The Interaction of Diabetes and Tuberculosis: Translating Research to Policy and Practice
Abstract
1. Introduction
2. Epidemiological Effects of Diabetes on Tuberculosis
2.1. Diabetes and Natural History of TB
2.2. Diabetes and Risk of M. tuberculosis Infection
2.3. Intermediate Hyperglycaemia and TB
2.4. Effect of Diabetes on TB Disease Risk, Presentation and Treatment Outcomes
3. Screening and Diagnosis of Combined Diabetes and Tuberculosis
4. Treatment of Combined Diabetes and Tuberculosis
4.1. Tuberculosis Treatment in Patients with Comorbid Diabetes
4.2. Optimising Diabetes Management in Patients with Combined Tuberculosis
4.3. Glycaemic Control
4.4. Adjustment of Treatment According to Patient Characteristics and Local Circumstances
4.5. Cardiovascular Risk Assessment and Management
5. Control of Diabetes-Associated Tuberculosis at a Population Level
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Root, H.F. Diabetic Coma and Pulmonary Tuberculosis. Trans. Am. Clin. Clim. Assoc. 1934, 50, 210–217. [Google Scholar]
- Luntz, G. Tuberculous diabetics: The Birmingham Regional Service. Lancet 1954, 266, 973–974. [Google Scholar] [CrossRef]
- Hu, F.B. Globalization of Diabetes: The role of diet, lifestyle, and genes. Diabetes Care 2011, 34, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hong, Y.; Lew, W.; Yang, S.; Lee, E. Incidence of pulmonary tuberculosis among diabetics. Tuber. Lung Dis. 1995, 76, 529–533. [Google Scholar] [CrossRef]
- Pablos-Méndez, A.; Blustein, J.; Knirsch, C.A. The role of diabetes mellitus in the higher prevalence of tuberculosis among His-panics. Am. J. Public Health 1997, 87, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, C.R.; Critchley, J.A.; Forouhi, N.G.; Roglic, G.; Williams, B.G.; Dye, C.; Unwin, N.C. Diabetes and the risk of tuberculosis: A neglected threat to public health? Chronic Illn. 2007, 3, 228–245. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.Y.; Murray, M.B. Diabetes mellitus increases the risk of active tuberculosis: A systematic review of 13 observational studies. PLoS Med. 2008, 5, e152. [Google Scholar]
- Kapur, A.; Harries, A.D.; Lönnroth, K.; Wilson, P.; Sulistyowati, L.S. Diabetes and tuberculosis co-epidemic: The Bali Declaration. Lancet Diabetes Endocrinol. 2016, 4, 8–10. [Google Scholar] [CrossRef]
- van Crevel, R.; Dockrell, H.M. TANDEM: Understanding diabetes and tuberculosis. Lancet Diabetes Endocrinol. 2014, 2, 270–272. [Google Scholar] [CrossRef]
- Lin, Y.; Harries, A.D.; Kumar, A.M.V.; Critchley, J.A.; Van Crevel, R.; Owiti, P.; Dlodlo, R.A.; Kapur, A. Tackling diabetes mellitus and tuberculosis: A new Union guide on the management of diabetes-tuberculosis. Int. J. Tuberc. Lung Dis. 2019, 23, 771–772. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; Fernandes, J.D.R.; Ohlrogge, A.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pr. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- van Crevel, R.; van de Vijver, S.; Moore, D.A.J. The global diabetes epidemic: What does it mean for infectious diseases in tropical countries? Lancet Diabetes Endocrinol. 2016, 5, 457–468. [Google Scholar] [CrossRef]
