Reduced Bacterial Counts from a Sewage Treatment Plant but Increased Counts and Antibiotic Resistance in the Recipient Stream in Accra, Ghana—A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
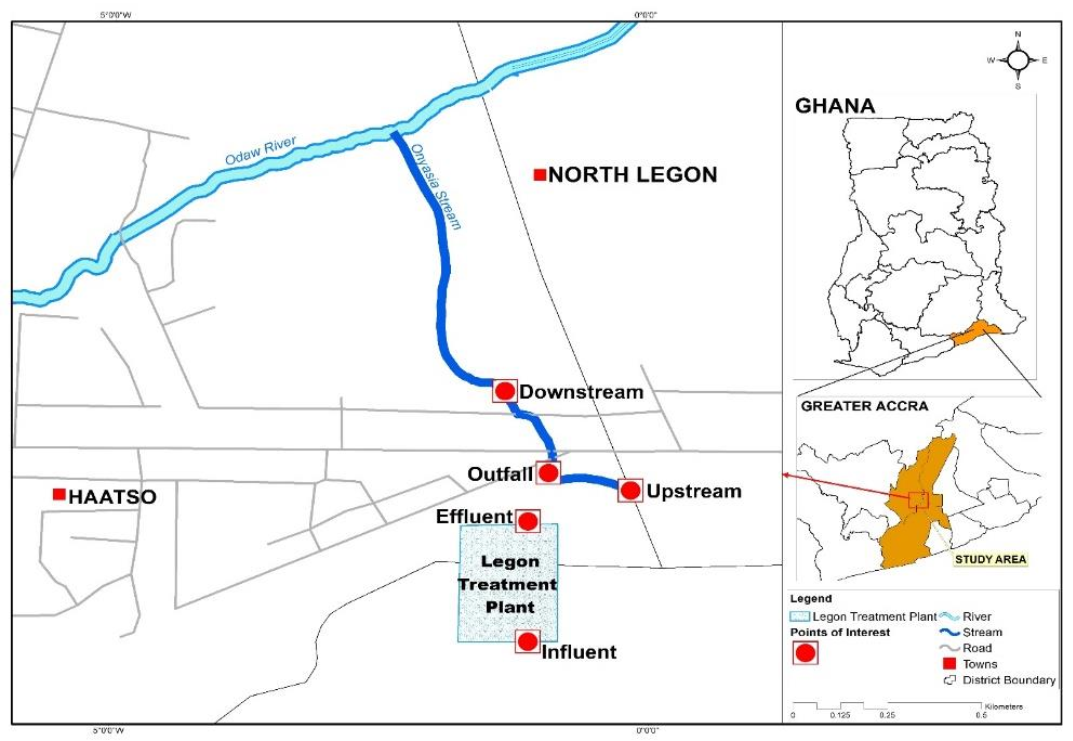
2.2. Study Setting
2.3. Water Sample Collection and Laboratory Analysis and Biochemical Identification
2.4. Quality Control Procedures
2.5. Study Inclusions and Period
2.6. Data Collection, Source of Data, and Validation
2.7. Statistical Analysis
3. Results
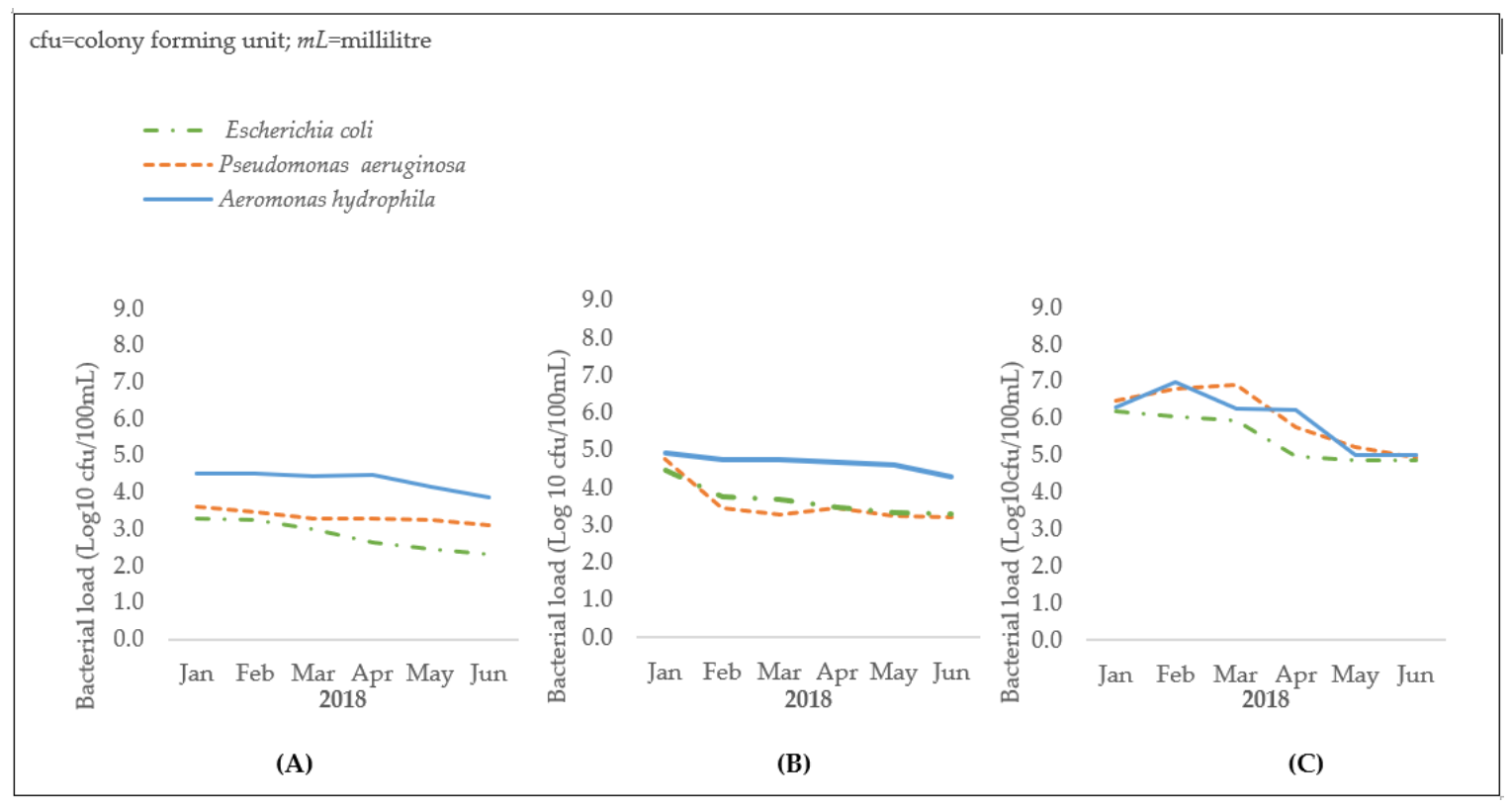
3.1. Sewage: Bacterial Loads of E. coli, P. aeruginosa, and A. hydrophila
3.2. Sewage: Antibiotic Resistance in E. coli, A. hydrophila, and P. aeruginosa in Influent and Effluent Samples
3.3. Onyasia Stream: Bacterial Counts of E. coli, P. aeruginosa, and A. hydrophila in Water Samples
3.4. Onyasia Stream: Antibiotic Resistance in E. coli, A. hydrophila, and P. aeruginosa in Upstream, Outfall and Downstream Water Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sxerwecińska, L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed]
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Dölz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial use in aquaculture re-examined: Its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, P.M.; Loureiro, L.; Matos, A.J.F. Transfer of multidrug-resistant bacteria between intermingled ecological niches: The interface between humans, animals and the environment. Int. J. Environ. Res. Public Health 2013, 10, 278–294. [Google Scholar] [CrossRef]
- Lundborg, C.S.; Tamhankar, A.J. Antibiotic residues in the environment of South East Asia. BMJ 2017, 358, 42–45. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef]
- Bouki, C.; Venieri, D.; Diamadopoulos, E. Detection and fate of antibiotic resistant bacteria in wastewater treatment plants: A review. Ecotoxicol. Environ. Saf. 2013, 91, 1–9. [Google Scholar] [CrossRef]
- WHO. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- García-Vello, P.; González-Zorn, B.; Saba, C.K.S. Antibiotic resistance patterns in human, animal, food and environmental isolates in ghana: A review. Pan Afr. Med. J. 2020, 35. [Google Scholar] [CrossRef]
- Opintan, J.A.; Newman, M.J. Prevalence of antimicrobial resistant pathogens from blood cultures: Results from a laboratory based nationwide surveillance in Ghana. Antimicrob. Resist. Infect. Control 2017, 6, 4–9. [Google Scholar] [CrossRef]
