Travel-Related Antimicrobial Resistance: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Study Assessment
2.4. Data Analysis and Visualisations
3. Results
3.1. AMR Associated with Planned Travel
3.2. AMR Bacteria Associated with Planned International Travel
3.3. Trends in the Movements of Travel-Associated AMR Bacteria
3.4. AMR Associated with Exposure to Healthcare Systems
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- The Review on Antimicrobial Resistance. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; HM Government: London, UK, 2016.
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. Available online: https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013–508.pdf (accessed on 1 December 2020).
- The Review on Antimicrobial Resistance. Antimicrobial Resistance: Tackling a Crisis for the Future Health and Wealth of Nations; HM Government: London, UK, 2014.
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Fouz, N.; Pangesti, K.N.A.; Yasir, M.; Al-Malki, A.L.; Azhar, E.I.; Hill-Cawthorne, G.A.; Abd El Ghany, M. The Contribution of Wastewater to the Transmission of Antimicrobial Resistance in the Environment: Implications of Mass Gathering Settings. Trop. Med. Infect. Dis. 2020, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Khachatourians, G.G. Agricultural use of antibiotics and the evolution and transfer of antibiotic-resistant bacteria. Can. Med. Assoc. J. 1998, 159, 1129–1136. [Google Scholar]
- Van der Bij, A.K.; Pitout, J.D.D. The role of international travel in the worldwide spread of multiresistant Enterobacteriaceae. J. Antimicrob. Chemother. 2012, 67, 2090–2100. [Google Scholar] [CrossRef]
- Robertson, J.; Iwamoto, K.; Hoxha, I.; Ghazaryan, L.; Abilova, V.; Cvijanovic, A.; Pyshnik, H.; Darakhvelidze, M.; Makalkina, L.; Jakupi, A.; et al. Antimicrobial Medicines Consumption in Eastern Europeand Central Asia—An Updated Cross-National Study and Assessment of Quantitative Metrics for Policy Action. Front. Pharmacol. 2019, 9, 1156. [Google Scholar] [CrossRef]
- Zhussupova, G.; Skvirskaya, G.; Reshetnikov, V.; Dragojevic-Simic, V.; Rancic, N.; Utepova, D.; Jakovljevic, M. The Evaluation of Antibiotic Consumption at the Inpatient Level in Kazakhstan from 2011 to 2018. Antibiotics 2020, 9. [Google Scholar] [CrossRef]
- Godman, B.; Haque, M.; Islam, S.; Iqbal, S.; Urmi, U.L.; Kamal, Z.M.; Shuvo, S.A.; Rahman, A.; Kamal, M.; Haque, M.; et al. Rapid Assessment of Price Instability and Paucity of Medicines and Protection for COVID-19 Across Asia: Findings and Public Health Implications for the Future. Front. Public Health 2020, 8, 585832. [Google Scholar] [CrossRef]
- Jakovljevic, M.B.; Djordjevic, N.; Jurisevic, M.; Jankovic, S. Evolution of the Serbian pharmaceutical market alongside socioeconomic transition. Expert Rev. Pharm. Outcomes Res. 2015, 15, 521–530. [Google Scholar] [CrossRef]
- Miljković, N.; Godman, B.; van Overbeeke, E.; Kovačević, M.; Tsiakitzis, K.; Apatsidou, A.; Nikopoulou, A.; Yubero, C.G.; Portillo Horcajada, L.; Stemer, G.; et al. Risks in Antibiotic Substitution Following Medicine Shortage: A Health-Care Failure Mode and Effect Analysis of Six European Hospitals. Front. Med. 2020, 7, 157. [Google Scholar] [CrossRef]
- World Tourism Organization. UNWTO Annual Report 2017; United Nations World Tourism Organization: Madrid, Spain, 2018. [Google Scholar]
- World Tourism Organization. UNWTO World Tourism Barometer and Statistical Annex, January 2019; United Nations World Tourism Organization: Madrid, Spain, 2019; pp. 1–40. [Google Scholar] [CrossRef]
- Nelson, R. Infectious risks of medical tourism. Lancet Infect. Dis. 2014, 14, 680–681. [Google Scholar] [CrossRef]
- United Nations High Commissioner for Refugees. Population Statistics: Time Series; United Nations High Commissioner for Refugees: Geneva, Switzerland, 2018. [Google Scholar]
- De Smalen, A.W.; Ghorab, H.; Abd El Ghany, M.; Hill-Cawthorne, G.A. Refugees and antimicrobial resistance: A systematic review. Travel Med. Infect. Dis. 2017, 15, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Jakovljevic, M.; Al Ahdab, S.; Jurisevic, M.; Mouselli, S. Antibiotic Resistance in Syria: A Local Problem Turns into a Global Threat. Front. Public Health 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Jakovljevic, M.M.; Netz, Y.; Buttigieg, S.C.; Adany, R.; Laaser, U.; Varjacic, M. Population aging and migration–History and UN forecasts in the EU-28 and its east and south near neighborhood–One century perspective 1950–2050. Glob. Health 2018, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Alirol, E.; Getaz, L.; Stoll, B.; Chappuis, F.; Loutan, L. Urbanisation and infectious diseases in a globalised world. Lancet Infect. Dis. 2011, 11, 131–141. [Google Scholar] [CrossRef]
- Moore, P.S.; Harrison, L.H.; Telzak, E.E.; Ajello, G.W.; Broome, C.V. Group a meningococcal carriage in travelers returning from Saudi Arabia. J. Am. Med. Assoc. 1988, 260, 2686–2689. [Google Scholar] [CrossRef]
- Mutreja, A.; Kim, D.W.; Thomson, N.R.; Connor, T.R.; Lee, J.H.; Kariuki, S.; Croucher, N.J.; Choi, S.Y.; Harris, S.R.; Lebens, M.; et al. Evidence for several waves of global transmission in the seventh cholera pandemic. Nature 2011, 477, 462–465. [Google Scholar] [CrossRef]
- Global Task Force on Cholera Control. Ending Cholera A Global Roadmap to 2030; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Yezli, S.; Assiri, A.M.; Alhakeem, R.F.; Turkistani, A.M.; Alotaibi, B. Meningococcal disease during the Hajj and Umrah mass gatherings. Int. J. Infect. Dis. 2016, 47, 60–64. [Google Scholar] [CrossRef]
- Arcilla, M.S.; van Hattem, J.M.; Haverkate, M.R.; Bootsma, M.C.J.; van Genderen, P.J.J.; Goorhuis, A.; Grobusch, M.P.; Lashof, A.M.O.; Molhoek, N.; Schultsz, C.; et al. Import and spread of extended-spectrum b-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): A prospective, multicentre cohort study. Lancet Infect. Dis. 2017, 17, 78–85. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Nurjadi, D.; Olalekan, A.O.; Layer, F.; Shittu, A.O.; Alabi, A.; Ghebremedhin, B.; Schaumburg, F.; Hofmann-Eifler, J.; Van Genderen, P.J.; Caumes, E.; et al. Emergence of trimethoprim resistance gene dfrG in Staphylococcus aureus causing human infection and colonization in sub-Saharan Africa and its import to Europe. J. Antimicrob. Chemother. 2014, 69, 2361–2368. [Google Scholar] [CrossRef]
- Nurjadi, D.; Friedrich-Janicke, B.; Schafer, J.; Van Genderen, P.J.; Goorhuis, A.; Perignon, A.; Neumayr, A.; Mueller, A.; Kantele, A.; Schunk, M.; et al. Skin and soft tissue infections in intercontinental travellers and the import of multi-resistant Staphylococcus aureus to Europe. Clin. Microbiol. Infect. 2015, 21, 567.e1–567.e10. [Google Scholar] [CrossRef] [PubMed]
- Nurjadi, D.; Schafer, J.; Friedrich-Janicke, B.; Mueller, A.; Neumayr, A.; Calvo-Cano, A.; Goorhuis, A.; Molhoek, N.; Lagler, H.; Kantele, A.; et al. Predominance of dfrG as determinant of trimethoprim resistance in imported Staphylococcus aureus. Clin. Microbiol. Infect. 2015, 21, 1095.e5–1095.e9. [Google Scholar] [CrossRef] [PubMed]
- Nurjadi, D.; Fleck, R.; Lindner, A.; Schafer, J.; Gertler, M.; Mueller, A.; Lagler, H.; Van Genderen, P.J.J.; Caumes, E.; Boutin, S.; et al. Import of community-associated, methicillin-resistant Staphylococcus aureus to Europe through skin and soft-tissue infection in intercontinental travellers, 2011–2016. Clin. Microbiol. Infect. 2019, 25, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.; Collignon, P. Colonisation with Escherichia coli resistant to “critically important” antibiotics: A high risk for international travellers. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1501–1506. [Google Scholar] [CrossRef]
- Rogers, B.A.; Kennedy, K.J.; Sidjabat, H.E.; Jones, M.; Collignon, P.; Paterson, D.L. Prolonged carriage of resistant E. coli by returned travellers: Clonality, risk factors and bacterial characteristics. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2413–2420. [Google Scholar] [CrossRef]
- Dhanji, H.; Patel, R.; Wall, R.; Doumith, M.; Patel, B.; Hope, R.; Livermore, D.M.; Woodford, N. Variation in the genetic environments of blaCTX-M-15 in Escherichia coli from the faeces of travellers returning to the United Kingdom. J. Antimicrob. Chemother. 2011, 66, 1005–1012. [Google Scholar] [CrossRef]
- Hopkins, K.L.; Mushtaq, S.; Richardson, J.F.; Doumith, M.; De Pinna, E.; Cheasty, T.; Wain, J.; Livermore, D.M.; Woodford, N. In vitro activity of rifaximin against clinical isolates of Escherichia coli and other enteropathogenic bacteria isolated from travellers returning to the UK. Int. J. Antimicrob. Agents 2014, 43, 431–437. [Google Scholar] [CrossRef]
- Leangapichart, T.; Rolain, J.M.; Memish, Z.A.; Al-Tawfiq, J.A.; Gautret, P. Emergence of drug resistant bacteria at the Hajj: A systematic review. Travel Med. Infect. Dis. 2017, 18, 3–17. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 December 2020).
