Lopinavir and Nelfinavir Induce the Accumulation of Crystalloid Lipid Inclusions within the Reservosomes of Trypanosoma cruzi and Inhibit Both Aspartyl-Type Peptidase and Cruzipain Activities Detected in These Crucial Organelles
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasite Cultivation
2.2. Treatment of T. cruzi Epimastigotes with Aspartyl PIs
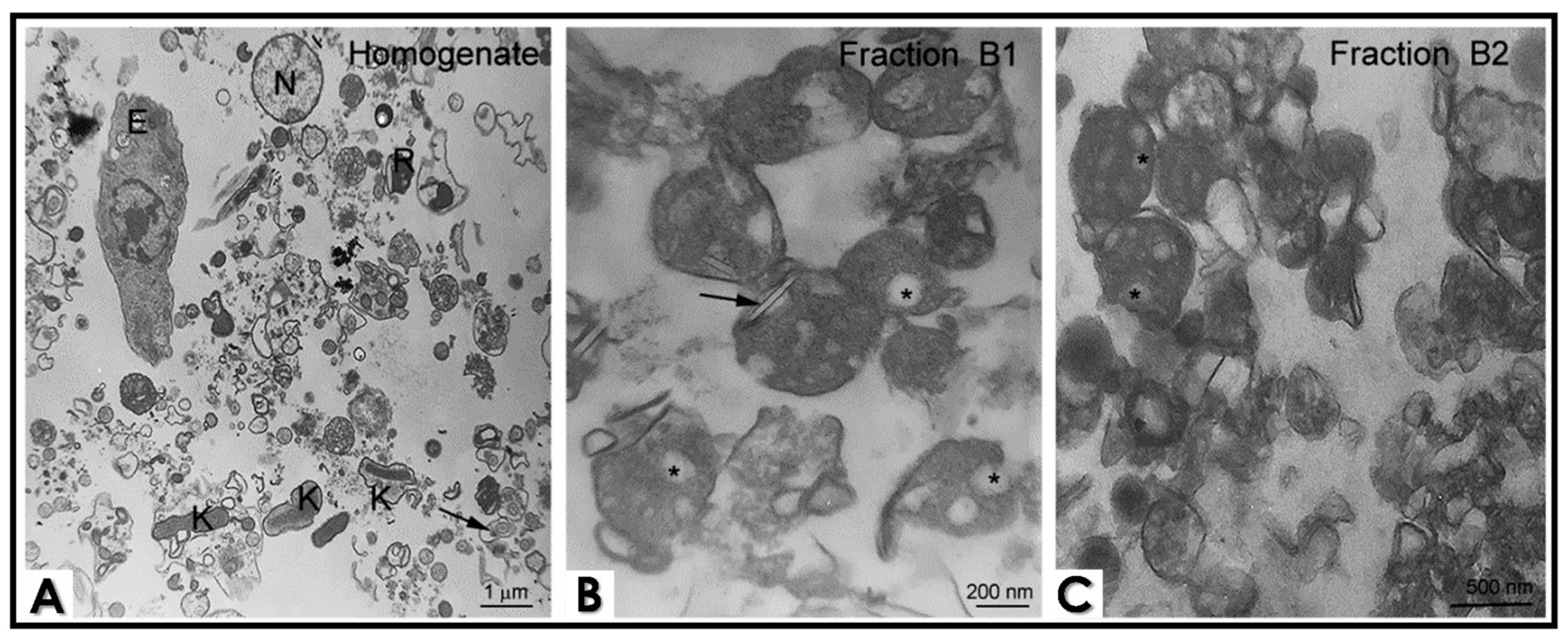
2.3. Cell Fractionation
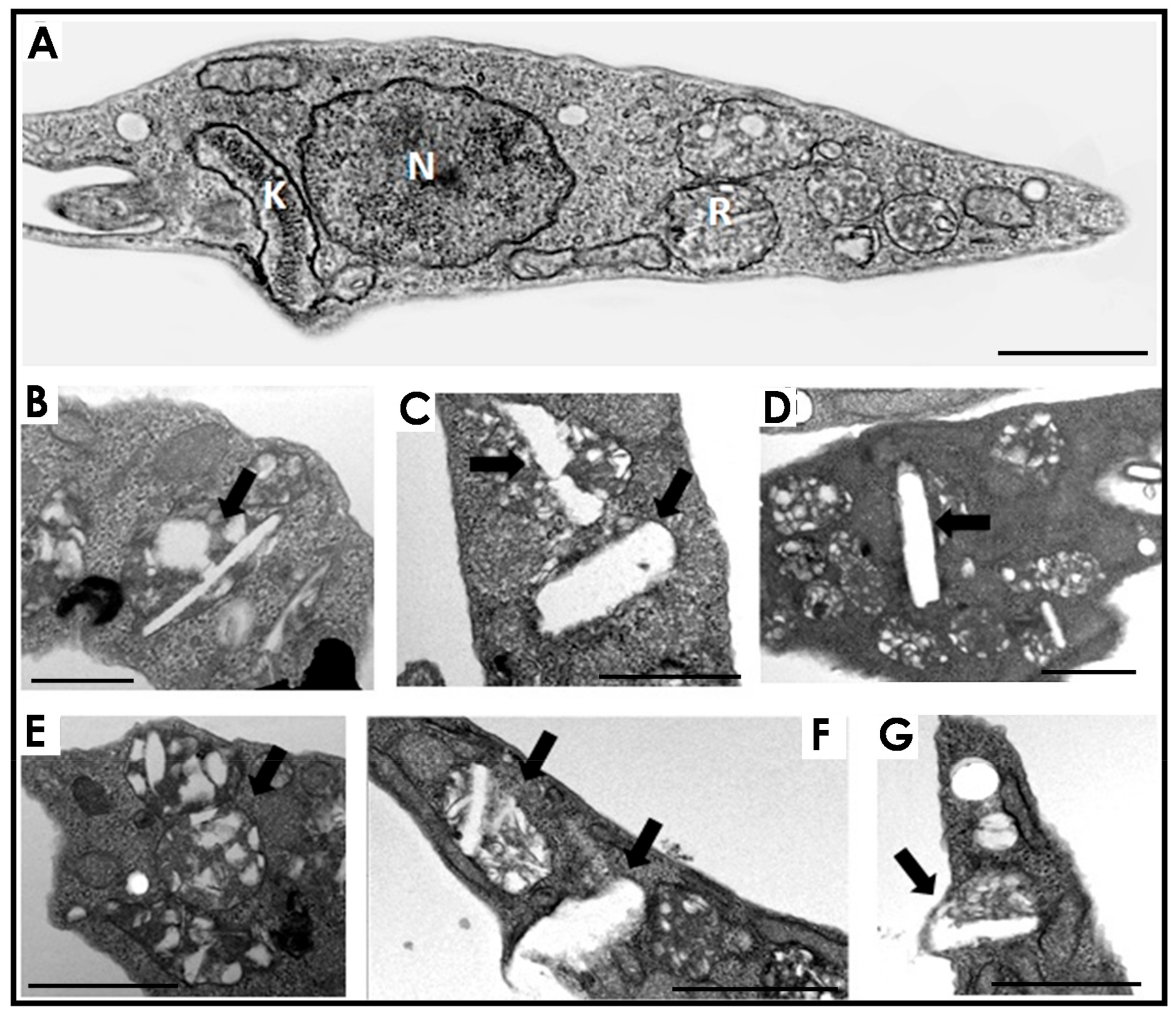
2.4. Transmission Electron Microscopy (TEM) of Epimastigotes and Cell Fractions
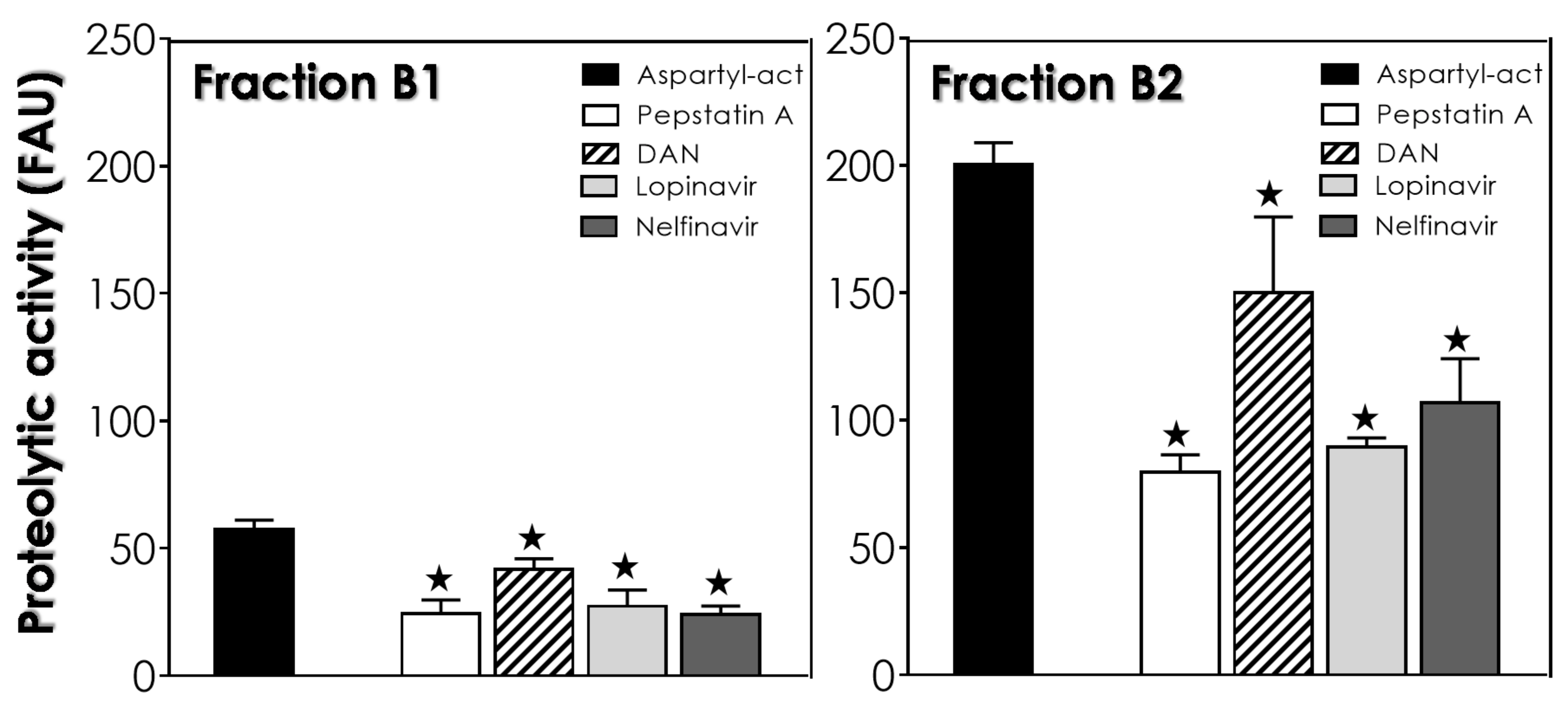
2.5. Aspartyl Peptidase Activity Assays in Reservosomes Fractions
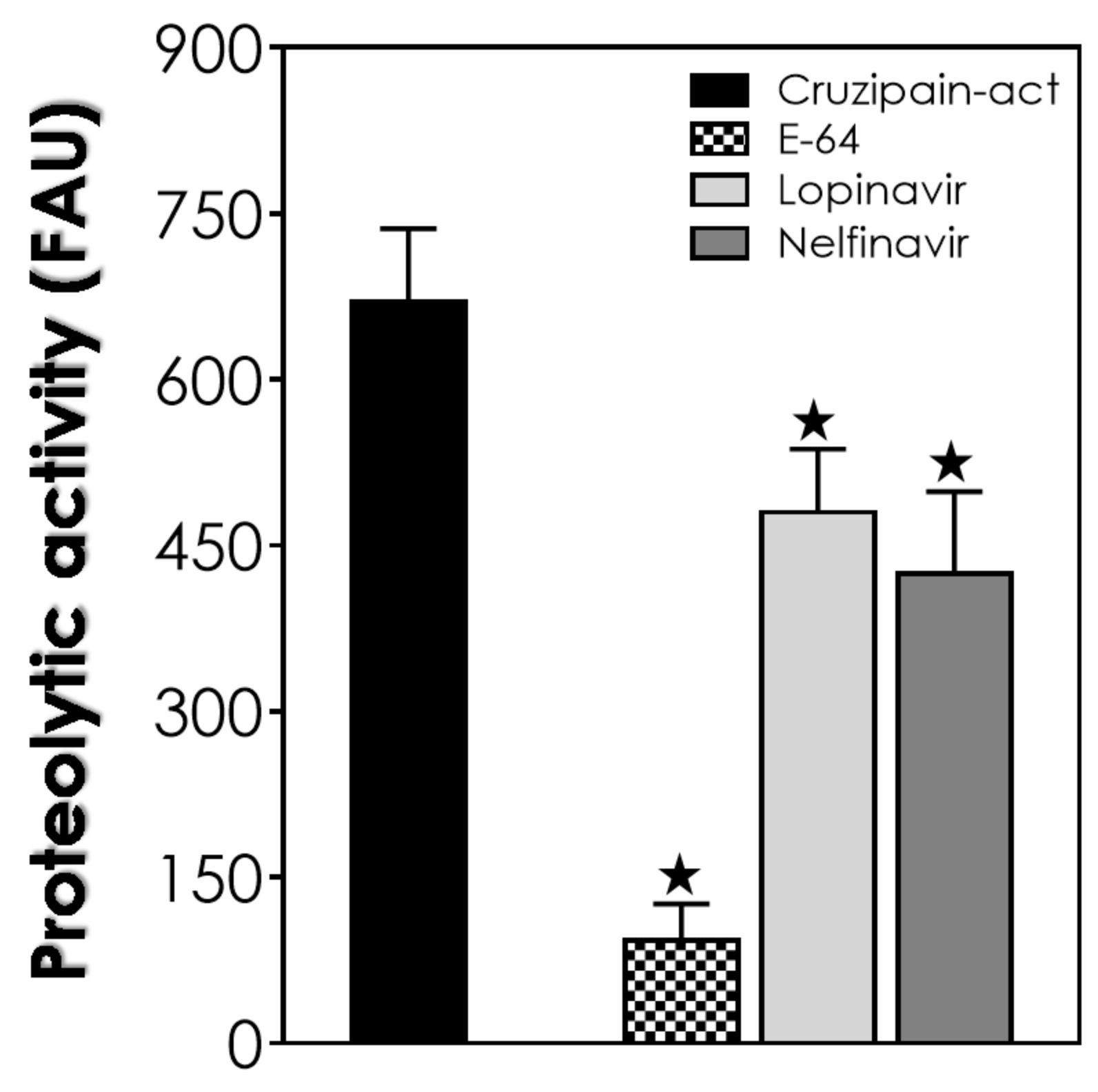
2.6. Cruzipain Activity Assay in Reservosome B2 Fraction Extract
2.7. Statistic and Graph Constructions
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsantrizos, Y.S. Peptidomimetic therapeutic agents targeting the protease enzyme of the human immunodeficiency virus and hepatitis C virus. Chem. Res. 2008, 41, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Palella, F.J.J.R.; Delaney, K.M.; Moorman, A.C.; Loveless, M.O.; Fuhrer, J.; Satten, G.A.; Aschman, D.J.; Holmberg, S.D. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV outpatient study investigators. N. Engl. J. Med. 1998, 338, 853–860. [Google Scholar] [CrossRef]
- Mastrolorenzo, A.; Rusconi, S.; Scozzafava, A.; Barbaro, G.; Supuran, C.T. Inhibitors of HIV-1 protease: Current state of the art 10 years after their introduction. From antiretroviral drugs to antifungal, antibacterial and antitumor agents based on aspartic protease inhibitors. Curr. Med. Chem. 2007, 14, 2734–2748. [Google Scholar] [CrossRef]