- Pearson-Stuttard, J.; Blundell, S.; Harris, T.; Cook, D.G.; Critchley, J.A. Diabetes and infection: Assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 2016, 4, 148–158. [Google Scholar] [CrossRef]
- Barron, E.; Bakhai, C.; Kar, P.; Weaver, A.; Bradley, D.; Ismail, H.; Knighton, P.; Holman, N.; Khunti, K.; Sattar, N.; et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: A whole-population study. Lancet Diabetes Endocrinol. 2020, 8, 813–822. [Google Scholar] [CrossRef]
- Carey, I.; Critchley, J.A.; Dewilde, S.; Harris, T.; Hosking, F.J.; Cook, D.G. Risk of Infection in Type 1 and Type 2 Diabetes Compared With the General Population: A Matched Cohort Study. Diabetes Care 2018, 41, 513–521. [Google Scholar] [CrossRef]
- Kamper-Jørgensen, Z.; Carstensen, B.; Norredam, M.; Bygbjerg, I.C.; Andersen, P.H.; Jørgensen, M.E. Diabetes-related tuberculosis in Denmark: Effect of ethnicity, diabetes duration and year of diagnosis. Int. J. Tuberc. Lung Dis. 2015, 19, 1169–1175. [Google Scholar] [CrossRef]
- Awad, S.F.; Dargham, S.R.; Omori, R.; Pearson, F.; Critchley, J.A.; Abu-Raddad, L.J. Analytical Exploration of Potential Pathways by which Diabetes Mellitus Impacts Tuberculosis Epidemiology. Sci. Rep. 2019, 9, 8494. [Google Scholar] [CrossRef]
- Lee, M.-R.; Huang, Y.-P.; Kuo, Y.-T.; Luo, C.H.; Shih, Y.J.; Shu, C.C.; Wang, J.-Y.; Ko, J.-C.; Yu, C.-J.; Lin, H.-H. Diabetes Mellitus and Latent Tuberculosis Infection: A Systematic Review and Metaa-nalysis. Clin. Infect. Dis. 2017, 64, 719–727. [Google Scholar] [CrossRef]
- Lin, C.-H.; Kuo, S.-C.; Hsieh, M.-C.; Ho, S.-Y.; Su, I.-J.; Lin, S.-H.; Chi, C.-Y.; Su, S.-L.; Liao, C.-Y.; Chen, Y.-C.; et al. Effect of diabetes mellitus on risk of latent TB infection in a high TB incidence area: A community-based study in Taiwan. BMJ Open 2019, 9, e029948. [Google Scholar] [CrossRef]
- Hensel, R.L.; Kempker, R.R.; Tapia, J.; Oladele, A.; Blumberg, H.M.; Magee, M.J. Increased risk of latent tuberculous infection among persons with pre-diabetes and diabetes mellitus. Int. J. Tuberc. Lung Dis. 2016, 20, 71–78. [Google Scholar] [CrossRef]
- Wang, L.; Gao, P.; Zhang, M.; Huang, Z.; Zhang, D.; Deng, Q.; Linhong, W.; Zhao, Z.; Qin, X.; Zhengjing, H.; et al. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA 2017, 317, 2515–2523. [Google Scholar] [CrossRef] [PubMed]
- Anjana, R.M.; Deepa, M.; Pradeepa, R.; Mahanta, J.; Narain, K.; Das, H.K.; Adhikari, P.; Rao, P.V.; Saboo, B.; Kumar, A.; et al. Prevalence of diabetes and prediabetes in 15 states of India: Results from the ICMR–INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017, 5, 585–596. [Google Scholar] [CrossRef]
- Mave, V.; Meshram, S.; Lokhande, R.; Kadam, D.; Dharmshale, S.; Bharadwaj, R.; Kagal, A.; Pradhan, N.; Deshmukh, S.; Atre, S.; et al. Prevalence of dysglycemia and clinical presentation of pulmonary tuberculosis in Western India. Int. J. Tuberc. Lung Dis. 2017, 21, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Al-Rifai, R.H.; Pearson, F.; Critchley, J.A.; Abu-Raddad, L.J. Association between diabetes mellitus and active tuberculosis: A sys-tematic review and meta-analysis. PLoS ONE 2017, 12, e0187967. [Google Scholar] [CrossRef] [PubMed]