- Modesti, P.A.; Reboldi, G.; Cappuccio, F.P.; Agyemang, C.; Remuzzi, G.; Rapi, S.; Perruolo, E.; Parati, G.; The European Society of Hypertension Working Group on Cardiovascular Risk in Low Resource Settings. Panethnic Differences in Blood Pressure in Europe: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0147601. [Google Scholar] [CrossRef]
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ Evid. Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef]
- Aardema, H.; Luijnenburg, E.M.; Salm, E.F.; Bijlmer, H.A.; Visser, C.E.; Van, T.W.J.W. Changing epidemiology of melioidosis? A case of acute pulmonary melioidosis with fatal outcome imported from Brazil. Epidemiol. Infect. 2005, 133, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Ackers, M.L.; Puhr, N.D.; Tauxe, R.V.; Mintz, E.D. Laboratory-based surveillance of Salmonella serotype Typhi infections in the United States: Antimicrobial resistance on the rise. J. Am. Med. Assoc. 2000, 283, 2668–2673. [Google Scholar] [CrossRef] [PubMed]
- Adelman, M.W.; Johnson, J.H.; Hohmann, E.L.; Gandhi, R.T. Ovarian endometrioma superinfected with Salmonella: Case report and review of the literature. Open Forum Infect. Dis. 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Ageevets, V.; Sopova, J.; Lazareva, I.; Malakhova, M.; Ilina, E.; Kostryukova, E.; Babenko, V.; Carattoli, A.; Lobzin, Y.; Uskov, A.; et al. Genetic environment of the blaKPC-2 gene in a Klebsiella pneumoniae isolate that may have been imported to Russia from Southeast Asia. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Ageevets, V.A.; Partina, I.V.; Lisitsyna, E.S.; Ilina, E.N.; Lobzin, Y.V.; Shlyapnikov, S.A.; Sidorenko, S.V. Emergence of carbapenemase-producing Gram-negative bacteria in Saint Petersburg, Russia. Int. J. Antimicrob. Agents 2014, 44, 152–155. [Google Scholar] [CrossRef]
- Ahmad Hatib, N.A.; Chong, C.Y.; Thoon, K.C.; Tee, N.W.; Krishnamoorthy, S.S.; Tan, N.W. Enteric Fever in a Tertiary Paediatric Hospital: A Retrospective Six-Year Review. Ann. Acad Med. Singap. 2016, 45, 297–302. [Google Scholar]
- Ahmed-Bentley, J.; Chandran, A.U.; Joffe, A.M.; French, D.; Peirano, G.; Pitout, J.D. Gram-negative bacteria that produce carbapenemases causing death attributed to recent foreign hospitalization. Antimicrob. Agents Chemother. 2013, 57, 3085–3091. [Google Scholar] [CrossRef][Green Version]
- Al Naiemi, N.; Zwart, B.; Rijnsburger, M.C.; Roosendaal, R.; Debets-Ossenkopp, Y.J.; Mulder, J.A.; Fijen, C.A.; Maten, W.; Vandenbroucke-Grauls, C.M.; Savelkoul, P.H. Extended-spectrum-beta-lactamase production in a Salmonella enterica serotype Typhi strain from the Philippines. J. Clin. Microbiol. 2008, 46, 2794–2795. [Google Scholar] [CrossRef]
- Alcoba-Florez, J.; Perz-Roth, E.; Gonzalez-Linares, S.; Mendez-Alvarez, S. Outbreak of Shigella sonnei in a rural hotel in La Gomera, Canary Islands, Spain. Int. Microbiol. 2005, 8, 133–136. [Google Scholar]
- Alexander, D.C.; Hao, W.; Gilmour, M.W.; Zittermann, S.; Sarabia, A.; Melano, R.G.; Peralta, A.; Lombos, M.; Warren, K.; Amatnieks, Y.; et al. Escherichia coli O104:H4 infections and international travel. Emerg. Infect. Dis. 2012, 18, 473–476. [Google Scholar] [CrossRef]
- Ali, H.; Nash, J.Q.; Kearns, A.M.; Pichon, B.; Vasu, V.; Nixon, Z.; Burgess, A.; Weston, D.; Sedgwick, J.; Ashford, G.; et al. Outbreak of a South West Pacific clone Panton-Valentine leucocidin-positive meticillin-resistant Staphylococcus aureus infection in a UK neonatal intensive care unit. J. Hosp. Infect. 2012, 80, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Allyn, J.; Angue, M.; Belmonte, O.; Lugagne, N.; Traversier, N.; Vandroux, D.; Lefort, Y.; Allou, N. Delayed diagnosis of high drug-resistant microorganisms carriage in repatriated patients: Three cases in a French intensive care unit. J. Travel Med. 2015, 22, 215–217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Allyn, J.; Coolen-Allou, N.; De Parseval, B.; Galas, T.; Belmonte, O.; Allou, N.; Miltgen, G. Medical evacuation from abroad of critically ill patients: A case report and ethical issues. Medicine 2018, 97. [Google Scholar] [CrossRef] [PubMed]
- Al-Mashhadani, M.; Hewson, R.; Vivancos, R.; Keenan, A.; Beeching, N.J.; Wain, J.; Parry, C.M. Foreign travel and decreased ciprofloxacin susceptibility in Salmonella enterica infections. Emerg. Infect. Dis. 2011, 17, 123–125. [Google Scholar] [CrossRef]
- Angue, M.; Allou, N.; Belmonte, O.; Lefort, Y.; Lugagne, N.; Vandroux, D.; Montravers, P.; Allyn, J. Risk Factors for Colonization with Multidrug-Resistant Bacteria Among Patients Admitted to the Intensive Care Unit After Returning From Abroad. J. Travel Med. 2015, 22, 300–305. [Google Scholar] [CrossRef]
- Arai, Y.; Nakano, T.; Katayama, Y.; Aoki, H.; Hirayama, T.; Ooi, Y.; Eda, J.; Imura, S.; Kashiwagi, E.; Sano, K. Epidemiological evidence of multidrug-resistant Shigella sonnei colonization in India by sentinel surveillance in a Japanese quarantine station. Kansenshogaku Zasshi 2008, 82, 322–327. [Google Scholar] [CrossRef][Green Version]
- Artzi, O.; Sinai, M.; Solomon, M.; Schwartz, E. Recurrent furunculosis in returning travelers: Newly defined entity. J. Travel Med. 2015, 22, 21–25. [Google Scholar] [CrossRef][Green Version]
- Baker, K.S.; Dallman, T.J.; Ashton, P.M.; Day, M.; Hughes, G.; Crook, P.D.; Gilbart, V.L.; Zittermann, S.; Allen, V.G.; Howden, B.P.; et al. Intercontinental dissemination of azithromycin-resistant shigellosis through sexual transmission: A cross-sectional study. Lancet Infect. Dis. 2015, 15, 913–921. [Google Scholar] [CrossRef]
- Barlow, R.S.; Debess, E.E.; Winthrop, K.L.; Lapidus, J.A.; Vega, R.; Cieslak, P.R. Travel-associated antimicrobial drug-resistant nontyphoidal Salmonellae, 2004–2009. Emerg. Infect. Dis. 2014, 20, 603–611. [Google Scholar] [CrossRef]
- Bathoorn, E.; Friedrich, A.W.; Zhou, K.; Arends, J.P.; Borst, D.M.; Grundmann, H.; Rossen, J.W. Latent introduction to the Netherlands of multiple antibiotic resistance including NDM-1 after hospitalisation in Egypt, August 2013. Eurosurveillance 2013, 18. [Google Scholar] [CrossRef][Green Version]
- Bengtsson-Palme, J.; Angelin, M.; Huss, M.; Kjellqvist, S.; Kristiansson, E.; Palmgren, H.; Larsson, D.G.; Johansson, A. The Human Gut Microbiome as a Transporter of Antibiotic Resistance Genes between Continents. Antimicrob. Agents Chemother. 2015, 59, 6551–6560. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, O.J.; Kuenzli, E.; Pires, J.; Tinguely, R.; Carattoli, A.; Hatz, C.; Perreten, V.; Endimiani, A. Travelers can import colistin-resistant Enterobacteriaceae, including those possessing the plasmid-mediated mcr-1 gene. Antimicrob. Agents Chemother. 2016, 60, 5080–5084. [Google Scholar] [CrossRef] [PubMed]
- Birgand, G.; Armand-Lefevre, L.; Lepainteur, M.; Lolom, I.; Neulier, C.; Reibel, F.; Yazdanpanah, Y.; Andremont, A.; Lucet, J.C. Introduction of highly resistant bacteria into a hospital via patients repatriated or recently hospitalized in a foreign country. Clin. Microbiol. Infect. 2014, 20, O887–O890. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blomfeldt, A.; Larssen, K.W.; Moghen, A.; Gabrielsen, C.; Elstrom, P.; Aamot, H.V.; Jorgensen, S.B. Emerging multidrug-resistant Bengal Bay clone ST772-MRSA-V in Norway: Molecular epidemiology 2004–2014. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- Bochet, M.; Francois, P.; Longtin, Y.; Gaide, O.; Renzi, G.; Harbarth, S. Community-acquired methicillin-resistant Staphylococcus aureus infections in two scuba divers returning from the Philippines. J. Travel Med. 2008, 15, 378–381. [Google Scholar] [CrossRef][Green Version]
- Bodilsen, J.; Vammen, S.; Fuursted, K.; Hjort, U. Mycotic aneurysm caused by Burkholderia pseudomallei in a previously healthy returning traveller. BMJ Case Rep. 2014, 2014, bcr2013202824. [Google Scholar] [CrossRef]
- Bottieau, E.; Clerinx, J.; Vlieghe, E.; Van Esbroeck, M.; Jacobs, J.; Van Gompel, A.; Van Den Ende, J. Epidemiology and outcome of Shigella, Salmonella and Campylobacter infections in travellers returning from the tropics with fever and diarrhoea. Acta Clin. Belg. 2011, 66, 191–195. [Google Scholar] [CrossRef]
- Bourgeois, A.L.; Gardiner, C.H.; Thornton, S.A.; Batchelor, R.A.; Burr, D.H.; Escamilla, J.; Echeverria, P.; Blacklow, N.R.; Herrmann, J.E.; Hyams, K.C. Etiology of acute diarrhea among United States military personnel deployed to South America and west Africa. Am. J. Trop. Med. Hyg. 1993, 48, 243–248. [Google Scholar] [CrossRef]
- Bowen, A.; Hurd, J.; Hoover, C.; Khachadourian, Y.; Traphagen, E.; Harvey, E.; Libby, T.; Ehlers, S.; Ongpin, M.; Norton, J.C.; et al. Importation and domestic transmission of Shigella sonnei resistant to ciprofloxacin—United States, May 2014–February 2015. Morb. Mortal. Wkly. Rep. 2015, 64, 318–320. [Google Scholar]
- Boyd, D.A.; Mataseje, L.F.; Pelude, L.; Mitchell, R.; Bryce, E.; Roscoe, D.; Embree, J.; Katz, K.; Kibsey, P.; Lavallee, C.; et al. Results from the Canadian Nosocomial Infection Surveillance Program for detection of carbapenemase-producing Acinetobacter spp. in Canadian hospitals, 2010–2016. J. Antimicrob. Chemother. 2019, 74, 315–320. [Google Scholar] [CrossRef]
- Brown, A.C.; Chen, J.C.; Watkins, L.K.F.; Campbell, D.; Folster, J.P.; Tate, H.; Wasilenko, J.; Van Tubbergen, C.; Friedman, C.R. CTX-M-65 Extended-Spectrum b-Lactamase-Producing Salmonella enterica Serotype Infantis, United States. Emerg. Infect. Dis. 2018, 24, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, R.; Ruiz, J.; Marco, F.; Oliveira, I.; Arroyo, M.; Aladuena, A.; Usera, M.A.; Jimenez De Anta, M.T.; Gascon, J.; Vila, J. Mechanism of resistance to several antimicrobial agents in Salmonella clinical isolates causing traveler’s diarrhea. Antimicrob. Agents Chemother. 2004, 48, 3934–3939. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, R.; Ruiz, J.; Sanchez-Cespedes, J.; Goni, P.; Gomez-Lus, R.; Jimenez De Anta, M.T.; Gascon, J.; Vila, J. Characterization of the enzyme aac(3)-id in a clinical isolate of Salmonella enterica serovar Haifa causing traveler’s diarrhea. Enfermedades Infecciosas y Microbiologia Clinica 2009, 27, 453–456. [Google Scholar] [CrossRef] [PubMed]
