Quality Assessment of an Antimicrobial Resistance Surveillance System in a Province of Nepal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Settings
2.2.1. General Setting
2.2.2. Specific Setting
2.3. Study Population and Period
2.4. Data Variables, Sources of Data, and Data Collection
2.4.1. Variables
2.4.2. Sources of Data
2.4.3. Data Collection
2.5. Data Analysis and Statistics
2.6. Ethical Approval
| AMR 1 data | Detailed identification and antibiotic susceptibility data of specific bacterial isolates, along with unique identifiers, specimen, origin, date of sampling, and demographic data from surveillance sites’ microbiology laboratory records. |
| Origin | Place: “Hospital” or “Community” origin. |
| Timeliness of data | AMR data, for a particular month, received within the 15th working day of the following month. |
| Duplicate data | AMR data occurring within a month i.e., repeated isolates of the same bacterial species isolated from a patient within thirty days, regardless of specimen type. |
| Specimen–pathogen combination | Combination of priority specimens (namely, blood, urine, stool, or genital swabs) with priority pathogens according to the GLASS2. |
| Pathogen–antibacterial combination | Combination of eight priority pathogens and the relevant listed antibiotics according to the GLASS. |
| Consistency of data | Data is considered consistent when the variables used to generate the report are as directed by the GLASS manual i.e., four priority specimens and eight priority pathogens. |
| Completeness of data | Completeness signifies no missing variables required according to GLASS criteria: age, sex, pathogen, origin, specimen, antibiotic susceptibility results, and date. |
| Non-reporting sites | The surveillance sites that have not sent any AMR laboratory data to the NPHL3 for ninety days consecutively. |
| Basic infrastructure | Basic facilities and equipment required by the AMR surveillance site to send the AMR reports to the NPHL. |