- Alfonso, Y.; Monzote, L.L. HIV protease inhibitors: Effect on the opportunistic protozoan parasites. Open Med. Chem. J. 2011, 5, 40–50. [Google Scholar] [CrossRef]
- Santos, A.L.S.; d’Avila-Levy, C.M.; Kneipp, L.F.; Sodré, C.L.; Sangenito, L.S.; Branquinha, M.H. The widespread anti-protozoal action of HIV aspartic peptidase inhibitors: Focus on Plasmodium spp., Leishmania spp. and Trypanosoma cruzi. Curr. Top. Med. Chem. 2017, 17, 1303–1317. [Google Scholar] [PubMed]
- Sangenito, L.S.; Menna-Barreto, R.F.S.; d’Avila-Levy, C.M.; Branquinha, M.H.; Santos, A.L.S. Repositioning of HIV aspartyl peptidase inhibitors for combating the neglected human pathogen Trypanosoma cruzi. Curr. Med. Chem. 2019, 26, 6590–6613. [Google Scholar] [CrossRef]
- Falutz, J. Management of fat accumulation in patients with HIV infection. Curr. HIV AIDS Rep. 2011, 8, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Capel, E.; Caron-Debarle, A.M.; Capeau, J. Effects of ritonavir-boosted darunavir, atazanavir and lopinavir on adipose functions and insulin sensitivity in murine and human adipocytes. Antivir. Ther. 2012, 17, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.O.; Marinho, F.A.; Altoé, E.F.; Vitório, B.S.; Alves, C.R.; Britto, C.; Motta, M.C.; Branquinha, M.H.; Santos, A.L.S.; d’Avila-Levy, C.M. HIV aspartyl peptidase inhibitors interfere with cellular proliferation, ultrastructure and macrophage infection of Leishmania amazonenses. PLoS ONE 2009, 4, e4918. [Google Scholar] [CrossRef]