- The Campylobacter Sentinel Surveillance Scheme Collaborators. Ciprofloxacin resistance in Campylobacter jejuni: Case-case analysis as a tool for elucidating risks at home and abroad. J. Antimicrob. Chemother. 2002, 50, 561–568. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cha, I.; Kim, N.O.; Nam, J.G.; Choi, E.S.; Chung, G.T.; Kang, Y.H.; Hong, S. Genetic diversity of Campylobacter jejuni isolates from Korea and travel-associated cases from east and southeast Asian countries. Jpn. J. Infect. Dis. 2014, 67, 490–494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chan, H.L.E.; Poon, L.M.; Chan, S.G.; Teo, J.W.P. The perils of medical tourism: NDM-1-positive Escherichia coli causing febrile neutropenia in a medical tourist. Singap. Med. J. 2011, 52, 299–302. [Google Scholar]
- Chan, W.W.; Peirano, G.; Smyth, D.J.; Pitout, J.D. The characteristics of Klebsiella pneumoniae that produce KPC-2 imported from Greece. Diagn. Microbiol. Infect. Dis. 2013, 75, 317–319. [Google Scholar] [CrossRef]
- Chatham-Stephens, K.; Medalla, F.; Hughes, M.; Appiah, G.D.; Aubert, R.D.; Caidi, H.; Angelo, K.M.; Walker, A.T.; Hatley, N.; Masani, S.; et al. Emergence of Extensively Drug-Resistant Salmonella Typhi Infections Among Travelers to or from Pakistan—United States, 2016–2018. Morb. Mortal. Wkly. Rep. 2019, 68, 11–13. [Google Scholar] [CrossRef]
- Christenson, B.; Ardung, B.; Sylvan, S. Methicillin-resistant Staphylococcus aureus infections in Uppsala County, Sweden. Open Infect. Dis. J. 2011, 5, 107–114. [Google Scholar] [CrossRef]
- Chua, K.Y.L.; Lindsay Grayson, M.; Burgess, A.N.; Lee, J.Y.H.; Howden, B.P. The growing burden of multidrugresistant infections among returned Australian travellers. Med. J. Aust. 2014, 200, 116–118. [Google Scholar] [CrossRef][Green Version]
- Cohen, M.B.; Hawkins, J.A.; Weckbach, L.S.; Staneck, J.L.; Levine, M.M.; Heck, J.E. Colonization by enteroaggregative Escherichia coli in travelers with and without diarrhea. J. Clin. Microbiol. 1993, 31, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, L.R.; Tran, V.; Tlamsa, A.; Chung, P.; Grossberg, R.; Weston, G.; Sarwar, U.N. Rapidly growing Mycobacterium infections after cosmetic surgery in medical tourists: The Bronx experience and a review of the literature. Int. J. Infect. Dis. 2017, 63, 1–6. [Google Scholar] [CrossRef]
- Dall, L.B.; Lausch, K.R.; Gedebjerg, A.; Fuursted, K.; Storgaard, M.; Larsen, C.S. Do probiotics prevent colonization with multi-resistant Enterobacteriaceae during travel? A randomized controlled trial. Travel Med. Infect. Dis. 2019, 27, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Daniels, N.A.; Neimann, J.; Karpati, A.; Parashar, U.D.; Greene, K.D.; Wells, J.G.; Srivastava, A.; Tauxe, R.V.; Mintz, E.D.; Quick, R. Traveler’s diarrhea at sea: Three outbreaks of waterborne enterotoxigenic Escherichia coli on cruise ships. J. Infect. Dis. 2000, 181, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Date, K.A.; Newton, A.E.; Medalla, F.; Blackstock, A.; Richardson, L.; McCullough, A.; Mintz, E.D.; Mahon, B.E. Changing Patterns in Enteric Fever Incidence and Increasing Antibiotic Resistance of Enteric Fever Isolates in the United States, 2008–2012. Clin. Infect. Dis. 2016, 63, 322–329. [Google Scholar] [CrossRef]
- Dave, J.; Warburton, F.; Freedman, J.; de Pinna, E.; Grant, K.; Sefton, A.; Crawley-Boevey, E.; Godbole, G.; Holliman, R.; Balasegaram, S. What were the risk factors and trends in antimicrobial resistance for enteric fever in London 2005–2012? J. Med. Microbiol. 2017, 66, 698–705. [Google Scholar] [CrossRef][Green Version]
- Day, M.; Doumith, M.; Jenkins, C.; Dallman, T.J.; Hopkins, K.L.; Elson, R.; Godbole, G.; Woodford, N. Antimicrobial resistance in Shiga toxin-producing Escherichia coli serogroups O157 and O26 isolated from human cases of diarrhoeal disease in England, 2015. J. Antimicrob. Chemother. 2017, 72, 145–152. [Google Scholar] [CrossRef]
- Decousser, J.W.; Jansen, C.; Nordmann, P.; Emirian, A.; Bonnin, R.A.; Anais, L.; Merle, J.C.; Poirel, L. Outbreak of NDM-1-producing Acinetobacter baumannii in France, January to May 2013. Eurosurveillance 2013, 18. [Google Scholar] [CrossRef]
- Denis, O.; Deplano, A.; De Beenhouwer, H.; Hallin, M.; Huysmans, G.; Garrino, M.G.; Glupczynski, Y.; Malaviolle, X.; Vergison, A.; Struelens, M.J. Polyclonal emergence and importation of community-acquired methicillin-resistant Staphylococcus aureus strains harbouring Panton-Valentine leucocidin genes in Belgium. J. Antimicrob. Chemother. 2005, 56, 1103–1106. [Google Scholar] [CrossRef]
- Di Ruscio, F.; Bjørnholt, J.V.; Leegaard, T.M.; Moen, A.E.F.; De Blasio, B.F. MRSA infections in Norway: A study of the temporal evolution, 2006–2015. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Drews, S.J.; Lau, C.; Andersen, M.; Ferrato, C.; Simmonds, K.; Stafford, L.; Fisher, B.; Everett, D.; Louie, M. Laboratory based surveillance of travel-related Shigella sonnei and Shigella flexneri in Alberta from 2002 to 2007. Glob. Health 2010, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Ellington, M.J.; Ganner, M.; Warner, M.; Boakes, E.; Cookson, B.D.; Hill, R.L.; Kearns, A.M. First international spread and dissemination of the virulent Queensland community-associated methicillin-resistant Staphylococcus aureus strain. Clin. Microbiol. Infect. 2010, 16, 1009–1012. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Engsbro, A.L.; Riis Jespersen, H.S.; Goldschmidt, M.I.; Mollerup, S.; Worning, P.; Pedersen, M.S.; Westh, H.; Schneider, U.V. Ceftriaxone-resistant Salmonella enterica serotype Typhi in a pregnant traveller returning from Karachi, Pakistan to Denmark, 2019. Eurosurveillance 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Epelboin, L.; Robert, J.; Tsyrina-Kouyoumdjian, E.; Laouira, S.; Meyssonnier, V.; Caumes, E.; Group, M.-G.T.W. High Rate of Multidrug-Resistant Gram-Negative Bacilli Carriage and Infection in Hospitalized Returning Travelers: A Cross-Sectional Cohort Study. J. Travel Med. 2015, 22, 292–299. [Google Scholar] [CrossRef]
- Espenhain, L.; Jørgensen, S.B.; Leegaard, T.M.; Lelek, M.M.; Hänsgen, S.H.; Nakstad, B.; Sunde, M.; Steinbakk, M. Travel to Asia is a strong predictor for carriage of cephalosporin resistant E. coli and Klebsiella spp. but does not explain everything; Prevalence study at a Norwegian hospital 2014–2016. Antimicrob. Resist. Infect. Control 2018, 7. [Google Scholar] [CrossRef]
- Espinal, P.; Miró, E.; Segura, C.; Gómez, L.; Plasencia, V.; Coll, P.; Navarro, F. First Description of blaNDM-7 Carried on an IncX4 Plasmid in Escherichia coli ST679 Isolated in Spain. Microb. Drug Resist. 2018, 24, 113–119. [Google Scholar] [CrossRef]
- Evans, M.R.; Northey, G.; Sarvotham, T.S.; Hopkins, A.L.; Rigby, C.J.; Thomas, D.R. Risk factors for ciprofloxacin-resistant Campylobacter infection in Wales. J. Antimicrob. Chemother. 2009, 64, 424–427. [Google Scholar] [CrossRef]
- Eyre, D.W.; Town, K.; Street, T.; Barker, L.; Sanderson, N.; Cole, M.J.; Mohammed, H.; Pitt, R.; Gobin, M.; Irish, C.; et al. Detection in the United Kingdom of the Neisseria gonorrhoeae FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin, October to December 2018. Eurosurveillance 2019, 24. [Google Scholar] [CrossRef]
- Ferstl, P.G.; Reinheimer, C.; Jozsa, K.; Zeuzem, S.; Kempf, V.A.J.; Waidmann, O.; Grammatikos, G. Severe infection with multidrug-resistant Salmonella choleraesuis in a young patient with primary sclerosing cholangitis. World J. Gastroenterol. 2017, 23, 2086–2089. [Google Scholar] [CrossRef]
- Fischer, D.; Veldman, A.; Diefenbach, M.; Schafer, V. Bacterial Colonization of Patients Undergoing International Air Transport: A Prospective Epidemiologic Study. J. Travel Med. 2004, 11, 44–48. [Google Scholar] [CrossRef][Green Version]
- FitzGerald, R.P.; Rosser, A.J.; Perera, D.N. Non-toxigenic penicillin-resistant cutaneous C. diphtheriae infection: A case report and review of the literature. J. Infect. Public Health 2015, 8, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Flateau, C.; Duron-Martinaud, S.; Haus-Cheymol, R.; Bousquet, A.; Delaune, D.; Ficko, C.; Merens, A.; Rapp, C. Prevalence and risk factors for Extended-Spectrum Beta-Lactamase-producing- Enterobacteriaceae in French military and civilian travelers: A cross-sectional analysis. Travel Med. Infect. Dis. 2018, 23, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Flateau, C.; Janvier, F.; Delacour, H.; Males, S.; Ficko, C.; Andriamanantena, D.; Jeannot, K.; Merens, A.; Rapp, C. Recurrent pyelonephritis due to NDM-1 metallo-beta-lactamase producing Pseudomonas aeruginosa in a patient returning from Serbia, France, 2012. Eurosurveillance 2012, 17, 20311. [Google Scholar] [CrossRef] [PubMed]
- Fleming, H.; Fowler, S.V.; Nguyen, L.; Hofinger, D.M. Lactococcus garvieae multi-valve infective endocarditis in a traveler returning from South Korea. Travel Med. Infect. Dis. 2012, 10, 101–104. [Google Scholar] [CrossRef]
- Frickmann, H.; Wiemer, D.; Frey, C.; Hagen, R.M.; Hinz, R.; Podbielski, A.; Köller, T.; Warnke, P. Low Enteric Colonization with Multidrug-Resistant Pathogens in Soldiers Returning from Deployments- Experience from the Years 2007–2015. PLoS ONE 2016, 11, e0162129. [Google Scholar] [CrossRef]
- Geissler, A.L.; Bustos Carrillo, F.; Swanson, K.; Patrick, M.E.; Fullerton, K.E.; Bennett, C.; Barrett, K.; Mahon, B.E. Increasing Campylobacter Infections, Outbreaks, and Antimicrobial Resistance in the United States, 2004–2012. Clin. Infect. Dis. 2017, 65, 1624–1631. [Google Scholar] [CrossRef]