| Specific requirements | Requirements other than the basic infrastructure to send the AMR reports regularly to the NPHL. |
3. Results
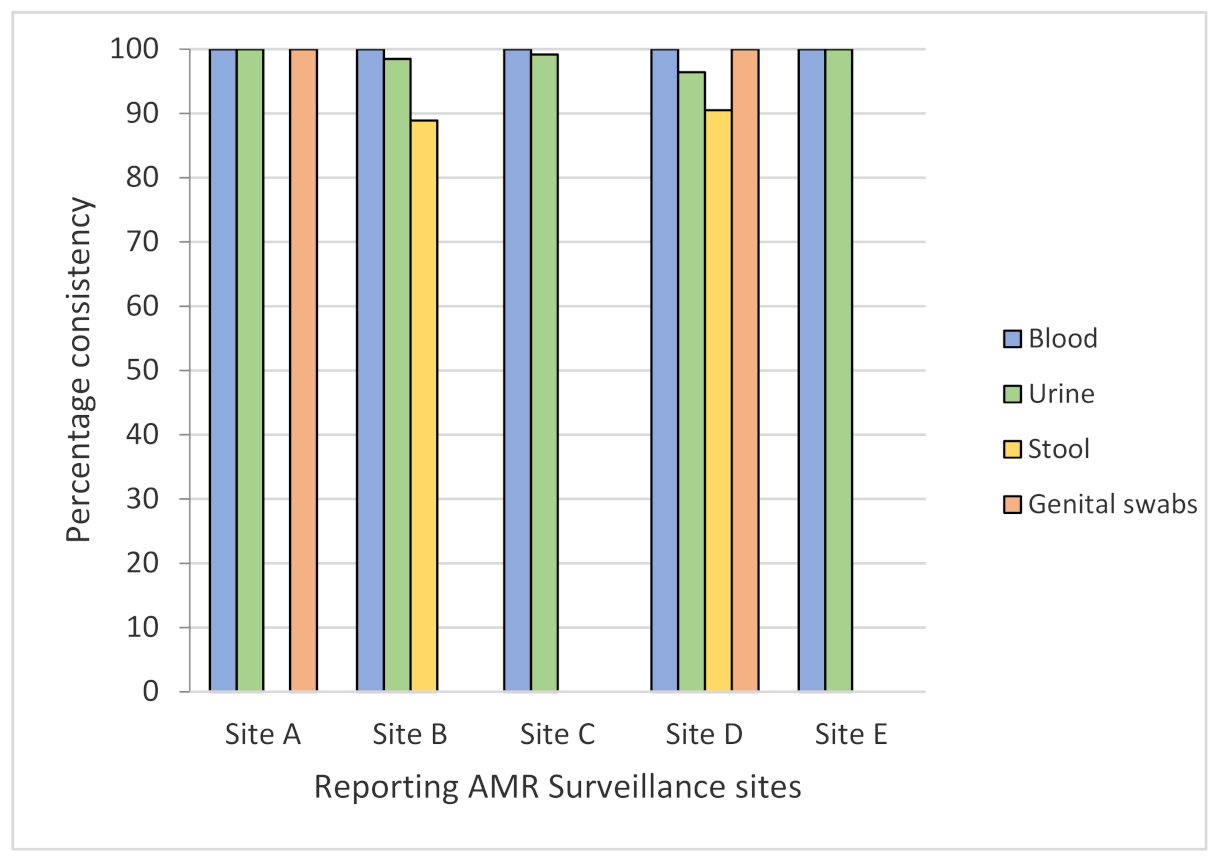
3.1. Consistency, Completeness, and Timeliness of AMR Surveillance Data
3.2. Barriers in Reporting AMR Surveillance Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Open Access Statement and Disclaimer
References
- WHO. Sixty-Eighth World Health Assembly; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- WHO. WHO Library Cataloguing-in-Publication Data Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- WHO. Global Antimicrobial Resistance Surveillance System (GLASS) Report 2016-17; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Yam, E.L.Y.; Hsu, L.Y.; Yap, E.P.; Yeo, T.W.; Lee, V.; Schlundt, J.; Lwin, M.O.; Limmathurotsakul, D.; Jit, M.; Dedon, P.; et al. Antimicrobial Resistance in the Asia Pacific region: A meeting report. Antimicrob. Resist. Infect. Control 2019, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Nsubuga, P.; White, M.E.; Thacker, S.B.; Anderson, M.A.; Blount, S.B.; Broome, C.V.; Chiller, T.M.; Espitia, V.; Imtiaz, R.; Sosin, D.; et al. Public Health Surveillance: A Tool for Targeting and Monitoring Interventions. In Disease Control Priorities in Developing Countries, 2nd ed.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2006; Chapter 53; pp. 997–1016. [Google Scholar]
- NPHL. Antimicrobial Resistance Surveillance Programme, Monthly Bulletin April 2017. Available online: https://www.nphl.gov.np/images/post-pictures/1495344122-amr-bulletin-april.PDF (accessed on 1 April 2017).
- DoHS. Annual Report 2073-74; Department of Health Services: Kathmandu, Nepal, 2018. [Google Scholar]
- WHO. Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2017-18; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- WHO. WHONET Software. Available online: http://www.who.int/medicines/areas/rational_use/AMR_WHONET_SOFTWARE/en/ (accessed on 11 February 2021).