- Rebello, K.M.; Andrade-Neto, V.V.; Zuma, A.A.; Motta, M.C.M.; Gomes, C.G.B.; de Souza, M.V.N.; Atella, G.C.; Branquinha, M.H.; Santos, A.L.S.; Torres-Santos, E.C.; et al. Lopinavir, an HIV-1 peptidase inhibitor, induces alteration on the lipid metabolism of Leishmania amazonensis promastigotes. Parasitology 2018, 145, 1304–1310. [Google Scholar] [CrossRef]
- Soares, M.J.; De Souza, W. Endocytosis of gold-labeled proteins and LDL by Trypanosoma cruzi. Parasitol. Res. 1991, 77, 461–469. [Google Scholar] [CrossRef]
- Porto Carreiro, I.A.; Miranda, K.; Attias, M.; De Souza, W.; Cunha-e-Silva, N.L. Trypanosoma cruzi epimastigotes endocytic pathway: Cargo enters the cytostome and passes through an early endosomal network before reservosome storage. Eur. J. Cell. Biol. 2000, 79, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, C.; Nakayasu, E.S.; Pereira, M.G.; Lourenco, D.; de Souza, W.; Almeida, I.C.; Cunha, E.S.N.L. Subcellular proteomics of Trypanosoma cruzi reservosomes. Proteomics 2009, 9, 1782–1794. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.G.; Nakayasu, E.S.; Sant’Anna, C.; De Cicco, N.N.; Atella, G.C.; de Souza, W.; Almeida, I.C.; Cunha-e-Silva, N. Trypanosoma cruzi epimastigotes are able to store and mobilize high amounts of cholesterol in reservosome lipid inclusions. PLoS ONE 2011, 6, e22359. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.G.; Visbal, G.; Salgado, L.T.; Vidal, J.C.; Godinho, J.L.; De Cicco, N.N.; Atella, G.C.; de Souza, W.; Cunha-e-Silva, N. Trypanosoma cruzi epimastigotes are able to manage internal cholesterol levels under nutritional lipid stress conditions. PLoS ONE 2015, 6, e0128949. [Google Scholar] [CrossRef]
- Pereira, M.G.; Visbal, G.; Costa, T.F.R.; Frases, S.; de Souza, W.; Atella, G.; Cunha-E-Silva, N. Trypanosoma cruzi epimastigotes store cholesteryl esters in lipid droplets after cholesterol endocytosis. Mol. Biochem. Parasitol. 2018, 224, 6–16. [Google Scholar] [CrossRef]
- Soares, M.J.; Souto-Padron, T.; Bonaldo, M.C.; Goldenberg, S.; De Souza, W. A stereological study of the differentiation process in Trypanosoma cruzi. Parasitol. Res. 1989, 75, 522–527. [Google Scholar] [CrossRef]
- Sangenito, L.S.; Menna-Barreto, R.F.S.; d’Avila-Levy, C.M.; Santos, A.L.S.; Branquinha, M.H. Decoding the anti-Trypanosoma cruzi action of HIV peptidase inhibitors using epimastigotes as a model. PLoS ONE 2014, 12, e113957. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cunha-e-Silva, N.L.; Atella, G.C.; Porto-Carreiro, I.A.; Morgado-Diaz, J.A.; Pereira, M.G.; de Souza, W. Isolation and characterization of a reservosome fraction from Trypanosoma cruzi. FEMS Microbiol. Lett. 2002, 214, 7–12. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 264–275. [Google Scholar] [CrossRef]
- Cazzulo, J.J.; Cazzulo, F.M.C.; Martínez, J.; de Cazzulo, B.M.F. Some kinetic properties of a cysteine proteinase (cruzipain) from Trypanosoma cruzi. Biochim. Biophys. Acta. 1990, 1037, 186–191. [Google Scholar] [CrossRef]