- Gilmour, M.W.; Martel-Laferriere, V.; Levesque, S.; Gaudreau, C.; Bekal, S.; Nadon, C.; Bourgault, A.M. Vibrio cholerae in traveler from Haiti to Canada. Emerg. Infect. Dis. 2011, 17, 1124–1125. [Google Scholar] [CrossRef]
- Guiral, E.; Mendez-Arancibia, E.; Soto, S.M.; Salvador, P.; Fabrega, A.; Gascon, J.; Vila, J. CTX-M-15-producing enteroaggregative Escherichia coli as cause of travelers’ diarrhea. Emerg. Infect. Dis. 2011, 17, 1950–1953. [Google Scholar] [CrossRef]
- Gunell, M.; Aulu, L.; Jalava, J.; Lukinmaa-Aberg, S.; Osterblad, M.; Ollgren, J.; Huovinen, P.; Siitonen, A.; Hakanen, A.J. Cefotaxime-resistant Salmonella enterica in travelers returning from Thailand to Finland. Emerg. Infect. Dis. 2014, 20, 1214–1217. [Google Scholar] [CrossRef]
- Gupta, S.K.; Medalla, F.; Omondi, M.W.; Whichard, J.M.; Fields, P.I.; Gerner-Smidt, P.; Patel, N.J.; Cooper, K.L.F.; Chiller, T.M.; Mintz, E.D. Laboratory-based surveillance of paratyphoid fever in the United States: Travel and antimicrobial resistance. Clin. Infect. Dis. 2008, 46, 1656–1663. [Google Scholar] [CrossRef]
- Hakanen, A.; Jousimies-Somer, H.; Siitonen, A.; Huovinen, P.; Kotilainent, P. Fluoroquinolone resistance in Campylobacter jejuni isolates in travelers returning to Finland: Association of ciprofloxacin resistance to travel destination. Emerg. Infect. Dis. 2003, 9, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Hakanen, A.; Kotilainen, P.; Huovinen, P.; Helenius, H.; Siitonen, A. Reduced fluoroquinolone susceptibility in Salmonella enterica serotypes in travelers returning from Southeast Asia. Emerg. Infect. Dis. 2001, 7, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Haley, B.J.; Kim, S.W.; Haendiges, J.; Keller, E.; Torpey, D.; Kim, A.; Crocker, K.; Myers, R.A.; Van Kessel, J.A.S. Salmonella enterica serovar Kentucky recovered from human clinical cases in Maryland, USA (2011–2015). Zoonoses Public Health 2019, 66, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, J.K.; Hogan, T.R.; Buckley, C.; Trembizki, E.; Mitchell, H.; Lau, C.L.; Whiley, D.M.; Lahra, M.M. Emergence and spread of ciprofloxacin-resistant Neisseria gonorrhoeae in New South Wales, Australia: Lessons from history. J. Antimicrob. Chemother. 2019, 74, 2214–2219. [Google Scholar] [CrossRef] [PubMed]
- Harnett, N.; McLeod, S.; AuYong, Y.; Wan, J.; Alexander, S.; Khakhria, R.; Krishnan, C. Molecular characterization of multiresistant strains of Salmonella typhi from South Asia isolated in Ontario, Canada. Can. J. Microbiol. 1998, 44, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, A.; Nagamatsu, M.; Ohmagari, N.; Hayakawa, K.; Kato, Y.; Kirikae, T. Isolation of OXA-48 carbapenemase-producing Klebsiella pneumoniae ST101 from an overseas traveler returning to Japan. Jpn. J. Infect. Dis. 2014, 67, 120–121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hassing, R.J.; Goessens, W.H.; van Pelt, W.; Mevius, D.J.; Stricker, B.H.; Molhoek, N.; Verbon, A.; van Genderen, P.J. Salmonella subtypes with increased MICs for azithromycin in travelers returned to The Netherlands. Emerg. Infect. Dis. 2014, 20, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Hassing, R.J.; Goessens, W.H.F.; Mevius, D.J.; Van Pelt, W.; Mouton, J.W.; Verbon, A.; Van Genderen, P.J. Decreased ciprofloxacin susceptibility in Salmonella Typhi and Paratyphi infections in ill-returned travellers: The impact on clinical outcome and future treatment options. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1295–1301. [Google Scholar] [CrossRef]
- Hassing, R.J.; Menezes, G.A.; Van Pelt, W.; Petit, P.L.; Van Genderen, P.J.; Goessens, W.H.F. Analysis of mechanisms involved in reduced susceptibility to ciprofloxacin in Salmonella enterica serotypes Typhi and Paratyphi A isolates from travellers to Southeast Asia. Int. J. Antimicrob. Agents 2011, 37, 240–243. [Google Scholar] [CrossRef][Green Version]
- Haukka, K.; Siitonen, A. Emerging resistance to newer antimicrobial agents among Shigella isolated from Finnish foreign travellers. Epidemiol. Infect. 2008, 136, 476–482. [Google Scholar] [CrossRef]
- Hebbelstrup Jensen, B.; Adler Sorensen, C.; Hebbelstrup Rye Rasmussen, S.; Rejkjaer Holm, D.; Friis-Moller, A.; Engberg, J.; Mirsepasi-Lauridsen, H.C.; Struve, C.; Hammerum, A.M.; Porsbo, L.J.; et al. Characterization of Diarrheagenic Enteroaggregative Escherichia coli in Danish Adults-Antibiotic Treatment Does Not Reduce Duration of Diarrhea. Front. Cell. Infect. Microbiol. 2018, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, R.S.; Le Hello, S.; Bortolaia, V.; Pulsrikarn, C.; Nielsen, E.M.; Pornruangmong, S.; Chaichana, P.; Svendsen, C.A.; Weill, F.X.; Aarestrup, F.M. Characterization of isolates of Salmonella enterica serovar Stanley, a serovar endemic to Asia and associated with travel. J. Clin. Microbiol. 2012, 50, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, R.S.; Leekitcharoenphon, P.; Mikoleit, M.; Jensen, J.D.; Kaas, R.S.; Roer, L.; Joshi, H.B.; Pornruangmong, S.; Pulsrikarn, C.; Gonzalez-Aviles, G.D.; et al. Genomic dissection of travel-associated extended-spectrum-beta-lactamase-producing Salmonella enterica serovar typhi isolates originating from the philippines: A one-off occurrence or a threat to effective treatment of typhoid fever? J. Clin. Microbiol. 2015, 53, 677–680. [Google Scholar] [CrossRef][Green Version]
- Herrera-Leon, S.; Llorente, M.T.; Sanchez, S. Plasmid-mediated quinolone resistance in different diarrheagenic Escherichia coli pathotypes responsible for complicated, noncomplicated, and traveler’s diarrhea cases. Antimicrob. Agents Chemother. 2016, 60, 1950–1951. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hrabak, J.; Stolbova, M.; Studentova, V.; Fridrichova, M.; Chudackova, E.; Zemlickova, H. NDM-1 producing Acinetobacter baumannii isolated from a patient repatriated to the Czech Republic from Egypt, July 2011. Eurosurveillance 2012, 17, 20085. [Google Scholar] [CrossRef]
- Huang, D.B.; Jiang, Z.D.; Ericsson, C.D.; Adachi, J.; Dupont, H.L. Emergence of trimethoprim-resistant Escherichia coli in healthy persons in the absence of prophylactic or therapeutic antibiotics during travel to Guadalajara, Mexico. Scand. J. Infect. Dis. 2001, 33, 812–814. [Google Scholar]
- Huang, Y.C.; Su, L.H.; Wu, T.L.; Lin, T.Y. Methicillin-resistant Staphylococcus aureus nasal carriage in international medical conference attendees. J. Microbiol. Immunol. Infect. 2019, 52, 242–247. [Google Scholar] [CrossRef]
- Huber, K.; Thoma, B.; Loscher, T.; Wieser, A. Primary skin melioidosis in a returning traveler. Infection 2015, 43, 507–508. [Google Scholar] [CrossRef][Green Version]
- Hume, S.; Schulz, T.; Vinton, P.; Korman, T.; Torresi, J. Increasing rates and clinical consequences of nalidixic acid-resistant isolates causing enteric fever in returned travellers: An 18-year experience. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 963–970. [Google Scholar] [CrossRef]
- Hussenet, C.; Jaureguiberry, S.; Robert, J.; Rouby, J.J.; Bricaire, F.; Caumes, E. Multidrug-resistant Acinetobacter baumannii infections in three returning travelers evacuated from Algeria, Thailand, and Turkey after hospitalization in local intensive care units. J. Travel Med. 2011, 18, 358–360. [Google Scholar] [CrossRef]
- Huynh, J.; Vosu, J.; Marais, B.J.; Britton, P.N. Multidrug-resistant tuberculous meningitis in a returned traveller. J. Paediatr. Child. Health 2019. [Google Scholar] [CrossRef] [PubMed]
- Ingle, D.J.; Easton, M.; Valcanis, M.; Seemann, T.; Kwong, J.C.; Stephens, N.; Carter, G.P.; Goncalves da Silva, A.; Adamopoulos, J.; Baines, S.L.; et al. Co-circulation of multidrug-resistant Shigella among men who have sex with men, Australia. Clin. Infect. Dis. 2019, 69, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Inkster, T.; Coia, J.; Meunier, D.; Doumith, M.; Martin, K.; Pike, R.; Imrie, L.; Kane, H.; Hay, M.; Wiuff, C.; et al. First outbreak of colonization by linezolid- and glycopeptide-resistant Enterococcus faecium harbouring the cfr gene in a UK nephrology unit. J. Hosp. Infect. 2017, 97, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Selvarangan, R.; Kanwar, N.; McHenry, R.; Chappell, J.D.; Halasa, N.; Wikswo, M.E.; Payne, D.C.; Azimi, P.H.; McDonald, L.C.; et al. Intestinal Carriage of Third-Generation Cephalosporin-Resistant and Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Healthy US Children. J. Pediatric Infect. Dis. Soc. 2018, 7, 234–240. [Google Scholar] [CrossRef]
- Iverson, C.J.; Wang, S.A.; Lee, M.V.; Ohye, R.G.; Trees, D.L.; Knapp, J.S.; Effler, P.V.; O’Connor, N.P.; Levine, W.C. Fluoroquinolone resistance among Neisseria gonorrhoeae isolates in Hawaii, 1990–2000: Role of foreign importation and increasing endemic spread. Sex. Transm Dis. 2004, 31, 702–708. [Google Scholar] [CrossRef]
- Izumiya, H.; Tada, Y.; Ito, K.; Morita-Ishihara, T.; Ohnishi, M.; Terajima, J.; Watanabe, H. Characterization of Shigella sonnei isolates from travel-associated cases in Japan. J. Med. Microbiol. 2009, 58, 1486–1491. [Google Scholar] [CrossRef]
- Jamal, W.Y.; Albert, M.J.; Rotimi, V.O. High prevalence of New Delhi metallo-b-lactamase-1 (NDM-1) producers among carbapenem-resistant Enterobacteriaceae in Kuwait. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Jeon, Y.L.; Nam, Y.S.; Lim, G.; Cho, S.Y.; Kim, Y.T.; Jang, J.H.; Kim, J.; Park, M.; Lee, H.J. Quinolone-resistant Shigella flexneri isolated in a patient who travelled to India. Ann. Lab. Med. 2012, 32, 366–369. [Google Scholar] [CrossRef]
- Jiang, Z.D.; Lowe, B.; Verenkar, M.P.; Ashley, D.; Steffen, R.; Tornieporth, N.; von Sonnenburg, F.; Waiyaki, P.; DuPont, H.L. Prevalence of enteric pathogens among international travelers with diarrhea acquired in Kenya (Mombasa), India (Goa), or Jamaica (Montego Bay). J. Infect. Dis. 2002, 185, 497–502. [Google Scholar] [CrossRef]
