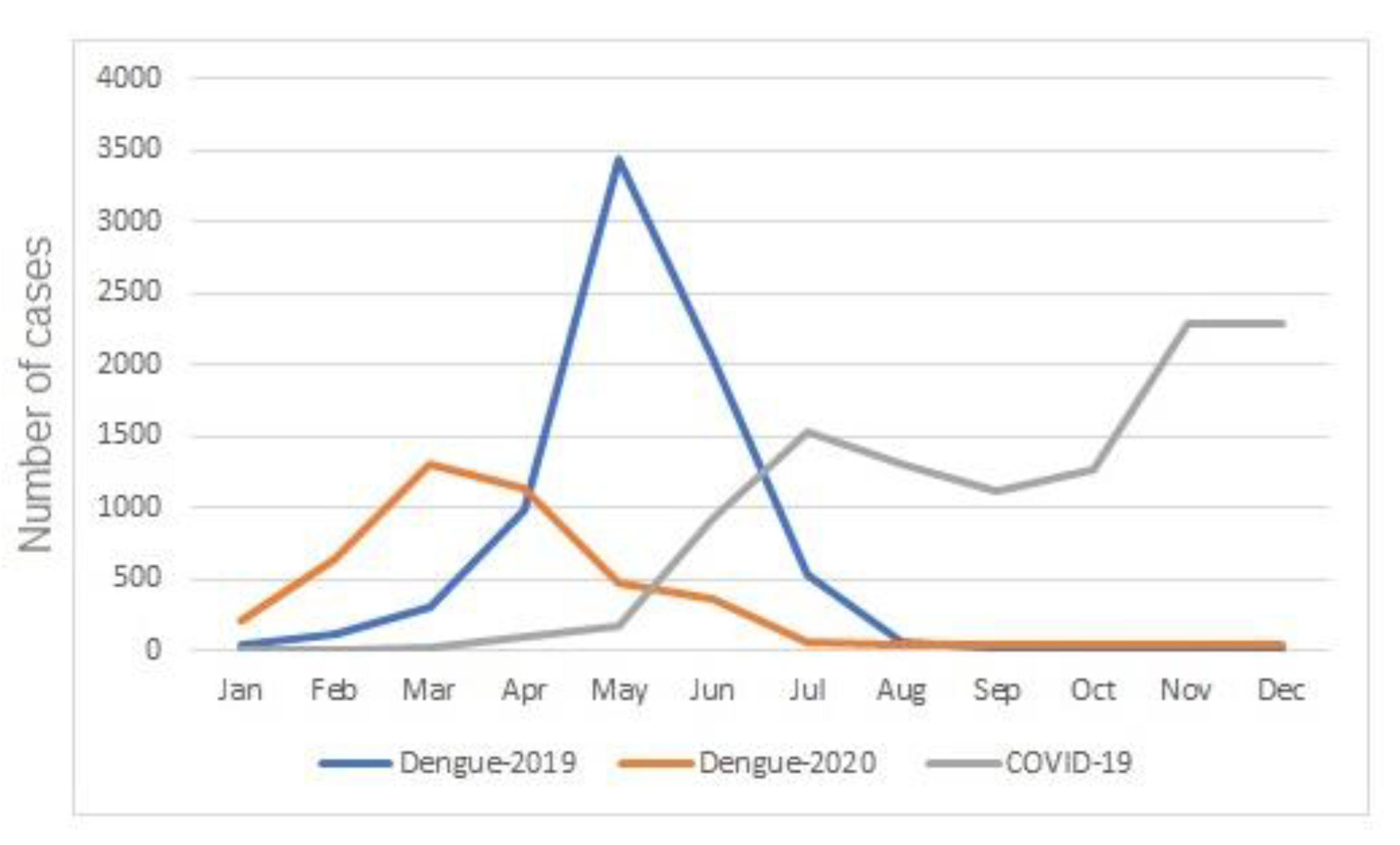
3.3. Dengue in the Context of COVID-19: Temporal Distribution of Cases of Dengue and COVID-19 in the Urban Area of Presidente Prudentein 2020
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lorenz, C.; Azevedo, T.S.; Chiaravalloti-Neto, F. COVID-19 and dengue fever: A dangerous combination for the health system in Brazil. Travel. Med. Infect. Dis. 2020, 35, 101659. [Google Scholar] [CrossRef] [PubMed]
- Reported Cases and Deaths by Country or Territory. Available online: https://www.worldometers.info/coronavirus/#countries (accessed on 3 July 2021).