- Sangenito, L.S.; de Guedes, A.A.; Gonçalves, D.S.; Seabra, S.H.; d’Avila-Levy, C.M.; Santos, A.L.S.; Branquinha, M.H. Deciphering the effects of nelfinavir and lopinavir on epimastigote forms of Trypanosoma cruzi. Parasitol. Int. 2017, 66, 529–536. [Google Scholar] [CrossRef]
- Sangenito, L.S.; Menna-Barreto, R.F.S.; Oliveira, A.C.; d’Avila-Levy, C.M.; Branquinha, M.H.; Santos, A.L.S. Primary evidence of the mechanisms of action of HIV aspartyl peptidase inhibitors on Trypanosoma cruzi trypomastigote forms. Int. J. Antimicrob. Agents 2018, 52, 185–194. [Google Scholar] [CrossRef]
- Sant’Anna, C.; Pereira, M.G.; Lemgruber, L.; de Souza, W.; Cunha e Silva, N.L. New insights into the morphology of Trypanosoma cruzi reservosome. Microsc. Res. Tech. 2008, 71, 599–605. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.M.; Myler, P.J.; Bartholomeu, D.C.; Nilsson, D.; Aggarwal, G.; Tran, A.N.; Ghedin, E.; Worthey, E.A.; Delcher, A.L.; Blandin, G.; et al. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas’ disease. Science 2005, 309, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.O.; Garcia-Gomes, A.S.; Catanho, M.; Sodre, C.L.; Santos, A.L.S.; Branquinha, M.H.; d’Avila-Levy, C.M. Aspartic peptidases of human pathogenic trypanosomatids: Perspectives and trends for chemotherapy. Curr. Med. Chem. 2013, 20, 3116–3133. [Google Scholar] [CrossRef] [PubMed]