- Andoh, L.A.; Ahmed, S.; Olsen, J.E.; Obiri-Danso, K.; Newman, M.J.; Opintan, J.A.; Barco, L.; Dalsgaard, A. Prevalence and characterization of Salmonella among humans in Ghana. Trop. Med. Health 2017, 45, 1–11. [Google Scholar] [CrossRef]
- African Development Fund. Accra Sewerage Improvement Project [Appraisal Report]; African Development Fund: Tunis, Tunisia, 2005. [Google Scholar]
- Andoh, B.F. Effects of Anthropogenic Activities on Water Quality of Streams: A Case Study of the Onyasia Stream in the Greater Accra Region. Master’s Thesis, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, 2014. [Google Scholar]
- Amarasiri, M.; Sano, D.; Suzuki, S. Understanding human health risks caused by antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARG) in water environments: Current knowledge and questions to be answered. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2016–2059. [Google Scholar] [CrossRef]
- WHO. Antimicrobial Resistance: An Emerging Water, Sanitation and Hygiene Issue; Briefing Note WHO/FWC/WSH/14.07; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- WHO. Critically Important Antimicrobials for Human Medicine; 5th Revision; World Health Organization: Geneva, Switzerland, 2017; ISBN 9789241512220. [Google Scholar]
- Igbinosa, I.H.; Okoh, A.I. Antibiotic susceptibility profile of aeromonas species isolated from wastewater treatment plant. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef]
- GSS Ghana Stastical Service, Population by Regions. Available online: https://www.statsghana.gov.gh/regionalpopulation.php?population=MTM0NTk2MjQzOS4yMDE1&&GreaterAccra®id=3 (accessed on 3 March 2021).
- Mohammed, M.; Egyir, I.S.; Donkor, A.K.; Amoah, P.; Nyarko, S.; Boateng, K.K.; Ziwu, C. Feasibility study for biogas integration into waste treatment plants in Ghana. Egypt. J. Pet. 2017, 26, 695–703. [Google Scholar] [CrossRef]
- Sagoe, G.; Danquah, F.S.; Amofa-Sarkodie, E.S.; Appiah-Effah, E.; Ekumah, E.; Mensah, E.K.; Karikari, K.S. GIS-aided optimisation of faecal sludge management in developing countries: The case of the Greater Accra Metropolitan Area, Ghana. Heliyon 2019, 5, e02505. [Google Scholar] [CrossRef]
- Bridgewater, L.; American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Cheesbrough, M. District Laboratory Practice in Tropical Countries, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006; ISBN 9780511543470. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017; ISBN 1-56238-1-56238-805-3. [Google Scholar]