- Johansen, T.B.; Scheffer, L.; Jensen, V.K.; Bohlin, J.; Feruglio, S.L. Whole-genome sequencing and antimicrobial resistance in Brucella melitensis from a Norwegian perspective. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Jorgensen, S.B.; Samuelsen, O.; Sundsfjord, A.; Bhatti, S.A.; Jorgensen, I.; Sivapathasundaram, T.; Leegaard, T.M. High prevalence of faecal carriage of ESBL-producing Enterobacteriaceae in Norwegian patients with gastroenteritis. Scand. J. Infect. Dis. 2014, 46, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Josseaume, J.; Verner, L.; Brady, W.J.; Duchateau, F.X. Multidrug-resistant bacteria among patients treated in foreign hospitals: Management considerations during medical repatriation. J. Travel Med. 2013, 20, 22–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kantele, A.; Laaveri, T.; Mero, S.; Vilkman, K.; Pakkanen, S.H.; Ollgren, J.; Antikainen, J.; Kirveskari, J. Antimicrobials increase travelers’ risk of colonization by extended-spectrum betalactamase-producing Enterobacteriaceae. Clin. Infect. Dis. 2015, 60, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Kantele, A.; Mero, S.; Kirveskari, J.; Lääveri, T. Fluoroquinolone antibiotic users select fluoroquinolone-resistant ESBL-producing Enterobacteriaceae (ESBL-PE)—Data of prospective traveller study. Travel Med. Infect. Dis. 2017, 16, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Karp, B.E.; Campbell, D.; Chen, J.C.; Folster, J.P.; Friedman, C.R. Plasmid-mediated quinolone resistance in human non-typhoidal Salmonella infections: An emerging public health problem in the United States. Zoonoses Public Health 2018, 65, 838–849. [Google Scholar] [CrossRef]
- Kaspar, T.; Schweiger, A.; Droz, S.; Marschall, J. Colonization with resistant microorganisms in patients transferred from abroad: Who needs to be screened? Antimicrob. Resist. Infect. Control 2015, 4. [Google Scholar] [CrossRef]
- Kassenborg, H.D.; Smith, K.E.; Vugia, D.J.; Rabatsky-Ehr, T.; Bates, M.R.; Carter, M.A.; Dumas, N.B.; Cassidy, M.P.; Marano, N.; Tauxe, R.V.; et al. Fluoroquinolone-resistant Campylobacter infections: Eating poultry outside of the home and foreign travel are risk factors. Clin. Infect. Dis. 2004, 38, S279–S284. [Google Scholar] [CrossRef]
- Khawaja, T.; Kirveskari, J.; Johansson, S.; Väisänen, J.; Djupsjöbacka, A.; Nevalainen, A.; Kantele, A. Patients hospitalized abroad as importers of multiresistant bacteria—A cross-sectional study. Clin. Microbiol. Infect. 2017. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, J.J.; Kim, S.J.; Jeon, S.E.; Seo, K.Y.; Choi, J.K.; Kim, N.O.; Hong, S.; Chung, G.T.; Yoo, C.K.; et al. Outbreak of Ciprofloxacin-Resistant Shigella sonnei Associated with Travel to Vietnam, Republic of Korea. Emerg. Infect. Dis. 2015, 21, 1247–1250. [Google Scholar] [CrossRef]
- Kishore, A.; Mehdi, S.; Sapundzieski, M.; Prudham, R.C.; Limdi, J.K. Fever and abdominal pain in a returning traveller. Can. J. Infect. Dis. Med. Microbiol. 2010, 21, 157–158. [Google Scholar] [CrossRef]
- Klein, S.; Menz, M.D.; Zanger, P.; Heeg, K.; Nurjadi, D. Increase in the prevalence of Panton-Valentine leukocidin and clonal shift in community-onset methicillin-resistant Staphylococcus aureus causing skin and soft-tissue infections in the Rhine-Neckar Region, Germany, 2012–2016. Int. J. Antimicrob. Agents 2019, 53, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Klemm, E.J.; Shakoor, S.; Page, A.J.; Qamar, F.N.; Judge, K.; Saeed, D.K.; Wong, V.K.; Dallman, T.J.; Nair, S.; Baker, S.; et al. Emergence of an Extensively Drug-Resistant Salmonella enterica Serovar Typhi Clone Harboring a Promiscuous Plasmid Encoding Resistance to Fluoroquinolones and Third-Generation Cephalosporins. mBio 2018, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Knaapila, J.; Kallio, H.; Hakanen, A.J.; Syvänen, K.; Ettala, O.; Kähkönen, E.; Lamminen, T.; Seppänen, M.; Jambor, I.; Rannikko, A.; et al. Antibiotic susceptibility of intestinal Escherichia coli in men undergoing transrectal prostate biopsies: A prospective, registered, multicentre study. BJU Int. 2018, 122, 203–210. [Google Scholar] [CrossRef]
- Ko, J.; Chung, D.R.; Park, S.Y.; Baek, J.Y.; Kim, S.H.; Kang, C.I.; Peck, K.R.; Lee, N.Y.; Song, J.H. First imported case of skin infection caused by PVL-positive ST30 community-associated methicillin-resistant Staphylococcus aureus clone in a returning Korean traveler from the Philippines. J. Korean Med. Sci. 2013, 28, 1100–1102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kohler, P.P.; Melano, R.G.; Patel, S.N.; Shafinaz, S.; Faheem, A.; Coleman, B.L.; Green, K.; Armstrong, I.; Almohri, H.; Borgia, S.; et al. Emergence of Carbapenemase-Producing Enterobacteriaceae, South-Central Ontario, Canada. Emerg. Infect. Dis. 2018, 24, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Fang, Y.; Zhang, M.; Hong, J.; Tan, Z.; Yuan, Z.; Zhu, F.; Mao, X.; Jin, Z.; Zhu, Y.; et al. Melioidosis acquired by a traveler from Papua New Guinea. Travel Med. Infect. Dis. 2016, 14, 267–270. [Google Scholar] [CrossRef]
- Kubota, K.; Barrett, T.J.; Ackers, M.L.; Brachman, P.S.; Mintz, E.D. Analysis of Salmonella enterica serotype Typhi pulsed-field gel electrophoresis patterns associated with international travel. J. Clin. Microbiol. 2005, 43, 1205–1209. [Google Scholar] [CrossRef][Green Version]
- Landelle, C.; Legrand, P.; Lesprit, P.; Cizeau, F.; Ducellier, D.; Gouot, C.; Brehaut, P.; Soing-Altrach, S.; Girou, E.; Brun-Buisson, C. Protracted outbreak of multidrug-resistant Acinetobacter baumannii after intercontinental transfer of colonized patients. Infect. Control Hosp. Epidemiol. 2013, 34, 119–124. [Google Scholar] [CrossRef]
- Lane, C.R.; Sutton, B.; Valcanis, M.; Kirk, M.; Walker, C.; Lalor, K.; Stephens, N. Travel Destinations and Sexual Behavior as Indicators of Antibiotic Resistant Shigella Strains-Victoria, Australia. Clin. Infect. Dis. 2015, 62, 722–729. [Google Scholar] [CrossRef]
- Langelier, C.; Graves, M.; Kalantar, K.; Caldera, S.; Durrant, R.; Fisher, M.; Backman, R.; Tanner, W.; DeRisi, J.L.; Leung, D.T. Microbiome and Antimicrobial Resistance Gene Dynamics in International Travelers. Emerg. Infect. Dis. 2019, 25, 1380–1383. [Google Scholar] [CrossRef]
- Larsen, A.R.; Bocher, S.; Stegger, M.; Goering, R.; Pallesen, L.V.; Skov, R. Epidemiology of European community-associated methicillin-resistant Staphylococcus aureus clonal complex 80 type IV strains isolated in Denmark from 1993 to 2004. J. Clin. Microbiol. 2008, 46, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Laupland, K.B.; Church, D.L.; Vidakovich, J.; Mucenski, M.; Pitout, J.D.D. Community-onset extended-spectrum b-lactamase (ESBL) producing Escherichia coli: Importance of international travel. J. Infect. 2008, 57, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Lausch, K.R.; Fuursted, K.; Larsen, C.S.; Storgaard, M. Colonisation with multi-resistant Enterobacteriaceae in hospitalised Danish patients with a history of recent travel: A cross-sectional study. Travel Med. Infect. Dis. 2013, 11, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Le Hello, S.; Hendriksen, R.S.; Doublet, B.; Fisher, I.; Nielsen, E.M.; Whichard, J.M.; Bouchrif, B.; Fashae, K.; Granier, S.A.; Jourdan-Da Silva, N.; et al. International spread of an epidemic population of Salmonella enterica serotype Kentucky ST198 resistant to ciprofloxacin. J. Infect. Dis. 2011, 204, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Chung, H.S.; Lee, H.; Yum, J.H.; Yong, D.; Jeong, S.H.; Lee, K.; Chong, Y. CTX-M-55-type extended-spectrum b-lactamase-producing Shigella sonnei isolated from a Korean patient who had travelled to China. Ann. Lab. Med. 2013, 33, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Tewari, D.; Yealy, C.C.; Fardig, D.; M’Ikanatha, N.M. Surveillance for travel and domestically acquired multidrug-resistant human Shigella infections—Pennsylvania, 2006–2014. Health Secur. 2016, 14, 143–151. [Google Scholar] [CrossRef]
- Lindgren, M.M.; Kotilainen, P.; Huovinen, P.; Hurme, S.; Lukinmaa, S.; Webber, M.A.; Piddock, L.J.; Siitonen, A.; Hakanen, A.J. Reduced fluoroquinolone susceptibility in Salmonella enterica isolates from travelers, Finland. Emerg. Infect. Dis. 2009, 15, 809–812. [Google Scholar] [CrossRef]
- Lo, W.U.; Cheung, Y.Y.; Lai, E.; Lung, D.; Que, T.L.; Ho, P.L. Complete sequence of an IncN plasmid, pIMP-HZ1, carrying blaIMP-4 in a Klebsiella pneumoniae strain associated with medical travel to China. Antimicrob. Agents Chemother. 2013, 57, 1561–1562. [Google Scholar] [CrossRef]
- Lubbert, C.; Straube, L.; Stein, C.; Makarewicz, O.; Schubert, S.; Mossner, J.; Pletz, M.W.; Rodloff, A.C. Colonization with extended-spectrum beta-lactamase-producing and carbapenemase-producing Enterobacteriaceae in international travelers returning to Germany. Int. J. Med. Microbiol. 2015, 305, 148–156. [Google Scholar] [CrossRef]
- Macaux, L.; Ndoye, O.; Cordel, H.; Pomares, T.B.; Seytre, D.; Bouchaud, O.; Cohen, Y.; Zahar, J.R.; Carbonnelle, E. Extensively-drug-resistant bacteria carriers among overseas travellers: One-third had not been hospitalized previously. Int. J. Antimicrob. Agents 2018, 52, 385–389. [Google Scholar] [CrossRef]
- Maier, J.; Melzl, H.; Reischl, U.; Drubel, I.; Witte, W.; Lehn, N.; Linde, H. Panton-Valentine leukocidin-positive methicillin-resistant Staphylococcus aureus in Germany associated with travel or foreign family origin. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Mataseje, L.F.; Boyd, D.A.; Fuller, J.; Haldane, D.; Hoang, L.; Lefebvre, B.; Melano, R.G.; Poutanen, S.; Van Caeseele, P.; Mulvey, M.R. Characterization of OXA-48-like carbapenemase producers in Canada, 2011–14. J. Antimicrob. Chemother. 2018, 73, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Mataseje, L.F.; Peirano, G.; Church, D.L.; Conly, J.; Mulvey, M.; Pitout, J.D. Colistin-Nonsusceptible Pseudomonas aeruginosa Sequence Type 654 with blaNDM-1 Arrives in North America. Antimicrob. Agents Chemother. 2016, 60, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Mawatari, M.; Kato, Y.; Hayakawa, K.; Morita, M.; Yamada, K.; Mezaki, K.; Kobayashi, T.; Fujiya, Y.; Kutsuna, S.; Takeshita, N.; et al. Salmonella enterica serotype Paratyphi A carrying CTX-M-15 type extended-spectrum beta-lactamase isolated from a Japanese traveller returning from India, Japan, July 2013. Eurosurveillance 2013, 18. [Google Scholar] [CrossRef]