- Castilho, V.V.S.; Gonçalves, K.C.S.; Rebello, K.M.; Baptista, L.P.R.; Sangenito, L.S.; Santos, H.L.C.; Branquinha, M.H.; Santos, A.L.S.; Menna-Barreto, R.F.S.; Guimarães, A.C.; et al. Docking simulation between HIV peptidase inhibitors and Trypanosoma cruzi aspartyl peptidase. BMC Res. Notes 2018, 11, 825. [Google Scholar] [CrossRef] [PubMed]
- Lechuga, G.C.; Napoleão-Pêgo, P.; Bottino, C.C.G.; Pinho, R.T.; Provance-Jr, D.W.; De-Simone, S.G. Trypanosoma cruzi presenilin-like transmembrane aspartyl protease: Characterization and cellular localization. Biomolecules 2020, 10, 1564. [Google Scholar] [CrossRef]
- White, R.E.; Powell, D.J.; Berry, C. HIV proteinase inhibitors target the Ddi1-like protein of Leishmania parasites. FASEB J. 2011, 25, 1729–1736. [Google Scholar] [CrossRef]
- Branquinha, M.H.; Oliveira, S.S.; Sangenito, L.S.; Sodre, C.L.; Kneipp, L.F.; d’Avila-Levy, C.M.; Santos, A.L.S. Cruzipain: An update on its potential as chemotherapy target against the human pathogen Trypanosoma cruzi. Curr. Med. Chem. 2015, 22, 2225–2235. [Google Scholar] [CrossRef]
- Bellera, C.L.; Sbaraglini, M.L.; Balcazar, D.E.; Fraccaroli, L.; Vanrell, M.C.; Casassa, A.F.; Labriola, C.A.; Romano, P.S.; Carrillo, C.; Talevi, A. High-throughput drug repositioning for the discovery of new treatments for Chagas disease. Mini. Rev. Med. Chem. 2015, 3, 182–193. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangenito, L.S.; Pereira, M.G.; Souto-Padron, T.; Branquinha, M.H.; Santos, A.L.S. Lopinavir and Nelfinavir Induce the Accumulation of Crystalloid Lipid Inclusions within the Reservosomes of Trypanosoma cruzi and Inhibit Both Aspartyl-Type Peptidase and Cruzipain Activities Detected in These Crucial Organelles. Trop. Med. Infect. Dis. 2021, 6, 120. https://doi.org/10.3390/tropicalmed6030120