- Riza, A.L.; Pearson, F.; Ugarte-Gil, C.; Alisjahbana, B.; Van De Vijver, S.; Panduru, N.M.; Hill, P.C.; Ruslami, R.; Moore, D.; Aarnoutse, R.; et al. Clinical management of concurrent diabetes and tuberculosis and the implications for patient services. Lancet Diabetes Endocrinol. 2014, 2, 740–753. [Google Scholar] [CrossRef]
- Tornheim, J.A.; Dooley, K.E. Tuberculosis Associated with HIV Infection. Microbiol. Spectr. 2017, 5, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, P.; Ugarte-Gil, C.; Golub, J.; Pearson, F.; Critchley, J. The effects of diabetes on tuberculosis treatment outcomes: An updated systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 2019, 23, 783–796. [Google Scholar] [CrossRef]
- Huaman, M.A.; Kryscio, R.J.; Fichtenbaum, C.J.; Henson, D.; Salt, E.; Sterling, T.R.; Garvy, B.A. Tuberculosis and risk of acute myocardial infarction: A propensity score-matched analysis. Epidemiology Infect. 2017, 145, 1363–1367. [Google Scholar] [CrossRef]
- Chung, W.-S.; Lin, C.-L.; Hung, C.-T.; Chu, Y.-H.; Sung, F.-C.; Kao, C.-H.; Yeh, J.-J. Tuberculosis increases the subsequent risk of acute coronary syndrome: A nationwide population-based cohort study. Int. J. Tuberc. Lung Dis. 2014, 18, 79–83. [Google Scholar] [CrossRef]
- Sheu, J.-J.; Chiou, H.-Y.; Kang, J.-H.; Chen, Y.-H.; Lin, H.-C. Tuberculosis and the risk of ischemic stroke: A 3-year follow-up study. Stroke 2010, 41, 244–249. [Google Scholar] [CrossRef]
- Faurholt-Jepsen, D.; Range, N.; PrayGod, G.A.; Jeremiah, K.; Faurholt-Jepsen, M.; Aabye, M.G.; Changalucha, J.; Christensen, D.L.; Grewal, H.M.S.; Martinussen, T.; et al. Diabetes is a strong predictor of mortality during tuberculosis treatment: A prospective cohort study among tuberculosis patients from Mwanza, Tanzania. Trop. Med. Int. Health 2013, 18, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.W.; Choi, H.; Lee, S.Y.; Lee, M.; Kim, Y.; Park, H.; Lee, J.; Zhan, X.; Kang, H.; Hwang, S.; et al. Impact of Diabetes and Smoking on Mortality in Tuberculosis. PLoS ONE 2013, 8, e58044. [Google Scholar] [CrossRef] [PubMed]
- Salindri, A.D.; Wang, J.-Y.; Lin, H.-H.; Magee, M.J. Post-tuberculosis incidence of diabetes, myocardial infarction, and stroke: Ret-rospective cohort analysis of patients formerly treated for tuberculosis in Taiwan, 2002–2013. Int. J. Infect. Dis. 2019, 84, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Huaman, M.A.; Ticona, E.; Miranda, G.; Kryscio, R.J.; Mugruza, R.; Aranda, E.; Rondan, P.L.; Henson, D.; Ticona, C.; Sterling, T.R.; et al. The Relationship Between Latent Tuberculosis Infection and Acute Myocardial Infarction. Clin. Infect. Dis. 2017, 66, 886–892. [Google Scholar] [CrossRef]
- Magee, M.J.; Salindri, A.D.; Gujral, U.P.; Auld, S.C.; Bao, J.; Haw, J.S.; Lin, H.-H.; Kornfeld, H. Convergence of non-communicable diseases and tuberculosis: A two-way street? Int. J. Tuberc. Lung Dis. 2018, 22, 1258–1268. [Google Scholar] [CrossRef]