- EPA. Sector Specific Effluent Quality Guidelines for Effluent Discharge Quality for Discharges; Ghana Environmental Protection Agency: Accra, Ghana, 2000. [Google Scholar]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbrouckef, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Bull. World Health Organ. 2007, 85, 867–872. [Google Scholar] [CrossRef]
- Ashbolt, N.J.; Grabow, W.O.K.; Snozzi, M. Indicators of microbial water quality. In Water Quality: Guidelines, Standards and Health; World Health Organization (WHO): Geneva, Switzerland, 2001; ISBN 1900222280. [Google Scholar]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef]
- Wellington, E.M.H.; Boxall, A.B.A.; Cross, P.; Feil, E.J.; Gaze, W.H.; Hawkey, P.M.; Johnson-Rollings, A.S.; Jones, D.L.; Lee, N.M.; Otten, W.; et al. The role of the natural environment in the emergence of antibiotic resistance in Gram-negative bacteria. Lancet Infect. Dis. 2013, 13, 155–165. [Google Scholar] [CrossRef]
- Kwabla, T.A. Assessing Willingness to Reuse Treated Wastewater for Crops Irrigation, and the Consumption of Crops Irrigated with Treated Wastewater: A Case Study of Students from University of Ghana and Ashiaman Municipality, Ghana. Master’s Thesis, University of Ghana, Accra, Ghana, 2017. [Google Scholar]
- Guo, Y.-R.; Cao, Q.-D.; Hong, Z.-S.; Tan, Y.-Y.; Chen, S.-D.; Jin, H.-J.; Tan, K.-S.; Wang, D.-Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med. Res. 2020, 7, 11. [Google Scholar] [CrossRef]
- Bhilwadikar, T.; Pounraj, S.; Manivannan, S.; Rastogi, N.K.; Negi, P.S. Decontamination of Microorganisms and Pesticides from Fresh Fruits and Vegetables: A Comprehensive Review from Common Household Processes to Modern Techniques. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1003–1038. [Google Scholar] [CrossRef]
- Mena, K.D.; Gerba, C.P. Risk assessment of P. aeruginosa in water. Rev. Environ. Contam. Toxicol. 2009, 201, 71–115. [Google Scholar] [CrossRef] [PubMed]
- Allende, A.; Monaghan, J. Irrigation water quality for leafy crops: A perspective of risks and potential solutions. Int. J. Environ. Res. Public Health 2015, 12, 7457–7477. [Google Scholar] [CrossRef] [PubMed]


| Sample | E. coli | A. hydrophila | P. aeruginosa | |||
|---|---|---|---|---|---|---|
| (Mean cfu/100 mL) | p-Value 1 | (Mean cfu/100 mL) | p-Value 1 | (Mean fu/100 mL) | p-Value 1 | |