Sangenito LS, Pereira MG, Souto-Padron T, Branquinha MH, Santos ALS. Lopinavir and Nelfinavir Induce the Accumulation of Crystalloid Lipid Inclusions within the Reservosomes of Trypanosoma cruzi and Inhibit Both Aspartyl-Type Peptidase and Cruzipain Activities Detected in These Crucial Organelles. Tropical Medicine and Infectious Disease. 2021; 6(3):120. https://doi.org/10.3390/tropicalmed6030120
Chicago/Turabian StyleSangenito, Leandro S., Miria G. Pereira, Thais Souto-Padron, Marta H. Branquinha, and André L. S. Santos. 2021. "Lopinavir and Nelfinavir Induce the Accumulation of Crystalloid Lipid Inclusions within the Reservosomes of Trypanosoma cruzi and Inhibit Both Aspartyl-Type Peptidase and Cruzipain Activities Detected in These Crucial Organelles" Tropical Medicine and Infectious Disease 6, no. 3: 120. https://doi.org/10.3390/tropicalmed6030120
APA StyleSangenito, L. S., Pereira, M. G., Souto-Padron, T., Branquinha, M. H., & Santos, A. L. S. (2021). Lopinavir and Nelfinavir Induce the Accumulation of Crystalloid Lipid Inclusions within the Reservosomes of Trypanosoma cruzi and Inhibit Both Aspartyl-Type Peptidase and Cruzipain Activities Detected in These Crucial Organelles. Tropical Medicine and Infectious Disease, 6(3), 120. https://doi.org/10.3390/tropicalmed6030120