- Huangfu, P.; Laurence, Y.V.; Alisjahbana, B.; Ugarte-Gil, C.; Riza, A.-L.; Walzl, G.; Ruslami, R.; Moore, D.A.J.; Ioana, M.; McAllister, S.; et al. Point of care HbA1c level for diabetes mellitus management and its accuracy among tuberculosis patients: A study in four countries. Int. J. Tuberc. Lung Dis. 2019, 23, 283–292. [Google Scholar] [CrossRef]
- Shewade, H.D.; Jeyashree, K.; Mahajan, P.; Shah, A.N.; Kirubakaran, R.; Rao, R.; Kumar, A.M.V. Effect of glycemic control and type of diabetes treatment on unsuccessful TB treatment outcomes among people with TB-Diabetes: A systematic review. PLoS ONE 2017, 12, e0186697. [Google Scholar] [CrossRef]
- Mi, F.; Tan, S.; Liang, L.; Harries, A.D.; Hinderaker, S.G.; Lin, Y.; Yue, W.; Chen, X.; Liang, B.; Gong, F.; et al. Diabetes mellitus and tuberculosis: Pattern of tuberculosis, two-month smear conversion and treatment outcomes in Guangzhou, China. Trop. Med. Int. Health 2013, 18, 1379–1385. [Google Scholar] [CrossRef]
- Nandakumar, K.V.; Duraisamy, K.; Balakrishnan, S.; Sunilkumar, M.; Sagili, K.D.; Satyanarayana, S.; Kumar, A.M.V.; Enarson, D.A. Outcome of tuberculosis treatment in patients with diabetes mellitus treated in the revised national tuberculosis control programme in Malappuram District, Kerala, India. PLoS ONE 2013, 8, e76275. [Google Scholar]
- Magee, M.; Bloss, E.; Shin, S.S.; Contreras, C.; Huaman, H.A.; Ticona, J.C.; Bayona, J.; Bonilla, C.A.; Yagui, M.; Jave, O.; et al. Clinical characteristics, drug resistance, and treatment outcomes among tuberculosis patients with diabetes in Peru. Int. J. Infect. Dis. 2013, 17, e404–e412. [Google Scholar] [CrossRef]
- Bai, K.-J.; Lee, J.-J.; Chien, S.-T.; Suk, C.-W.; Chiang, C.-Y. The Influence of Smoking on Pulmonary Tuberculosis in Diabetic and Non-Diabetic Patients. PLoS ONE 2016, 11, e0156677. [Google Scholar] [CrossRef] [PubMed]
- Wagnew, F.; Eshetie, S.; Alebel, A.; Dessie, G.; Leshargie, C.T.; Abajobir, A.A. Meta-analysis of the prevalence of tuberculosis in diabetic patients and its association with cigarette smoking in African and Asian countries. BMC Res. Notes 2018, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.; Wilkinson, E.; Rinu, P.K.; Maung, T.M.; Bachani, D.; Punia, J.S.; Jain, S.; Yadav, T.; Jarhyan, P.; Mohan, S.; et al. Tuberculosis-diabetes screening: How well are we doing? A mixed-methods study from North India. Public Health Action 2019, 9, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Grint, D.; Alisjhabana, B.; Ugarte-Gil, C.; Riza, A.-L.; Walzl, G.; Pearson, F.; Ruslami, R.; Moore, D.A.J.; Ioana, M.; McAllister, S.; et al. Accuracy of diabetes screening methods used for people with tuberculosis, Indonesia, Peru, Romania, South Africa. Bull. World Health Organ. 2018, 96, 738–749. [Google Scholar] [CrossRef]
- Berkowitz, N.; Okorie, A.; Goliath, R.; Levitt, N.; Wilkinson, K.A.; Oni, T. The prevalence and determinants of active tuberculosis among diabetes patients in Cape Town, South Africa, a high HIV/TB burden setting. Diabetes Res. Clin. Pr. 2018, 138, 16–25. [Google Scholar] [CrossRef]
- Soe, K.T.; Satyanarayana, S.; Saw, S.; San, C.C.; Aung, S.T. Gaps in Implementing Bidirectional Screening for Tuberculosis and Diabetes Mellitus in Myanmar: An Operational Research Study. Trop Med. Infect. Dis. 2020, 5, 19. [Google Scholar]