- Vernet, G.; Mary, C.; Altmann, D.M.; Doumbo, O.; Morpeth, S.; Bhutta, Z.A.; Klugman, K.P. Surveillance for antimicrobial drug resistance in under-resourced countries. Emerg. Infect. Dis. 2014, 20, 434–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnall, J.; Rajkhowa, A.; Ikuta, K.; Rao, P.; Moore, C.E. Surveillance and monitoring of antimicrobial resistance: Limitations and lessons from the GRAM project. BMC Med. 2019, 17, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vong, S.; Anciaux, A.; Hulth, A.; Stelling, J.; Thamlikitkul, V.; Gupta, S.; Fuks, J.M.; Walia, K.; Rattanumpawan, P.; Eremin, S.; et al. Using information technology to improve surveillance of antimicrobial resistance in South East Asia. BMJ 2017, 358, j3781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malla, S.; Dumre, S.P.; Shakya, G.; Kansakar, P.; Rai, B.; Hossain, A.; Nair, G.B.; Albert, M.J.; Sack, D.; Baker, S.; et al. The challenges and successes of implementing a sustainable antimicrobial resistance surveillance programme in Nepal. BMC Public Health 2014, 14, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillonetto, M.; Jordao, R.T.S.; Andraus, G.S.; Bergamo, R.; Rocha, F.B.; Onishi, M.C.; de Almeida, B.M.M.; Nogueira, K.D.S.; Dal Lin, A.; Dias, V.; et al. The Experience of Implementing a National Antimicrobial Resistance Surveillance System in Brazil. Front. Public Health 2020, 8, 575536. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, A.; Suzuki, S. Japan nosocomial infections surveillance (JANIS): A model of sustainable national antimicrobial resistance surveillance based on hospital diagnostic microbiology laboratories. BMC Health Serv. Res. 2018, 18, 799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirijatuphat, R.; Sripanidkulchai, K.; Boonyasiri, A.; Rattanaumpawan, P.; Supapueng, O.; Kiratisin, P.; Thamlikitkul, V. Implementation of global antimicrobial resistance surveillance system (GLASS) in patients with bacteremia. PLoS ONE 2018, 13, e0190132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajihara, T.; Yahara, K.; Stelling, J.; Eremin, S.R.; Tornimbene, B.; Thamlikitkul, V.; Hirabayashi, A.; Anzai, E.; Wakai, S.; Matsunaga, N.; et al. Comparison of de-duplication methods used by WHO Global Antimicrobial Resistance Surveillance System (GLASS) and Japan Nosocomial Infections Surveillance (JANIS) in the surveillance of antimicrobial resistance. PLoS ONE 2020, 15, e0228234. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.N.; Bhatta, D.R.; Ansari, M.T.; Tiwari, H.K.; Mathuria, J.P.; Gaur, A.; Supram, H.S.; Gokhale, S. Application of WHONET in the Antimicrobial Resistance Surveillance of Uropathogens: A First User Experience from Nepal. J. Clin. Diagn. Res. 2013, 7, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Hazim, C.; Abubeker Ibrahim, R.; Westercamp, M.; Belete, G.A.; Amare Kibret, B.; Kanter, T.; Yimer, G.; Adem, T.S.; Stevenson, K.B.; Urrego, M.; et al. Establishment of a Sentinel Laboratory-Based Antimicrobial Resistance Surveillance Network in Ethiopia. Health Secur. 2018, 16, S30–S36. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.A.; Teshal, A.M.; Dinku, S.F.; Abera, N.A.; Negeri, A.A.; Desta, F.G.; Seyum, E.T.; Gemeda, A.W.; Keficho, W.M. Antimicrobial resistance surveillance in Ethiopia: Implementation experiences and lessons learned. Afr. J. Lab. Med. 2018, 7, 770. [Google Scholar] [CrossRef] [Green Version]