- Medalla, F.; Sjolund-Karlsson, M.; Shin, S.; Harvey, E.; Joyce, K.; Theobald, L.; Nygren, B.N.; Pecic, G.; Gay, K.; Austin, J.; et al. Ciprofloxacin-resistant Salmonella enterica Serotype Typhi, United States, 1999–2008. Emerg. Infect. Dis. 2011, 17, 1095–1098. [Google Scholar] [CrossRef]
- Meltzer, E.; Stienlauf, S.; Leshem, E.; Sidi, Y.; Schwartz, E. A large outbreak of Salmonella Paratyphi A infection among Israeli travelers to Nepal. Clin. Infect. Dis. 2014, 58, 359–364. [Google Scholar] [CrossRef]
- Mendez Arancibia, E.; Pitart, C.; Ruiz, J.; Marco, F.; Gascon, J.; Vila, J. Evolution of antimicrobial resistance in enteroaggregative Escherichia coli and enterotoxigenic Escherichia coli causing traveller’s diarrhoea. J. Antimicrob. Chemother. 2009, 64, 343–347. [Google Scholar] [CrossRef]
- Mensa, L.; Marco, F.; Vila, J.; Gascon, J.; Ruiz, J. Quinolone resistance among Shigella spp. isolated from travellers returning from India. Clin. Microbiol. Infect. 2008, 14, 279–281. [Google Scholar] [CrossRef]
- Mermin, J.H.; Townes, J.M.; Gerber, M.; Dolan, N.; Mintz, E.D.; Tauxe, R.V. Typhoid fever in the United States, 1985–1994: Changing risks of international travel and increasing antimicrobial resistance. Arch. Intern. Med. 1998, 158, 633–638. [Google Scholar] [CrossRef]
- Meyer, E.; Gastmeier, P.; Kola, A.; Schwab, F. Pet animals and foreign travel are risk factors for colonisation with extended-spectrum b-lactamase-producing Escherichia coli. Infection 2012, 40, 685–687. [Google Scholar] [CrossRef]
- Mina, N.; Bernard, K.; Burdz, T.; Wiebe, D.; Rai, J.S.; Hoang, L. Canada’s first case of a multi-drug resistant Corynebacterium diphtheriae, isolated from a skin abscess. Can. J. Infect. Dis. Med. Microbiol. 2011, 22, 10A. [Google Scholar]
- Mischlinger, J.; Lagler, H.; Harrison, N.; Ramharter, M. Dalbavancin for outpatient parenteral antimicrobial therapy of skin and soft tissue infections in a returning traveller: Proposal for novel treatment indications. Wiener Klinische Wochenschrift 2017, 129, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Mittal, J.; Szymczak, W.A.; Guo, Y.; Levi, M.H.; Chen, L.; Kreiswirth, B.N.; Riska, P.F.; Nori, P. Two for the price of one: Emerging carbapenemases in a returning traveller to New York City. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Muchena, G.; Shambira, G.; Masuka, N.; Juru, T.; Gombe, N.; Takundwa, L.; Bangure, D.; Tshimanga, M. Determinants of multidrug resistance among previously treated tuberculosis patients in Zimbabwe, 2014. Int. J. Tuberc. Lung Dis. 2017, 21, 1167–1172. [Google Scholar] [CrossRef]
- Mulvey, M.R.; Bharat, A.; Boyd, D.A.; Irwin, R.J.; Wylie, J. Characterization of a colistin-resistant Salmonella enterica 4,[5],12:i:-harbouring mcr-3.2 on a variant IncHI-2 plasmid identified in Canada. J. Med. Microbiol. 2018, 67, 1673–1675. [Google Scholar] [CrossRef]
- Murray, B.E.; Mathewson, J.J.; DuPont, H.L.; Ericsson, C.D.; Reves, R.R. Emergence of resistant fecal Escherichia coli in travelers not taking prophylactic antimicrobial agents. Antimicrob. Agents Chemother. 1990, 34, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Mutters, N.T.; Gunther, F.; Sander, A.; Mischnik, A.; Frank, U. Influx of multidrug-resistant organisms by country-to-country transfer of patients. BMC Infect. Dis. 2015, 15, 466. [Google Scholar] [CrossRef][Green Version]
- Navia, M.M.; Gascon, J.; Vila, J. Analysis of the mechanisms of resistance to several antimicrobial agents in Shigella spp. causing travellers’ diarrhoea. Clin. Microbiol. Infect. 2005, 11, 1044–1047. [Google Scholar] [CrossRef]
- Navia, M.M.; Ruiz, J.; Vila, J. Molecular characterization of the integrons in Shigella strains isolated from patients with traveler’s diarrhea. Diagn. Microbiol. Infect. Dis. 2004, 48, 175–179. [Google Scholar] [CrossRef]
- Nemeth, J.; Ledergerber, B.; Preiswerk, B.; Nobile, A.; Karrer, S.; Ruef, C.; Kuster, S.P. Multidrug-resistant bacteria in travellers hospitalized abroad: Prevalence, characteristics, and influence on clinical outcome. J. Hosp. Infect. 2012, 82, 254–259. [Google Scholar] [CrossRef]
- Niederer, L.; Kuhnert, P.; Egger, R.; Buttner, S.; Hachler, H.; Korczak, B.M. Genotypes and antibiotic resistances of Campylobacter jejuni and Campylobacter coli isolates from domestic and travel-associated human cases. Appl. Environ. Microbiol. 2012, 78, 288–291. [Google Scholar] [CrossRef]
- Nuesch-Inderbinen, M.; Abgottspon, H.; Sagesser, G.; Cernela, N.; Stephan, R. Antimicrobial susceptibility of travel-related Salmonella enterica serovar Typhi isolates detected in Switzerland (2002–2013) and molecular characterization of quinolone resistant isolates. BMC Infect. Dis. 2015, 15, 212. [Google Scholar] [CrossRef]
- Ny, S.; Löfmark, S.; Börjesson, S.; Englund, S.; Ringman, M.; Bergström, J.; Nauclér, P.; Giske, C.G.; Byfors, S. Community carriage of ESBL-producing Escherichia coli is associated with strains of low pathogenicity: A Swedish nationwide study. J. Antimicrob. Chemother. 2017, 72, 582–588. [Google Scholar] [CrossRef]
- O’Donnell, A.T.; Vieira, A.R.; Huang, J.Y.; Whichard, J.; Cole, D.; Karp, B.E. Quinolone-resistant Salmonella enterica serotype EnteritiDis. infections associated with international travel. Clin. Infect. Dis. 2014, 59, e139–e141. [Google Scholar] [CrossRef][Green Version]
- Oh, H.M.; Chew, S.K.; Monteiro, E.H. Multidrug-resistant typhoid fever in Singapore. Singap. Med. J. 1994, 35, 599–601. [Google Scholar]
- Ohtaka, M. Epidemiological approach to the prevention of imported infectious diseases in the age of globalization. Kansenshogaku Zasshi 1997, 71, 18–25. [Google Scholar] [CrossRef][Green Version]
- Olesen, B.; Jensen, C.; Olsen, K.; Fussing, V.; Gerner-Smidt, P.; Scheutz, F. VTEC O117:K1:H7 A new clonal group of E. coli associated with persistent diarrhoea in Danish travellers. Scand. J. Infect. Dis. 2005, 37, 288–294. [Google Scholar] [CrossRef]
- Östholm-Balkhed, Å.; Tärnberg, M.; Nilsson, M.; Nilsson, L.E.; Hanberger, H.; Hällgren, A.; on behalf of the Travel Study Group of Southeast Sweden. Travel-associated faecal colonization with ESBL-producing Enterobacteriaceae: Incidence and risk factors. J. Antimicrob. Chemother. 2013, 68, 2144–2153. [Google Scholar] [CrossRef]
- O’Sullivan, B.P.; Torres, B.; Conidi, G.; Smole, S.; Gauthier, C.; Stauffer, K.E.; Glass, M.B.; Gee, J.E.; Blaney, D.; Smith, T.L. Burkholderia pseudomallei infection in a child with cystic fibrosis: Acquisition in the Western Hemisphere. Chest 2011, 140, 239–242. [Google Scholar] [CrossRef]
- Ouyang-Latimer, J.; Jafri, S.; VanTassel, A.; Jiang, Z.D.; Gurleen, K.; Rodriguez, S.; Nandy, R.K.; Ramamurthy, T.; Chatterjee, S.; McKenzie, R.; et al. In vitro antimicrobial susceptibility of bacterial enteropathogens isolated from international travelers to Mexico, Guatemala, and India from 2006 to 2008. Antimicrob. Agents Chemother. 2011, 55, 874–878. [Google Scholar] [CrossRef]
- Paltansing, S.; Vlot, J.A.; Kraakman, M.E.; Mesman, R.; Bruijning, M.L.; Bernards, A.T.; Visser, L.G.; Veldkamp, K.E. Extended-spectrum b-lactamase-producing Enterobacteriaceae among travelers from the Netherlands. Emerg. Infect. Dis. 2013, 19, 1206–1213. [Google Scholar] [CrossRef]
- Pandey, P.; Bodhidatta, L.; Lewis, M.; Murphy, H.; Shlim, D.R.; Cave, W.; Rajah, R.; Springer, M.; Batchelor, T.; Sornsakrin, S.; et al. Travelers’ diarrhea in Nepal: An update on the pathogens and antibiotic resistance. J. Travel Med. 2011, 18, 102–108. [Google Scholar] [CrossRef]
- Parsonnet, J.; Gerber, A.R.; Greene, K.D.; Tauxe, R.V.; Vallejo Aguilar, O.J.; Blake, P.A. Shigella dysenteriae type 1 infections in US travellers to Mexico, 1988. Lancet 1989, 2, 543–545. [Google Scholar] [CrossRef]
- Patel, T.A.; Armstrong, M.; Morris-Jones, S.D.; Wright, S.G.; Doherty, T. Imported enteric fever: Case series from the hospital for tropical diseases, London, United Kingdom. Am. J. Trop. Med. Hyg. 2010, 82, 1121–1126. [Google Scholar] [CrossRef]
- Peirano, G.; Ahmed-Bentley, J.; Woodford, N.; Pitout, J.D. New Delhi metallo-b-lactamase from traveler returning to Canada. Emerg. Infect. Dis. 2011, 17, 242–244. [Google Scholar] [CrossRef]
- Pires, J.; Kraemer, J.G.; Kuenzli, E.; Kasraian, S.; Tinguely, R.; Hatz, C.; Endimiani, A.; Hilty, M. Gut microbiota dynamics in travelers returning from India colonized with extended-spectrum cephalosporin-resistant Enterobacteriaceae: A longitudinal study. Travel Med. Infect. Dis. 2019, 27, 72–80. [Google Scholar] [CrossRef]
- Pitout, J.D.D.; Campbell, L.; Church, D.L.; Gregson, D.B.; Laupland, K.B. Molecular characteristics of travel-related extended-spectrum-b-lactamase-producing Escherichia coli isolates from the Calgary Health Region. Antimicrob. Agents Chemother. 2009, 53, 2539–2543. [Google Scholar] [CrossRef]
- Pommelet, V.; Mariani, P.; Basmaci, R.; Tourdjman, M.; Morin, L.; Gaschignard, J.; de Lauzanne, A.; Lemaitre, C.; Bonacorsi, S.; Faye, A. Enteric fever among children: 50 cases in a French tertiary care centre. J. Travel Med. 2018, 25. [Google Scholar] [CrossRef]
- Pons, M.J.; Gomes, C.; Martinez-Puchol, S.; Ruiz, L.; Mensa, L.; Vila, J.; Gascon, J.; Ruiz, J. Antimicrobial resistance in Shigella spp. causing traveller’s diarrhoea (1995–2010): A retrospective analysis. Travel Med. Infect. Dis. 2013, 11, 315–319. [Google Scholar] [CrossRef]
- Pontali, E.; Feasi, M.; Usiglio, D.; Mori, M.; Cassola, G. Imported typhoid fever with hepatitis from Bangladesh: A case of delayed response to ceftriaxone? J. Travel Med. 2008, 15, 366–368. [Google Scholar] [CrossRef]