- Alisjahbana, B.; McAllister, S.M.; Ugarte-Gil, C.; Panduru, N.M.; Ronacher, K.; Koesoemadinata, R.C.; Zubiate, C.; Riza, A.L.; Malherbe, S.T.; Kleynhans, L.; et al. Screening diabetes mellitus patients for pulmonary tuberculosis: A multisite study in Indonesia, Peru, Romania and South Africa. Trans. R. Soc. Trop. Med. Hyg. 2020, 5, e152. [Google Scholar] [CrossRef]
- Mave, V.; Nimkar, S.; Prasad, H.; Kadam, D.; Meshram, S.; Lokhande, R.; Gupte, N.; Jain, D.; Gupta, A.; Golub, J.E. Tuberculosis screening among persons with diabetes mellitus in Pune, India. BMC Infect. Dis. 2017, 17, 388. [Google Scholar] [CrossRef]
- Majumder, A.; Carroll, B.; Bhana, S.; Tefu, D.; Syeda, S.; Martinson, N.; Golub, J. Screening for active tuberculosis in a diabetes mellitus clinic in Soweto, South Africa. Int. J. Tuberc. Lung Dis. 2016, 20, 992–993. [Google Scholar] [CrossRef]
- Ji, Y.; Cao, H.; Liu, Q.; Li, Z.; Song, H.; Xu, D.; Tian, D.; Qiu, B.; Wang, J.-M. Screening for pulmonary tuberculosis in high-risk groups of diabetic patients. Int. J. Infect. Dis. 2020, 93, 84–89. [Google Scholar] [CrossRef]
- Liu, Q.; Li, W.; Xue, M.; Chen, Y.; Du, X.; Wang, C.; Han, L.; Tang, Y.; Feng, Y.; Tao, C.; et al. Diabetes mellitus and the risk of multidrug resistant tuberculosis: A meta-analysis. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiao, H.; Sugawara, I. Tuberculosis complicated by diabetes mellitus at shanghai pulmonary hospital, china. Jpn. J. Infect. Dis. 2009, 62, 390–391. [Google Scholar] [PubMed]
- Wang, J.-Y.; Lee, M.-C.; Shu, C.-C.; Lee, C.H.; Lee, L.N.; Chao, K.M.; Chang, F.Y. Optimal duration of anti-TB treatment in patients with diabetes: Nine or six months? Chest 2015, 147, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Nijland, H.M.J.; Ruslami, R.; Stalenhoef, J.E.; Nelwan, E.J.; Alisjahbana, B.; Nelwan, R.H.H.; Van Der Ven, A.J.A.M.; Danusantoso, H.; Aarnoutse, R.E.; Van Crevel, R. Exposure to Rifampicin Is Strongly Reduced in Patients with Tuberculosis and Type 2 Diabetes. Clin. Infect. Dis. 2006, 43, 848–854. [Google Scholar] [CrossRef]
- Kumar, A.K.H.; Chandrasekaran, V.; Kannan, T.; Murali, A.L.; Lavanya, J.; Sudha, V.; Swaminathan, S.; Ramachandran, G. Anti-tuberculosis drug concentrations in tuberculosis patients with and without diabetes mellitus. Eur. J. Clin. Pharmacol. 2016, 73, 65–70. [Google Scholar] [CrossRef]
- Ruslami, R.; Nijland, H.M.J.; Adhiarta, I.G.N.; Kariadi, S.H.K.S.; Alisjahbana, B.; Aarnoutse, R.E.; Van Crevel, R. Pharmacokinetics of Antituberculosis Drugs in Pulmonary Tuberculosis Patients with Type 2 Diabetes. Antimicrob. Agents Chemother. 2009, 54, 1068–1074. [Google Scholar] [CrossRef]
- Alkabab, Y.; Keller, S.; Dodge, D.; Houpt, E.R.; Staley, D.; Heysell, S. Early interventions for diabetes related tuberculosis associate with hastened sputum microbiological clearance in Virginia, USA. BMC Infect. Dis. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Ruesen, C.; Chaidir, L.; Ugarte-Gil, C.; Van Ingen, J.; Critchley, J.A.; Hill, P.C.; Ruslami, R.; Santoso, P.; Huynen, M.A.; Dockrell, H.M.; et al. Diabetes is associated with genotypically drug-resistant tuberculosis. Eur. Respir. J. 2020, 55, 1901891. [Google Scholar] [CrossRef]