| Variables | Overall | Site A | Site B | Site C | Site D | Site E | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Consistent (n 3) | % | Total (n) | Consistent (n) | % | Total (n) | Consistent (n) | % | Total (n) | Consistent (n) | % | Total (n) | Consistent (n) | % | Total (n) | Consistent (n) | % | |
| Total number of records | 1584 | 1038 | 66 | 428 | 223 | 52 | 381 | 221 | 58 | 372 | 296 | 80 | 232 | 204 | 88 | 171 | 94 | 55 |
| Escherichia coli | 1020 | 641 | 63 | 354 | 172 | 49 | 220 | 110 | 50 | 218 | 199 | 91 | 109 | 93 | 85 | 119 | 67 | 56 |
| Klebsiella pneumoniae | 242 | 133 | 55 | 31 | 15 | 48 | 67 | 37 | 55 | 94 | 50 | 53 | 10 | 7 | 70 | 40 | 24 | 60 |
| Acinetobacter spp. 4 | 64 | 35 | 55 | 4 | 4 | 100 | 15 | 0 | 0 | 44 | 31 | 71 | 1 | 0 | 0 | 0 | N/A 5 | N/A |
| Staphylococcus aureus | 77 | 64 | 83 | 4 | 0 | 0 | 19 | 19 | 100 | 16 | 16 | 10 | 29 | 29 | 100 | 9 | 0 | 0 |
| Streptococcus pneumoniae | 1 | 0 | 0 | 0 | N/A | N/A | 0 | NA | N/A | 0 | NA | N/A | 1 | 0 | 0 | 0 | N/A | N/A |
| Salmonella spp. | 159 | 153 | 96 | 34 | 32 | 94 | 56 | 55 | 98 | 0 | N/A | N/A | 66 | 63 | 95 | 3 | 3 | 100 |
| Shigella spp. | 17 | 12 | 71 | 0 | N/A | N/A | 3 | 0 | 0 | 0 | N/A | N/A | 14 | 12 | 86 | 0 | N/A | N/A |
| Neisseria gonorrhoeae | 4 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | N/A | N/A | 2 | 0 | 0 | 0 | N/A | N/A |
| Variables | Site A | Site B | Site C | Site D | Site E | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n 1 | (%) | N | (%) | n | (%) | n | (%) | n | (%) | |
| Total records | 580 | 100 | 3164 | 100 | 810 | 100 | 265 | 100 | 341 | 100 |
| Age | 580 | 100 | 3164 | 100 | 810 | 100 | 264 | 99.6 | 341 | 100 |
| Sex | 580 | 100 | 3164 | 100 | 810 | 100 | 264 | 99.6 | 341 | 100 |
| Origin | 536 | 92.4 | MD 2 | 0 | MD | 0 | 122 | 46 | 341 | 100 |
| Date of isolation | 580 | 100 | 3164 | 100 | 810 | 100 | 232 | 87.5 | 341 | 100 |
| Specimen | 575 | 99.1 | 3164 | 100 | 810 | 100 | 232 | 87.5 | 289 | 84.8 |
| Isolate | 548 | 94.5 | 3164 | 100 | 810 | 100 | 232 | 87.5 | 289 | 84.8 |
| Antibiotic susceptibility results | 548 | 94.5 | 3126 | 98.8 | 810 | 100 | 232 | 87.5 | 273 | 80 |
| Specimen–pathogen combination | 428 | 73.8 | 1461 | 46.2 | 724 | 89.4 | 232 | 87.5 | 289 | 84.8 |
| Pathogen–antibacterial combination | 428 | 73.8 | 1490 | 47.1 | 724 | 89.4 | 232 | 87.9 | 289 | 84.8 |
| Variables | Site A | Site B | Site C | Site D | Site E | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n 1 | (%) | N | (%) | n | (%) | n | (%) | n | (%) | |
| Total records | 580 | 100 | 3164 | 100 | 810 | 100 | 265 | 100 | 341 | 100 |
| Age | 580 | 100 | 3164 | 100 | 810 | 100 | 264 | 99.6 | 341 | 100 |
| Sex | 580 | 100 | 3164 | 100 | 810 | 100 | 264 | 99.6 | 341 | 100 |
| Origin | 536 | 92.4 | MD 2 | 0 | MD | 0 | 122 | 46 | 341 | 100 |
| Date of isolation | 580 | 100 | 3164 | 100 | 810 | 100 | 232 | 87.5 | 341 | 100 |
| Specimen | 575 | 99.1 | 3164 | 100 | 810 | 100 | 232 | 87.5 | 289 | 84.8 |
| Isolate | 548 | 94.5 | 3164 | 100 | 810 | 100 | 232 | 87.5 | 289 | 84.8 |
| Antibiotic susceptibility results | 548 | 94.5 | 3126 | 98.8 | 810 | 100 | 232 | 87.5 | 273 | 80 |
| Specimen–pathogen combination | 428 | 73.8 | 1461 | 46.2 | 724 | 89.4 | 232 | 87.5 | 289 | 84.8 |