- Alcântara, E.; Mantovani, J.; Rotta, L.; Park, E.; Rodrigues, T.; Campos Carvalho, F.; Roberto Souza Filho, C. Investigating spatiotemporal patterns of the COVID-19 in São Paulo State, Brazil. Geospat. Health 2020, 26, 15. [Google Scholar]
- Nicolelis, M.A.L.; Raimundo, R.L.G.; Peixoto, P.S.; Andreazzi, C.S. The impact of super-spreader cities, highways, and intensive care availability in the early stages of the COVID-19 epidemic in Brazil. Sci. Rep. 2021, 11, 13001. [Google Scholar] [CrossRef] [PubMed]
- Mhango, M.; Dzobo, M.; Chitungo, I.; Dzinamarira, T. COVID-19 Risk Factors among Health Workers: A Rapid Review. Saf. Health Work 2020, 11, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.J.R.D.; Magalhães, J.J.F.; Pena, L. Simultaneous Circulation of DENV, CHIKV, ZIKV and SARS-CoV-2 in Brazil: An Inconvenient Truth. One Health 2020, 12, 100205. [Google Scholar] [CrossRef]
- Secretaria de Vigilância em Saúde, Ministério da Saúde. Monitoramento dos casos de arboviroses urbanas transmitidas pelo Aedes Aegypti (dengue, chikungunya e zika). Sem. Epidemiol. 2019, 2, 1–16. [Google Scholar]
- Secretaria de Vigilância em Saúde, Ministério da Saúde. Monitoramento dos casos de arboviroses urbanas transmitidas pelo Aedes Aegypti (dengue, chikungunya e zika). Sem. Epidemiol. 2020, 51, 1–3. [Google Scholar]
- Rabiu, A.T.; Mohan, A.; Çavdaroğlu, S.; Xenophontos, E.; Costa, A.C.S.; Tsagkaris, C.; Hashim, H.T.; Ahmad, S.; Essar, M.Y. Dengue and COVID-19: A double burden to Brazil. J. Med. Virol. 2021, 93, 4092–4093. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A.; Tissera, H.; Ooi, E.E.; Coloma, J.; Scott, T.W.; Gubler, D.J. Preventing Dengue Epidemics during the COVID-19 Pandemic. Am. J. Trop. Med. Hyg. 2020, 103, 570–571. [Google Scholar] [CrossRef]
- PAHO. Epidemiological Update Dengue in the Context of COVID-19; PAHO: Washington, DC, USA, 2020; pp. 1–10. [Google Scholar]
- Navarro, J.C.; Arrivillaga-Henríquez, J.; Salazar-Loor, J.; Rodriguez-Morales, A.J. COVID-19 and dengue, co-epidemics in Ecuador and other countries in Latin America: Pushing strained health care systems over the edge. Travel. Med. Infect. Dis. 2020, 37, 101656. [Google Scholar] [CrossRef] [PubMed]
- Prestes-Carneiro, L.E.; Daniel, L.A.F.; Almeida, L.C.; D’Andrea, L.Z.; Vieira, A.G.; Anjolete, I.R.; André, L.; Flores, E.F. Spatiotemporal analysis and environmental risk factors of visceral leishmaniasis in an urban setting in São Paulo State, Brazil. Parasit Vectors 2019, 12, 251. [Google Scholar] [CrossRef] [Green Version]
- Soares Santana, R.; Briguenti Souza, K.; Lussari, F.; Fonseca, E.S.; Andrade, C.O.; Meidas, M.M.K.; Zampieri D’Andrea, L.A.; Silva, F.A.; Flores, E.F.; Anjolete, I.R.; et al. Cases and distribution of visceral leishmaniasis in western São Paulo: A neglected disease in this region of Brazil. PLoS Negl. Trop. Dis. 2021, 15, e0009411. [Google Scholar] [CrossRef]