- Ronacher, K.; van Crevel, R.; Critchley, J.A.; Bremer, A.A.; Schlesinger, L.S.; Kapur, A.; Basaraba, R.; Kornfeld, H.; Restrepo, B.I. Defining a Research Agenda to Address the Converging Epidemics of Tuber-culosis and Diabetes: Part 2: Underlying Biologic Mechanisms. Chest 2017, 152, 174–180. [Google Scholar] [CrossRef]
- Maganga, E.; Smart, L.R.; Kalluvya, S.; Kataraihya, J.B.; Saleh, A.M.; Obeid, L.; Downs, J.A.; Fitzgerald, D.W.; Peck, R.N. Glucose Metabolism Disorders, HIV and Antiretroviral Therapy among Tanzanian Adults. PLoS ONE 2015, 10, e0134410. [Google Scholar] [CrossRef]
- Mohammed, A.E.; Shenkute, T.Y.; Gebisa, W.C. Diabetes mellitus and risk factors in human immunodeficiency virus-infected in-dividuals at Jimma University Specialized Hospital, Southwest Ethiopia. Diabetes Metab. Syndr. Obes. 2015, 8, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, Z.; Dave, J.A.; Maartens, G.; Levitt, N.S. Changes in blood pressure, glucose levels, insulin secretion and anthropometry after long term exposure to antiretroviral therapy in South African women. AIDS Res. Ther. 2015, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Werfalli, M.; Engel, M.E.; Musekiwa, A.; Kengne, A.P.; Levitt, N.S. The prevalence of type 2 diabetes among older people in Africa: A systematic review. Lancet Diabetes Endocrinol. 2016, 4, 72–84. [Google Scholar] [CrossRef]
- Ugarte-Gil, C.; Alisjahbana, B.; Ronacher, K.; Riza, A.L.; Koesoemadinata, R.C.; Malherbe, S.T.; Cioboata, R.; Llontop, J.C.; Kleynhans, L.; Lopez, S.; et al. Diabetes mellitus among pulmonary tuberculosis patients from four TB-endemic countries: The TANDEM study. Clin. Infect. Dis. 2019, 2, 56. [Google Scholar]
- Herman, W.H.; Petersen, M.; Kalyani, R.R. Response to Comment on American Diabetes Association. Standards of Medical Care in Diabetes-2017. Diabetes Care 2017;40(Suppl. 1):S1-S135. Diabetes Care 2017, 40, e94–e95. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Wilt, T.J.; Kansagara, D.; Kansagara, D.; Horwitch, C.; Barry, M.J.; Forciea, M.A.; Clinical Guidelines Committee of the American College of Physicians. Hemoglobin A 1cTargets for Glycemic Control With Pharmacologic Therapy for Nonpregnant Adults With Type 2 Diabetes Mellitus: A Guidance Statement Update From the American College of Physicians. Ann. Intern. Med. 2018, 168, 569. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A.W. 10-Year Follow-up of Intensive Glucose Control in Type 2 Diabetes. New Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef]
- Boillat-Blanco, N.; Ramaiya, K.L.; Mganga, M.; Minja, L.T.; Bovet, P.; Schindler, C.; Von Eckardstein, A.; Gagneux, S.; Daubenberger, C.; Reither, K.; et al. Transient Hyperglycemia in Patients With Tuberculosis in Tanzania: Impli-cations for Diabetes Screening Algorithms. J. Infect. Dis. 2016, 213, 1163–1172. [Google Scholar] [CrossRef]
- Alisjahbana, B.; Sahiratmadja, E.; Nelwan, E.J.; Purwa, A.M.; Ahmad, Y.; Ottenhoff, T.H.; Nelwan, R.H.H.; Parwati, I.; van der Meer, J.W.M.; van Crevel, R. The effect of type 2 diabetes mellitus on the presentation and treatment re-sponse of pulmonary tuberculosis. Clin. Infect. Dis. 2007, 45, 428–435. [Google Scholar] [CrossRef]