| Influent | 102,266,667 | <0.01 | 376,333 | <0.001 | 5,666,667 | 0.01 |
| Effluent | 710 | 9603 | 1550 | |||
| Antibiotics | Isolates Resistant to Antibiotics | |||||
|---|---|---|---|---|---|---|
| E. coli | A. hydrophila | |||||
| Influent (N = 30) | Effluent (N = 30) | p-Value 1 | Influent (N = 30) | Effluent (N = 30) | p-Value 1 | |
| n (%) | n (%) | n (%) | n (%) | |||
| Gentamicin 10 μg | 2(7) | 8 (27) | 0.04 | 4(13) | 5(17) | 0.5 |
| Amoxicillin/Clavulanate 20 μg | 15(50) | 15(50) | 0.5 | 18(60) | 29(97) | <0.001 |
| Tetracycline 30 μg | 24(80) | 11(37) | <0.001 | 27(90) | 7(23) | <0.001 |
| Ciprofloxacin 5 μg | 19(63) | 3(10) | <0.001 | 20(67) | 4(13) | <0.001 |
| Imipenem 10 μg | 1(3) | 5(17) | 0.1 | 3(10) | 10(33) | 0.03 |
| Cefuroxime- 30 μg | 15(50) | 14(47) | 0.5 | 22 (73) | 15(50) | <0.001 |
| Aztreonam 30 μg | 9(30) | 6(20) | 0.3 | 12(40) | 11(37) | 0.5 |
| Antibiotics | Isolate Resistance to Antibiotics | ||
|---|---|---|---|
| P. aeruginosa | |||
| Influent (N = 30) | Effluent (N = 30) | p-Value 1 | |
| n (%) | n (%) | ||
| Gentamicin 10 μg | 2(7) | 5(17) | 0.4 |
| Ciprofloxacin 5 μg | 9(30) | 4(13) | 0.2 |
| Imipenem 10 μg | 1(3) | 2(7) | 0.5 |
| Aztreonam 30 μg | 9(30) | 10(33) | 0.5 |
| Ceftazidime 30 μg | 6(20) | 1(3) | 0.02 |
| Sample ID | E. coli | A. hydrophila | P. aeruginosa | |||
|---|---|---|---|---|---|---|
| (Mean cfu/100 mL) | p Value 1 | (Mean cfu/100 mL) | p Value 1 | (Mean cfu/100 mL) | p Value 1 | |
| Upstream | 955 | 0.01 | 2350 | 0.03 | 24,433 | 0.05 |
| Outfall | 11,900 | 8033 | 52,233 | |||
| Downstream | 3,043,333 | 64,100 | 2,536,667 | |||
| Antibiotics | Isolates Resistant to Antibiotics | |||||||
|---|---|---|---|---|---|---|---|---|
| E. coli | A. hydrophila | |||||||
| Upstream (N = 30) | Outfall (N = 30) | Downstream (N = 30) | p-Value 1 | Upstream (N = 30) | Outfall (N = 30) | Downstream (N = 30) | p-Value 1 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||
| Gentamicin 10 μg | 1(3) | 6(20) | 8(27) | 0.03 | 0(0) | 2(7) | 3(10) | 0.2 |
| Amoxicillin/Clavulanate 20 μg | 14(47) | 18(60) | 22(73) | 0.03 | 8(27) | 23(77) | 24(80) | 0.2 |
| Tetracycline 30 μg | 10(30) | 9(50) | 17(40) | 0.41 | 3(10) | 4(13) | 10(33) | <0.001 |
| Ciprofloxacin 5 μg | 3(10) | 9(30) | 10(33) | 0.05 | 0(0) | 4(13) | 5(17) | 0.05 |
| Imipenem 10 μg | 0(0) | 4(13) | 7(23) | 0.005 | 0(0) | 5(16) | 8(27) | <0.001 |
| Cefuroxime 30 μg | 12(40) | 15(50) | 22(73) | 0.01 | 2(7) | 11(37) | 14(47) | <0.001 |