- Porter, C.K.; Riddle, M.S.; Tribble, D.R.; Putnam, S.D.; Rockabrand, D.M.; Frenck, R.W.; Rozmajzl, P.; Kilbane, E.; Fox, A.; Ruck, R.; et al. The epidemiology of travelers’ diarrhea in Incirlik, Turkey: A region with a predominance of heat-stabile toxin producing enterotoxigenic Escherichia coli. Diagn. Microbiol. Infect. Dis. 2010, 66, 241–247. [Google Scholar] [CrossRef]
- Post, A.; Martiny, D.; van Waterschoot, N.; Hallin, M.; Maniewski, U.; Bottieau, E.; Van Esbroeck, M.; Vlieghe, E.; Ombelet, S.; Vandenberg, O.; et al. Antibiotic susceptibility profiles among Campylobacter isolates obtained from international travelers between 2007 and 2014. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2101–2107. [Google Scholar] [CrossRef]
- Principe, L.; Mauri, C.; Conte, V.; Pini, B.; Giani, T.; Rossolini, G.M.; Luzzaro, F. First report of NDM-1-producing Klebsiella pneumoniae imported from Africa to Italy: Evidence of the need for continuous surveillance. J. Glob. Antimicrob. Resist. 2017, 8, 23–27. [Google Scholar] [CrossRef][Green Version]
- Reinheimer, C.; Kempf, V.A.; Jozsa, K.; Wichelhaus, T.A.; Hogardt, M.; O’Rourke, F.; Brandt, C. Prevalence of multidrug-resistant organisms in refugee patients, medical tourists and domestic patients admitted to a German university hospital. BMC Infect. Dis. 2017, 17, 17. [Google Scholar] [CrossRef]
- Reuland, E.A.; Al Naiemi, N.; Kaiser, A.M.; Heck, M.; Kluytmans, J.A.J.W.; Savelkoul, P.H.M.; Elders, P.J.M.; Vandenbroucke-Grauls, C.M.J.E. Prevalence and risk factors for carriage of ESBL-producing Enterobacteriaceae in Amsterdam. J. Antimicrob. Chemother. 2016, 71, 1076–1082. [Google Scholar] [CrossRef]
- Ricotta, E.E.; Palmer, A.; Wymore, K.; Clogher, P.; Oosmanally, N.; Robinson, T.; Lathrop, S.; Karr, J.; Hatch, J.; Dunn, J.; et al. Epidemiology and antimicrobial resistance of international travel-associated Campylobacter infections in the United States, 2005–2011. Am. J. Public Health 2014, 104, e108–e114. [Google Scholar] [CrossRef]
- Rieber, H.; Frontzek, A.; Pfeifer, Y. Molecular Investigation of Carbapenem-Resistant Acinetobacter spp. from Hospitals in North Rhine-Westphalia, Germany. Microb. Drug Resist. 2017, 23, 25–31. [Google Scholar] [CrossRef]
- Rodriguez, I.; Rodicio, M.R.; Guerra, B.; Hopkins, K.L. Potential international spread of multidrug-resistant invasive Salmonella enterica serovar enteritidis. Emerg. Infect. Dis. 2012, 18, 1173–1176. [Google Scholar] [CrossRef]
- Rogers, B.A.; Sidjabat, H.E.; Silvey, A.; Anderson, T.L.; Perera, S.; Li, J.; Paterson, D.L. Treatment options for New Delhi metallo-beta-lactamase-harboring Enterobacteriaceae. Microb. Drug Resist. 2013, 19, 100–103. [Google Scholar] [CrossRef]
- Ruiz, J.; Marco, F.; Oliveira, I.; Vila, J.; Gascon, J. Trends in antimicrobial resistance in Campylobacter spp. causing traveler’s diarrhea. Acta Pathologica Microbiologica Immunologica Scandinavica 2007, 115, 218–224. [Google Scholar] [CrossRef]
- Ruppe, E.; Armand-Lefevre, L.; Estellat, C.; Consigny, P.H.; El Mniai, A.; Boussadia, Y.; Goujon, C.; Ralaimazava, P.; Campa, P.; Girard, P.M.; et al. High Rate of Acquisition but Short Duration of Carriage of Multidrug-Resistant Enterobacteriaceae After Travel to the Tropics. Clin. Infect. Dis. 2015, 61, 593–600. [Google Scholar] [CrossRef]
- Sadouki, Z.; Day, M.R.; Doumith, M.; Chattaway, M.A.; Dallman, T.J.; Hopkins, K.L.; Elson, R.; Woodford, N.; Godbole, G.; Jenkins, C. Comparison of phenotypic and WGS-derived antimicrobial resistance profiles of Shigella sonnei isolated from cases of diarrhoeal disease in England and Wales, 2015. J. Antimicrob. Chemother. 2017, 72, 2496–2502. [Google Scholar] [CrossRef]
- Saitoh, T.; Morita, M.; Shimada, T.; Izumiya, H.; Kanayama, A.; Oishi, K.; Ohnishi, M.; Sunagawa, T. Increase in paratyphoid fever cases in Japanese travellers returning from Cambodia in 2013. Epidemiol. Infect. 2016, 144, 602–606. [Google Scholar] [CrossRef]
- Salazar-Austin, N.; Ordonez, A.A.; Hsu, A.J.; Benson, J.E.; Mahesh, M.; Menachery, E.; Razeq, J.H.; Salfinger, M.; Starke, J.R.; Milstone, A.M.; et al. Extensively drug-resistant tuberculosis in a young child after travel to India. Lancet Infect. Dis. 2015, 15, 1485–1491. [Google Scholar] [CrossRef]
- Samuelsen, Ø.; Overballe-Petersen, S.; Bjørnholt, J.V.; Brisse, S.; Doumith, M.; Woodford, N.; Hopkins, K.L.; Aasnæs, B.; Haldorsen, B.; Sundsfjord, A. Molecular and epidemiological characterization of carbapenemase-producing Enterobacteriaceae in Norway, 2007 to 2014. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Schaumburg, F.; Sertic, S.M.; Correa-Martinez, C.; Mellmann, A.; Kock, R.; Becker, K. Acquisition and colonization dynamics of antimicrobial-resistant bacteria during international travel: A prospective cohort study. Clin. Microbiol. Infect. 2019, 25, 1287.e1–1287.e7. [Google Scholar] [CrossRef]
- Schleucher, R.D.; Gaessler, M.; Knobloch, J. Panton-Valentine leukocidin-producing methicillin-Sensitive Staphylococcus aureus as a cause for recurrent, contagious skin infections in young, healthy travelers returned from a tropical country: A new worldwide public health problem? J. Travel Med. 2008, 15, 137–139. [Google Scholar] [CrossRef]
- Sekirov, I.; Croxen, M.A.; Ng, C.; Azana, R.; Chang, Y.; Mataseje, L.; Boyd, D.; Mangat, C.; Mack, B.; Tadros, M.; et al. Epidemiologic and genotypic review of carbapenemase-producing organisms in British Columbia, Canada, between 2008 and 2014. J. Clin. Microbiol. 2016, 54, 317–327. [Google Scholar] [CrossRef]
- Senok, A.; Somily, A.; Raji, A.; Gawlik, D.; Al-Shahrani, F.; Baqi, S.; Boswihi, S.; Skakni, L.; Udo, E.E.; Weber, S.; et al. Diversity of methicillin-resistant Staphylococcus aureus CC22-MRSA-IV from Saudi Arabia and the Gulf region. Int. J. Infect. Dis. 2016, 51, 31–35. [Google Scholar] [CrossRef][Green Version]
- Sharafeldin, E.; Soonawala, D.; Vandenbroucke, J.P.; Hack, E.; Visser, L.G. Health risks encountered by Dutch medical students during an elective in the tropics and the quality and comprehensiveness of pre-and post-travel care. BMC Med. Educ 2010, 10, 89. [Google Scholar] [CrossRef]
- Shiferaw, B.; Solghan, S.; Palmer, A.; Joyce, K.; Barzilay, E.J.; Krueger, A.; Cieslak, P. Antimicrobial susceptibility patterns of Shigella isolates in Foodborne Diseases Active Surveillance Network (FoodNet) sites, 2000–2010. Clin. Infect. Dis. 2012, 54, S458–S463. [Google Scholar] [CrossRef]
- Shin, E.; Hong, H.; Oh, Y.; Lee, Y. First Report and Molecular Characterization of a Campylobacter jejuni Isolate with Extensive Drug Resistance from a Travel-Associated Human Case. Antimicrob. Agents Chemother. 2015, 59, 6670–6672. [Google Scholar] [CrossRef][Green Version]
- Shrestha, R.K.; Padmanabhan, R.A.; Saravolatz, L.D.; Hall, G.S.; Gordon, S.M. Community-acquired methicillin-resistant Staphylococcus aureus in a returned traveler. Infect. Dis. Clin. Pract. 2005, 13, 139–141. [Google Scholar] [CrossRef]
- Siira, L.; Naseer, U.; Alfsnes, K.; Hermansen, N.O.; Lange, H.; Brandal, L.T. Whole genome sequencing of Salmonella Chester reveals geographically distinct clusters, Norway, 2000 to 2016. Eurosurveillance 2019, 24. [Google Scholar] [CrossRef]
- Silva, N.J.; Watrin, M.; Weill, F.X.; King, L.A.; Gouali, M.; Mailles, A.; van Cauteren, D.; Bataille, M.; Guettier, S.; Castrale, C.; et al. Outbreak of haemolytic uraemic syndrome due to Shiga toxin-producing Escherichia coli O104:H4 among French tourists returning from Turkey, September 2011. Eurosurveillance 2012, 17, 20120119. [Google Scholar]
- Skjot-Arkil, H.; Mogensen, C.B.; Lassen, A.T.; Johansen, I.S.; Chen, M.; Petersen, P.; Andersen, K.V.; Ellermann-Eriksen, S.; Moller, J.M.; Ludwig, M.; et al. Carrier prevalence and risk factors for colonisation of multiresistant bacteria in Danish emergency departments: A cross-sectional survey. BMJ Open 2019, 9, e029000. [Google Scholar] [CrossRef]
- Sole, M.; Pitart, C.; Oliveira, I.; Fabrega, A.; Munoz, L.; Campo, I.; Salvador, P.; Alvarez-Martinez, M.J.; Gascon, J.; Marco, F.; et al. Extended spectrum b-lactamase-producing Escherichia coli faecal carriage in Spanish travellers returning from tropical and subtropical countries. Clin. Microbiol. Infect. 2014, 20, O636–O639. [Google Scholar] [CrossRef][Green Version]
- Stenhem, M.; Ortqvist, A.; Ringberg, H.; Larsson, L.; Olsson-Liljequist, B.; Haeggman, S.; Kalin, M.; Ekdahl, K. Imported methicillin-resistant Staphylococcus aureus, Sweden. Emerg. Infect. Dis. 2010, 16, 189–196. [Google Scholar] [CrossRef]
- Strysko, J.P.; Mony, V.; Cleveland, J.; Siddiqui, H.; Homel, P.; Gagliardo, C. International travel is a risk factor for extended-spectrum b-lactamase-producing Enterobacteriaceae acquisition in children: A case-case-control study in an urban U.S. hospital. Travel Med. Infect. Dis. 2016, 14, 568–571. [Google Scholar] [CrossRef]
- Szabo, D.; Szentandrassy, J.; Juhasz, Z.; Katona, K.; Nagy, K.; Rokusz, L. Imported PER-1 producing Pseudomonas aeruginosa, PER-1 producing Acinetobacter baumanii and VIM-2-producing Pseudomonas aeruginosa strains in Hungary. Ann. Clin. Microbiol. Antimicrob. 2008, 7, 12. [Google Scholar] [CrossRef]
- Taguchi, M.; Kawahara, R.; Seto, K.; Inoue, K.; Hayashi, A.; Yamagata, N.; Kamakura, K.; Kashiwagi, E. Plasmid-mediated quinolone resistance in Salmonella isolated from patients with overseas travelers’ diarrhea in Japan. Jpn. J. Infect. Dis. 2009, 62, 312–314. [Google Scholar]
- Talan, D.A.; Takhar, S.S.; Krishnadasan, A.; Abrahamian, F.M.; Mower, W.R.; Moran, G.J. Fluoroquinolone-resistant and extended-spectrum β-lactamase-producing Escherichia coli infections in patients with pyelonephritis, United States. Emerg. Infect. Dis. 2016, 22, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Tangden, T.; Cars, O.; Melhus, A.; Lowdin, E. Foreign travel is a major risk factor for colonization with Escherichia coli producing CTX-M-type extended-spectrum b-lactamases: A prospective study with Swedish volunteers. Antimicrob. Agents Chemother. 2010, 54, 3564–3568. [Google Scholar] [CrossRef] [PubMed]