- Te Brake, L.H.M.; Yunivita, V.; Livia, R.; Soetedjo, N.; van Ewijk-Beneken Kolmer, E.; Koenderink, J.B.; Burger, D.M.; Prayudi Santoso, P.; van Crevel, R.; Alisjahbana, B.; et al. Rifampicin Alters Metformin Plasma Exposure but Not Blood Glucose Levels in Diabetic Tuberculosis Patients. Clin. Pharmacol. Ther. 2019, 105, 730–737. [Google Scholar] [CrossRef]
- Singhal, A.; Jie, L.; Kumar, P.; Hong, G.S.; Leow, M.K.-S.; Paleja, B.; Tsenova, L.; Kurepina, N.; Chen, J.; Zolezzi, F.; et al. Metformin as adjunct antituberculosis therapy. Sci. Transl. Med. 2014, 6, 263ra159. [Google Scholar] [CrossRef]
- Degner, N.; Wang, J.-Y.; Golub, E.J.; Karakousis, P.C. Metformin Use Reverses the Increased Mortality Associated With Diabetes Mellitus During Tuberculosis Treatment. Clin. Infect. Dis. 2017, 66, 198–205. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Lipska, K.J.; Mayo, H.; Bailey, C.J.; McGuire, D.K. Metformin in patients with type 2 diabetes and kidney disease: A systematic review. JAMA 2014, 312, 2668–2675. [Google Scholar] [CrossRef] [PubMed]
- Ruslami, R.; Aarnoutse, R.E.; Alisjahbana, B.; Van Der Ven, A.J.A.M.; Van Crevel, R. Implications of the global increase of diabetes for tuberculosis control and patient care. Trop. Med. Int. Health 2010, 15, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Beran, D.; Ewen, M.; Laing, R. Constraints and challenges in access to insulin: A global perspective. Lancet Diabetes Endocrinol. 2016, 4, 275–285. [Google Scholar] [CrossRef]
- Grant, P. Management of diabetes in resource-poor settings. Clin. Med. 2013, 13, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Allain, T.J.; Van Oosterhout, J.J.; Douglas, G.P.; Joukes, S.; Gadabu, O.J.; Darts, C.; Kapur, A.; Harries, A.D. Applying lessons learnt from the ‘DOTS’ Tuberculosis Model to monitoring and evaluating persons with diabetes mellitus in Blantyre, Malawi. Trop. Med. Int. Health 2011, 16, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- van Crevel, R.; Koesoemadinata, R.; Hill, P.C.; Harries, A.D. Clinical management of combined tuberculosis and diabetes. Int. J. Tuberc. Lung Dis. 2018, 22, 1404–1410. [Google Scholar] [CrossRef]
- Noubiap, J.J.; Nansseu, J.R.; Nyaga, U.F.; Nkeck, J.R.; Endomba, F.T.; Kaze, A.D.; Agbor, V.N.; Bigna, J.J. Global prevalence of diabetes in active tuberculosis: A systematic review and me-ta-analysis of data from 2·3 million patients with tuberculosis. Lancet Glob. Health 2019, 7, e448–e460. [Google Scholar] [CrossRef]
- Lo, H.-Y.; Yang, S.-L.; Lin, H.-H.; Bai, K.J.; Lee, J.J.; Lee, T.I.; Chiang, C.Y. Does enhanced diabetes management reduce the risk and improve the outcome of tuber-culosis? Int. J. Tuberc. Lung Dis. 2016, 20, 376–382. [Google Scholar] [CrossRef]
- Ben Romdhane, B.H.; Tlili, F.; Skhiri, A.; Zaman, S.; Phillimore, P. Health system challenges of NCDs in Tunisia. Int. J. Public Health 2015, 60 (Suppl. 1), S39–S46. [Google Scholar] [CrossRef] [PubMed]