| Aztreonam 30 μg | 4(13) | 8(27) | 10(33) | 0.06 | 0(0) | 5(17) | 12(40) | <0.001 |
| Antibiotics | Isolates Resistant to Antibiotics | |||
|---|---|---|---|---|
| P. aeruginosa | ||||
| Upstream (N = 30) | Outfall (N = 30) | Downstream (N = 30) | p-Value | |
| Gentamicin 10 μg | 1(3) | 6(20) | 8(27) | 0.02 |
| Ciprofloxacin 5 μg | 1(3) | 7(22) | 12(40) | <0.01 |
| Imipenem 10 μg | 0(0) | 1(3) | 2(7) | 0.2 |
| Aztreonam 30 μg | 6(20) | 15(50) | 16(53) | 0.01 |
| Ceftazidime 30 μg | 1(3) | 4(13) | 4(13) | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adomako, L.A.B.; Yirenya-Tawiah, D.; Nukpezah, D.; Abrahamya, A.; Labi, A.-K.; Grigoryan, R.; Ahmed, H.; Owusu-Danquah, J.; Annang, T.Y.; Banu, R.A.; et al. Reduced Bacterial Counts from a Sewage Treatment Plant but Increased Counts and Antibiotic Resistance in the Recipient Stream in Accra, Ghana—A Cross-Sectional Study. Trop. Med. Infect. Dis. 2021, 6, 79. https://doi.org/10.3390/tropicalmed6020079
Adomako LAB, Yirenya-Tawiah D, Nukpezah D, Abrahamya A, Labi A-K, Grigoryan R, Ahmed H, Owusu-Danquah J, Annang TY, Banu RA, et al. Reduced Bacterial Counts from a Sewage Treatment Plant but Increased Counts and Antibiotic Resistance in the Recipient Stream in Accra, Ghana—A Cross-Sectional Study. Tropical Medicine and Infectious Disease. 2021; 6(2):79. https://doi.org/10.3390/tropicalmed6020079
Chicago/Turabian StyleAdomako, Lady A. B., Dzidzo Yirenya-Tawiah, Daniel Nukpezah, Arpine Abrahamya, Appiah-Korang Labi, Ruzanna Grigoryan, Hawa Ahmed, Josiah Owusu-Danquah, Ted Yemoh Annang, Regina A. Banu, and et al. 2021. "Reduced Bacterial Counts from a Sewage Treatment Plant but Increased Counts and Antibiotic Resistance in the Recipient Stream in Accra, Ghana—A Cross-Sectional Study" Tropical Medicine and Infectious Disease 6, no. 2: 79. https://doi.org/10.3390/tropicalmed6020079
APA StyleAdomako, L. A. B., Yirenya-Tawiah, D., Nukpezah, D., Abrahamya, A., Labi, A.-K., Grigoryan, R., Ahmed, H., Owusu-Danquah, J., Annang, T. Y., Banu, R. A., Osei-Atweneboana, M. Y., Timire, C., Tweya, H., Ackon, S. E. D., Nartey, E., & Zachariah, R. (2021). Reduced Bacterial Counts from a Sewage Treatment Plant but Increased Counts and Antibiotic Resistance in the Recipient Stream in Accra, Ghana—A Cross-Sectional Study. Tropical Medicine and Infectious Disease, 6(2), 79. https://doi.org/10.3390/tropicalmed6020079