- Melo Costa, M.; Campos, K.B.; Brito, L.P.; Roux, E.; Melo Rodovalho, C.; Bellinato, D.F.; Lima, J.B.P.; Martins, A.J. Kdr genotyping in Aedes aegypti from Brazil on a nation-wide scale from 2017 to 2018. Sci. Rep. 2020, 10, 13267. [Google Scholar] [CrossRef]
- Rex, F.E.; Borges, C.A.S.; Käfer, P.S. Spatial analysis of the COVID-19 distribution pattern in São Paulo State, Brazil. Cien. Saude Colet. 2020, 25, 3377–3384. [Google Scholar] [CrossRef] [PubMed]
- Boton Pereira, D.H.; Primo, L.S.; Pelizari, G.; Flores, E.; de Moraes-Vasconcelos, D.; Condino-Neto, A.; Prestes-Carneiro, L.E. Primary Immunodeficiencies in a Mesoregion of São Paulo, Brazil: Epidemiologic, Clinical, and Geospatial Approach. Front. Immunol. 2020, 11, 862. [Google Scholar] [CrossRef]
- IBGE. Cidades e Estados, Presidente Prudente. 2020. Available online: https://www.ibge.gov.br/cidades-e-estados/sp/presidente-prudente.html (accessed on 3 July 2021).
- The R Core Team. A Language and Environment for Statistical Computing; Version 4.2.0.; The R Core Team: Vienna, Austria, 2021; pp. 1–3793. [Google Scholar]
- Verduyn, M.; Allou, N.; Gazaille, V.; Andre, M.; Desroche, T.; Jaffar, M.C.; Traversier, N.; Levin, C.; Lagrange-Xelot, M.; Moiton, M.-P.; et al. Co-infection of dengue and COVID-19: A case report. PLoS Negl. Trop. Dis. 2020, 14, e0008476. [Google Scholar] [CrossRef]
- Epelboin, L.; Blondé, R.; Nacher, M.; Combe, P.; Collet, L. COVID-19 and dengue co-infection in a returning traveller. J. Travel. Med. 2020, 27, taaa114. [Google Scholar] [CrossRef] [PubMed]
- Radisic, M.V.; Piro, M.A.; Mori, I.; Rotryng, F.; Santamarina, J.F. SARS-CoV-2 and Dengue virus Co-infection. A Case Report. Infez. Med. 2020, 28, 416–419. [Google Scholar] [PubMed]
- Carosella, L.M.; Pryluka, D.; Maranzana, A.; Barcan, L.; Cuini, R.; Freuler, C.; Martinez, A.; Equiza, T.R.; Peria, C.R.; Yahni, D.; et al. COVIDENGUE Study Group1. Characteristics of Patients Co-infected with Severe Acute Respiratory Syndrome Coronavirus 2 and Dengue Virus, Buenos Aires, Argentina, March–June 2020. Emerg. Infect. Dis. 2021, 27, 348–351. [Google Scholar] [CrossRef]
- World Health Organization. Immunization, Vaccines and Biologicals. Available online: https://www.who.int/immunization/diseases/dengue/en/ (accessed on 10 January 2021).
- Ministério da Saúde. Secretaria de Vigilância em Saúde. Doença pelo Coronavírus COVID-19. Boletim Epidemiológico Especial. Sem. Epidemiológica 2020, 49, 41. [Google Scholar]
- Conselho Federal de Medicina/Conselhos Regionais de Medicina. Memorial aos Médicos que se Foram Durante o Combate à COVID-19. Available online: https://memorial.cfm.org.br (accessed on 10 July 2021).