- Phillimore, P.; Zaman, S.; Ahmad, B.; Shoaibi, A.; Khatib, R.; Khatib, R.; Husseini, A.; Fouad, F.; Elias, M.; Maziak, W.; et al. Health system challenges of cardiovascular disease and diabetes in four Eastern Med-iterranean countries. Glob. Public Health 2013, 8, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Awad, S.F.; Critchley, J.A.; Abu-Raddad, L.J. Epidemiological impact of targeted interventions for people with diabetes mellitus on tuberculosis transmission in India: Modelling based predictions. Epidemics 2020, 30, 100381. [Google Scholar] [CrossRef] [PubMed]
- Soetedjo, N.N.M.; McAllister, S.M.; Ugarte-Gil, C.; Firanescu, A.G.; Ronacher, K.; Alisjahbana, B.; Costache, A.L.; Zubiate, C.; Malherbe, S.T.; Koesoemadinata, R.C.; et al. Disease characteristics and treatment of patients with diabetes mellitus attending government health services in Indonesia, Peru, Romania and South Africa. Trop. Med. Int. Health 2018, 23, 1118–1128. [Google Scholar] [CrossRef]
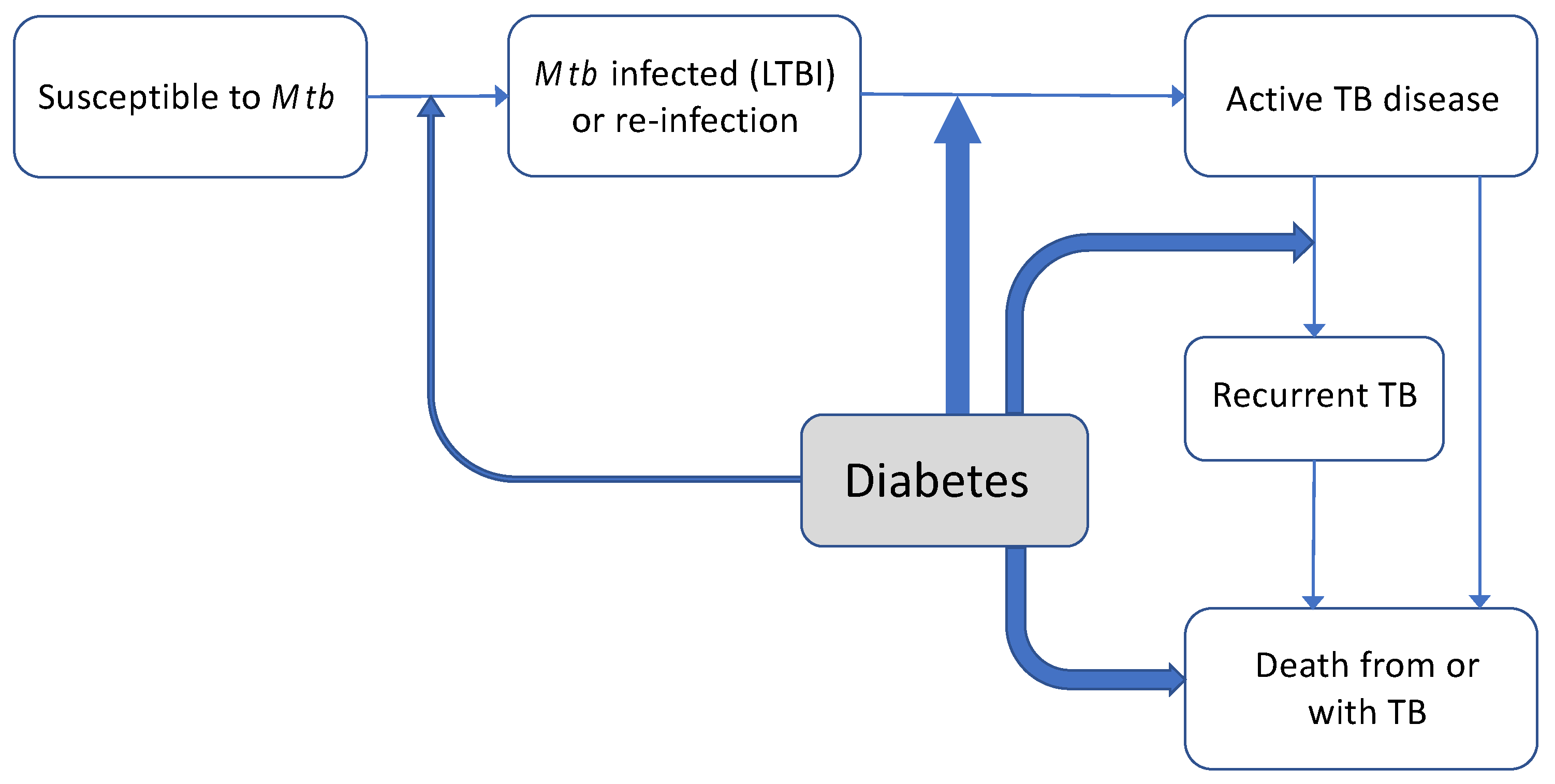
| Screening TB patients for DM |
|
| Screening DM patients for TB |
|
| Screening DM patients for LTBI |
|
| TB treatment |
|
| Glycaemic control |
|
| Cardiovascular disease (CVD) |
|
| Strategy | Pro | Con |
|---|---|---|
| DM screening among TB patients | Possible improvement of individual patient outcome | No expected population impact |
| Better management of combined TB and DM | Possible improvement of individual patient outcome | No expected population impact |
| A more effective TB vaccine | Massive impact, also beyond DM-associated TB | Not available yet |
| Better DM management | Massive impact on all DM complications and mortality, especially related to cardiovascular disease | Challenging, especially in low-resource settings |
| TB preventive therapy for people with DM and LTBI | Projected reduction of individual TB risk, with significant population impact in terms of TB control | No evidence from phase 3 trials yet |
| Prevent diabetes incidence | Potentially massive impact on population health, CVD risk and saving substantial health care resources | Requires substantial shift in population diet and reductions in obesity. Limited evidence how to scale up to whole populations in LMIC |
|
|
|
|
|
|
|
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Crevel, R.; Critchley, J.A. The Interaction of Diabetes and Tuberculosis: Translating Research to Policy and Practice. Trop. Med. Infect. Dis. 2021, 6, 8. https://doi.org/10.3390/tropicalmed6010008
van Crevel R, Critchley JA. The Interaction of Diabetes and Tuberculosis: Translating Research to Policy and Practice. Tropical Medicine and Infectious Disease. 2021; 6(1):8. https://doi.org/10.3390/tropicalmed6010008
Chicago/Turabian Stylevan Crevel, Reinout, and Julia A. Critchley. 2021. "The Interaction of Diabetes and Tuberculosis: Translating Research to Policy and Practice" Tropical Medicine and Infectious Disease 6, no. 1: 8. https://doi.org/10.3390/tropicalmed6010008
APA Stylevan Crevel, R., & Critchley, J. A. (2021). The Interaction of Diabetes and Tuberculosis: Translating Research to Policy and Practice. Tropical Medicine and Infectious Disease, 6(1), 8. https://doi.org/10.3390/tropicalmed6010008