| Pathogen–antibacterial combination | 428 | 73.8 | 1490 | 47.1 | 724 | 89.4 | 232 | 87.9 | 289 | 84.8 |
| Variable | Site A | Site B | Site C | Site D | Site E | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n 2 | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Completeness | 3826 | 99 | 3031 | 88 | 2976 | 89 | 1978 | 95 | 1539 | 100 |
| Consistency | 4803 | 92 | 21897 | 77 | 6308 | 87 | 2042 | 86 | 2793 | 91 |
| Timeliness | 0 | - | 0 | - | 1 | - | 0 | - | 1 | - |
| Mean delay (days) | 8 | - | 247 | - | 0 | - | 269 | - | 0 | - |
| Requirements | Response | |||
|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | Site 4 | |
| Number of microbiology staff | 15 | 8 | 4 | 7 |
| Number of rooms dedicated to data entry | One | One | None | None |
| Area of data entry room | <150 | <150 | N/A 2 | <150 |
| Availability of computer for data entry | Yes | No | No | Yes |
| Number of computers for data entry | 5 | One | N/A | 1 |
| Availability of Internet service | Yes | No | N/A | Yes |
| Speed of Internet service | >0.5 Mbps 3 | N/A | N/A | >0.5 Mbps |
| Availability of person for data entry | Yes | No | No | Yes |
| Qualification of data entry person | BSc.MLT 4 | BSc.MLT/CMLT 5 | N/A | BSc.MLT |
| Training received on AMR surveillance | Yes | Yes | Yes | No |
| When was AMR surveillance training received? | 2019 | 2019 | 2019 | N/A |
| Training received on data entry and analysis | Yes | Yes | Yes | No |
| When was data entry training received? | Every year | May 2019 | May 2019 | N/A |
| Training received on WHONET 6 | Yes | Yes | Yes | No |
| When was WHONET training received? | 2019 | 2019 | 2019 | N/A |
| Agreement/TOR 7 | Verbal | Verbal | Verbal | Verbal |
| Institutional restrictions on data sharing with the NPHL 8 | None | None | Verbal | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acharya, J.; Zolfo, M.; Enbiale, W.; Kyaw, K.W.Y.; Bhattachan, M.; Rijal, N.; Shrestha, A.; Shrestha, B.; Madhup, S.K.; Raghubanshi, B.R.; et al. Quality Assessment of an Antimicrobial Resistance Surveillance System in a Province of Nepal. Trop. Med. Infect. Dis. 2021, 6, 60. https://doi.org/10.3390/tropicalmed6020060
Acharya J, Zolfo M, Enbiale W, Kyaw KWY, Bhattachan M, Rijal N, Shrestha A, Shrestha B, Madhup SK, Raghubanshi BR, et al. Quality Assessment of an Antimicrobial Resistance Surveillance System in a Province of Nepal. Tropical Medicine and Infectious Disease. 2021; 6(2):60. https://doi.org/10.3390/tropicalmed6020060
Chicago/Turabian StyleAcharya, Jyoti, Maria Zolfo, Wendemagegn Enbiale, Khine Wut Yee Kyaw, Meika Bhattachan, Nisha Rijal, Anjana Shrestha, Basudha Shrestha, Surendra Kumar Madhup, Bijendra Raj Raghubanshi, and et al. 2021. "Quality Assessment of an Antimicrobial Resistance Surveillance System in a Province of Nepal" Tropical Medicine and Infectious Disease 6, no. 2: 60. https://doi.org/10.3390/tropicalmed6020060
APA StyleAcharya, J., Zolfo, M., Enbiale, W., Kyaw, K. W. Y., Bhattachan, M., Rijal, N., Shrestha, A., Shrestha, B., Madhup, S. K., Raghubanshi, B. R., Kattel, H. P., Rajbhandari, P., Bhandari, P., Thakur, S., Sharma, S., Singh, D. R., & Jha, R. (2021). Quality Assessment of an Antimicrobial Resistance Surveillance System in a Province of Nepal. Tropical Medicine and Infectious Disease, 6(2), 60. https://doi.org/10.3390/tropicalmed6020060