- Estofolete, C.F.; Machado, L.F.; Zini, N.; Luckemeyer, G.; Moraes, M.; Santos, T.; Santos, B.; Ruiz, L.; Vasilakis, N.; Lobo, S.; et al. Fatal stroke as presentation of SARS-CoV-2 and dengue virus coinfection. J. Med. Virol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Quental, K.N.; Leite, A.L.; Feitosa, A.D.N.A.; Oliveira, Z.N.P.; Tavares, L.V.S.; Tavares, W.G.S.; Pinheiro, E.F.; Lacsina, J.R.; DeSouza-Vieira, T.; Silva, J.B.N.F. SARS-CoV-2 co-infection with dengue virus in Brazil: A potential case of viral transmission by a health care provider to household members. Travel. Med. Infect. Dis. 2021, 40, 101975. [Google Scholar] [CrossRef] [PubMed]
- Phadke, R.; Mohan, A.; Çavdaroğlu, S.; Dapke, K.; Costa, A.C.D.S.; Riaz, M.M.A.; Hashim, H.T.; Essar, M.Y.; Ahmad, S. Dengue amidst COVID-19 in India: The mystery of plummeting cases. J. Med. Virol. 2021, 93, 4120–4121. [Google Scholar] [CrossRef]
- Niriella, M.A.; Ediriweera, D.S.; De Silva, A.P.; Premarathna, B.H.R.; Jayasinghe, S.; de Silva, H.J. Dengue and leptospirosis infection during the coronavirus 2019 outbreak in Sri Lanka. Trans. R. Soc. Trop. Med. Hyg. 2021, trab058. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.C.S.; Morita, L.H.M.; Da Silva, E.B.; Zardo, L.A.R.; Fontes, C.J.F.; Granzotto, D.C.T. Bayesian modeling of COVID-19 cases with a correction to account for under-reported cases. Infect. Dis. Model. 2020, 5, 699–713. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, S.M.; do Nascimento, C.T.; Ferro, R.S.; Flores, E.F.; Maldonado Bertacco, E.A.; Fonseca, E.d.S.; Prestes-Carneiro, L.E. Double Trouble: Dengue Followed by COVID-19 Infection Acquired in Two Different Regions: A Doctor’s Case Report and Spatial Distribution of Cases in Presidente Prudente, São Paulo, Brazil. Trop. Med. Infect. Dis. 2021, 6, 156. https://doi.org/10.3390/tropicalmed6030156
Pereira SM, do Nascimento CT, Ferro RS, Flores EF, Maldonado Bertacco EA, Fonseca EdS, Prestes-Carneiro LE. Double Trouble: Dengue Followed by COVID-19 Infection Acquired in Two Different Regions: A Doctor’s Case Report and Spatial Distribution of Cases in Presidente Prudente, São Paulo, Brazil. Tropical Medicine and Infectious Disease. 2021; 6(3):156. https://doi.org/10.3390/tropicalmed6030156
Chicago/Turabian StylePereira, Sérgio Munhoz, Charlene Troiani do Nascimento, Rodrigo Sala Ferro, Edilson Ferreira Flores, Elaine Aparecida Maldonado Bertacco, Elivelton da Silva Fonseca, and Luiz Euribel Prestes-Carneiro. 2021. "Double Trouble: Dengue Followed by COVID-19 Infection Acquired in Two Different Regions: A Doctor’s Case Report and Spatial Distribution of Cases in Presidente Prudente, São Paulo, Brazil" Tropical Medicine and Infectious Disease 6, no. 3: 156. https://doi.org/10.3390/tropicalmed6030156
APA StylePereira, S. M., do Nascimento, C. T., Ferro, R. S., Flores, E. F., Maldonado Bertacco, E. A., Fonseca, E. d. S., & Prestes-Carneiro, L. E. (2021). Double Trouble: Dengue Followed by COVID-19 Infection Acquired in Two Different Regions: A Doctor’s Case Report and Spatial Distribution of Cases in Presidente Prudente, São Paulo, Brazil. Tropical Medicine and Infectious Disease, 6(3), 156. https://doi.org/10.3390/tropicalmed6030156