- Tappe, D.; Schulze, M.H.; Oesterlein, A.; Turnwald, D.; Muller, A.; Vogel, U.; Stich, A. Panton-Valentine leukocidin-positive Staphylococcus aureus infections in returning travelers. Am. J. Trop. Med. Hyg. 2010, 83, 748–750. [Google Scholar] [CrossRef] [PubMed]
- Tappe, D.; Schulze, M.H.; Van Der Linden, M.; Ziegler, U.; Muller, A.; Stich, A. Travel-related Streptococcal Toxic Shock Syndrome caused by emm type 78 Streptococcus pyogenes. J. Clin. Microbiol. 2011, 49, 3094–3095. [Google Scholar] [CrossRef][Green Version]
- Tatavarthy, A.; Sanderson, R.; Peak, K.; Scilabro, G.; Davenhill, P.; Cannons, A.; Amusoa, P. Molecular typing and resistance analysis of travel-associated Salmonella enterica serotype typhi. J. Clin. Microbiol. 2012, 50, 2631–2638. [Google Scholar] [CrossRef] [PubMed]
- Tauxe, R.V.; Puhr, N.D.; Wells, J.G.; Hargrett-Bean, N.; Blake, P.A. Antimicrobial resistance of Shigella isolates in the USA: The importance of international travelers. J. Infect. Dis. 1990, 162, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Terry, L.M.; Barker, C.R.; Day, M.R.; Greig, D.R.; Dallman, T.J.; Jenkins, C. Antimicrobial resistance profiles of Shigella dysenteriae isolated from travellers returning to the UK, 2004–2017. J. Med. Microbiol. 2018, 67, 1022–1030. [Google Scholar] [CrossRef]
- Tham, J.; Walder, M.; Melander, E.; Odenholt, I. Duration of colonization with extended-spectrum beta-lactamase-producing Escherichia coli in patients with travellers’ diarrhoea. Scand. J. Infect. Dis. 2012, 44, 573–577. [Google Scholar] [CrossRef]
- Tojo, M.; Mawatari, M.; Hayakawa, K.; Nagamatsu, M.; Shimada, K.; Mezaki, K.; Sugiki, Y.; Kuroda, E.; Takeshita, N.; Kutsuna, S.; et al. Multidrug-resistant Acinetobactor baumannii isolated from a traveler returned from Brunei. J. Infect. Chemother. 2015, 21, 212–214. [Google Scholar] [CrossRef]
- Trojanek, M.; Dedicova, D.; Zemlickova, H.; Jakubu, V.; Malikova, E.; Reisingerova, M.; Gabrielova, A.; Papagiannitsis, C.C.; Hrabak, J.; Horova, B.; et al. Enteric fever imported to the Czech Republic: Epidemiology, clinical characteristics and antimicrobial susceptibility. Folia Microbiol. 2015, 60, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Ukah, U.V.; Glass, M.; Avery, B.; Daignault, D.; Mulvey, M.R.; Reid-Smith, R.J.; Parmley, E.J.; Portt, A.; Boerlin, P.; Manges, A.R. Risk factors for acquisition of multidrug-resistant Escherichia coli and development of community-acquired urinary tract infections. Epidemiol. Infect. 2018, 146, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Valentin, T.; Feierl, G.; Masoud-Landgraf, L.; Kohek, P.; Luxner, J.; Zarfel, G. Proteus mirabilis harboring carbapenemase NDM-5 and ESBL VEB-6 detected in Austria. Diagn. Microbiol. Infect. Dis. 2018, 91, 284–286. [Google Scholar] [CrossRef]
- Valverde, A.; Turrientes, M.C.; Norman, F.; San Martin, E.; Moreno, L.; Perez-Molina, J.A.; Lopez-Velez, R.; Canton, R. CTX-M-15-non-ST131 Escherichia coli isolates are mainly responsible of faecal carriage with ESBL-producing Enterobacteriaceae in travellers, immigrants and those visiting friends and relatives. Clin. Microbiol. Infect. 2015, 21, 252.e1–252.e4. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.; Gascon, J.; Abdalla, S.; Gomez, J.; Marco, F.; Moreno, A.; Corachan, M.; Jimenez de Anta, T. Antimicrobial resistance of Shigella isolates causing traveler’s diarrhea. Antimicrob. Agents Chemother. 1994, 38, 2668–2670. [Google Scholar] [CrossRef]
- Vila, J.; Ruiz, J.; Gallardo, F.; Vargas, M.; Soler, L.; Figueras, M.J.; Gascon, J. Aeromonas spp. and traveler’s diarrhea: Clinical features and antimicrobial resistance. Emerg. Infect. Dis. 2003, 9, 552–555. [Google Scholar] [CrossRef]
- Vila, J.; Vargas, M.; Ruiz, J.; Corachan, M.; Jimenez De Anta, M.T.; Gascon, J. Quinolone resistance in enterotoxigenic Escherichia coli causing diarrhea in travelers to India in comparison with other geographical areas. Antimicrob. Agents Chemother. 2000, 44, 1731–1733. [Google Scholar] [CrossRef]
- Vila, J.; Vargas, M.; Ruiz, J.; Espasa, M.; Pujol, M.; Corachan, M.; Jimenez de Anta, M.T.; Gascon, J. Susceptibility patterns of enteroaggregative Escherichia coli associated with traveller’s diarrhoea: Emergence of quinolone resistance. J. Med. Microbiol. 2001, 50, 996–1000. [Google Scholar] [CrossRef][Green Version]
- Vlieghe, E.R.; Jacobs, J.A.; Van Esbroeck, M.; Koole, O.; Van Gompel, A. Trends of norfloxacin and erythromycin resistance of Campylobacter jejuni/Campylobacter coli isolates recovered from international travelers, 1994 to 2006. J. Travel Med. 2008, 15, 419–425. [Google Scholar] [CrossRef]
- Von Dach, E.; Diene, S.M.; Fankhauser, C.; Schrenzel, J.; Harbarth, S.; Francois, P. Comparative Genomics of Community-Associated Methicillin-Resistant Staphylococcus aureus Shows the Emergence of Clone ST8-USA300 in Geneva, Switzerland. J. Infect. Dis. 2016, 213, 1370–1379. [Google Scholar] [CrossRef]
- Von Wintersdorff, C.J.; Penders, J.; Stobberingh, E.E.; Oude Lashof, A.M.; Hoebe, C.J.; Savelkoul, P.H.; Wolffs, P.F. High rates of antimicrobial drug resistance gene acquisition after international travel, The Netherlands. Emerg. Infect. Dis. 2014, 20, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.M.; Merker, M.; Knoblauch, A.M.; Helbling, P.; Schoch, O.D.; van der Werf, M.J.; Kranzer, K.; Fiebig, L.; Kroger, S.; Haas, W.; et al. A cluster of multidrug-resistant Mycobacterium tuberculosis among patients arriving in Europe from the Horn of Africa: A molecular epidemiological study. Lancet Infect. Dis. 2018, 18, 431–440. [Google Scholar] [CrossRef]
- Wang, S.J.; Chiu, S.H.; Lin, Y.C.; Tsai, Y.C.; Mu, J.J. Carbapenem resistant Enterobacteriaceae carrying New Delhi metallo-b-lactamase gene (NDM-1) in Taiwan. Diagn. Microbiol. Infect. Dis. 2013, 76, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Weyrich, P.; Borgmann, S.; Mayer, F.; Heeg, P.; Riessen, R.; Kotter, I. Fatal multidrug-resistant Acinetobacter baumannii sepsis in a patient with travel history and recent onset of systemic lupus erythematosus: A case report. Int. J. Hyg. Environ. Health 2006, 209, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Wielders, C.C.H.; van Hoek, A.H.A.M.; Hengeveld, P.D.; Veenman, C.; Dierikx, C.M.; Zomer, T.P.; Smit, L.A.M.; van der Hoek, W.; Heederik, D.J.; de Greeff, S.C.; et al. Extended-spectrum β-lactamase- and pAmpC-producing Enterobacteriaceae among the general population in a livestock-dense area. Clin. Microbiol. Infect. 2017, 23, 120.e1–120.e8. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.; Lew, T.E.; Fuller, A.; Spelman, D.W.; Jenney, A.W. A case of multi-drug resistant ESBL-producing Shigella sonnei acute acalculous cholecystitis and gastroenteritis in a returned traveller. J. Travel Med. 2018, 25. [Google Scholar] [CrossRef]
- Williamson, D.A.; Lane, C.R.; Easton, M.; Valcanis, M.; Strachan, J.; Veitch, M.G.; Kirk, M.D.; Howden, B.P. Increasing Antimicrobial Resistance in Nontyphoidal Salmonella Isolates in Australia from 1979 to 2015. Antimicrob. Agents Chemother. 2018, 62, 2. [Google Scholar] [CrossRef]
- Wybo, I.; Blommaert, L.; De Beer, T.; Soetens, O.; De Regt, J.; Lacor, P.; Pierard, D.; Lauwers, S. Outbreak of multidrug-resistant Acinetobacter baumannii in a Belgian university hospital after transfer of patients from Greece. J. Hosp. Infect. 2007, 67, 374–380. [Google Scholar] [CrossRef]
- Yaita, K.; Aoki, K.; Suzuki, T.; Nakaharai, K.; Yoshimura, Y.; Harada, S.; Ishii, Y.; Tachikawa, N. Epidemiology of extended-spectrum b-lactamase producing Escherichia coli in the stools of returning Japanese travelers, and the risk factors for colonization. PLoS ONE 2014, 9, e98000. [Google Scholar] [CrossRef]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a new metallo-beta-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef]
- Abd El Ghany, M.; Fouz, N.; Hill-Cawthorne, G.A. Human Movement and Transmission of Antimicrobial-Resistant Bacteria. In Antibiotic Resistance in the Environment: A Worldwide Overview; Manaia, C.M., Donner, E., Vaz-Moreira, I., Hong, P., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Riddle, M.S. Travel, Diarrhea, Antibiotics, Antimicrobial Resistance and Practice Guidelines—A Holistic Approach to a Health Conundrum. Curr. Infect. Dis. Rep. 2020, 22, 8. [Google Scholar] [CrossRef]
- Greenwood, Z.; Black, J.; Weld, L.; O’Brien, D.; Leder, K.; Von Sonnenburg, F.; Pandey, P.; Schwartz, E.; Connor, B.A.; Brown, G.; et al. Gastrointestinal Infection Among International Travelers Globally. J. Travel Med. 2008, 15, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, C.D. Travellers’ diarrhoea. Int. J. Antimicrob. Agents 2003, 21, 116–124. [Google Scholar] [CrossRef]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018, 31. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.A.; Aminzadeh, Z.; Hayashi, Y.; Paterson, D.L. Country-to-Country Transfer of Patients and the Risk of Multi-Resistant Bacterial Infection. Clin. Infect. Dis. 2011, 53, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, L.; Labonté, R.; Runnels, V.; Packer, C. Medical tourism today: What is the state of existing knowledge? J. Public Health Policy 2010, 31, 185–198. [Google Scholar] [CrossRef]
- United States Department of Transportation. 2018 Traffic Data for U.S Airlines and Foreign Airlines U.S. Flights; Bureau of Transportation Statistics: Washington, DC, USA, 2019.
- The World Bank. Research and Development Expenditure (% of GDP). Available online: https://data.worldbank.org/indicator/GB.XPD.RSDV.GD.ZS (accessed on 6 October 2018).
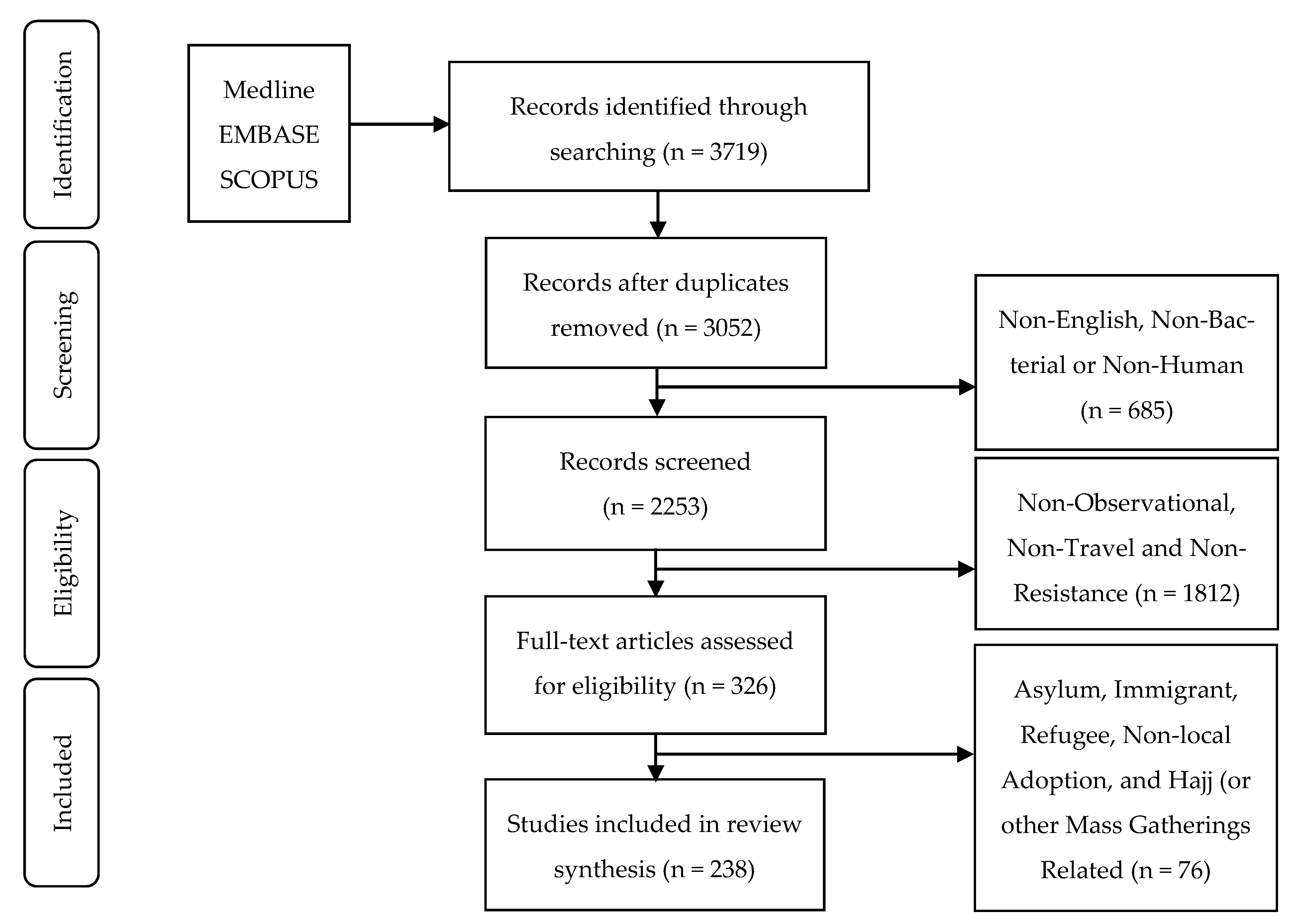
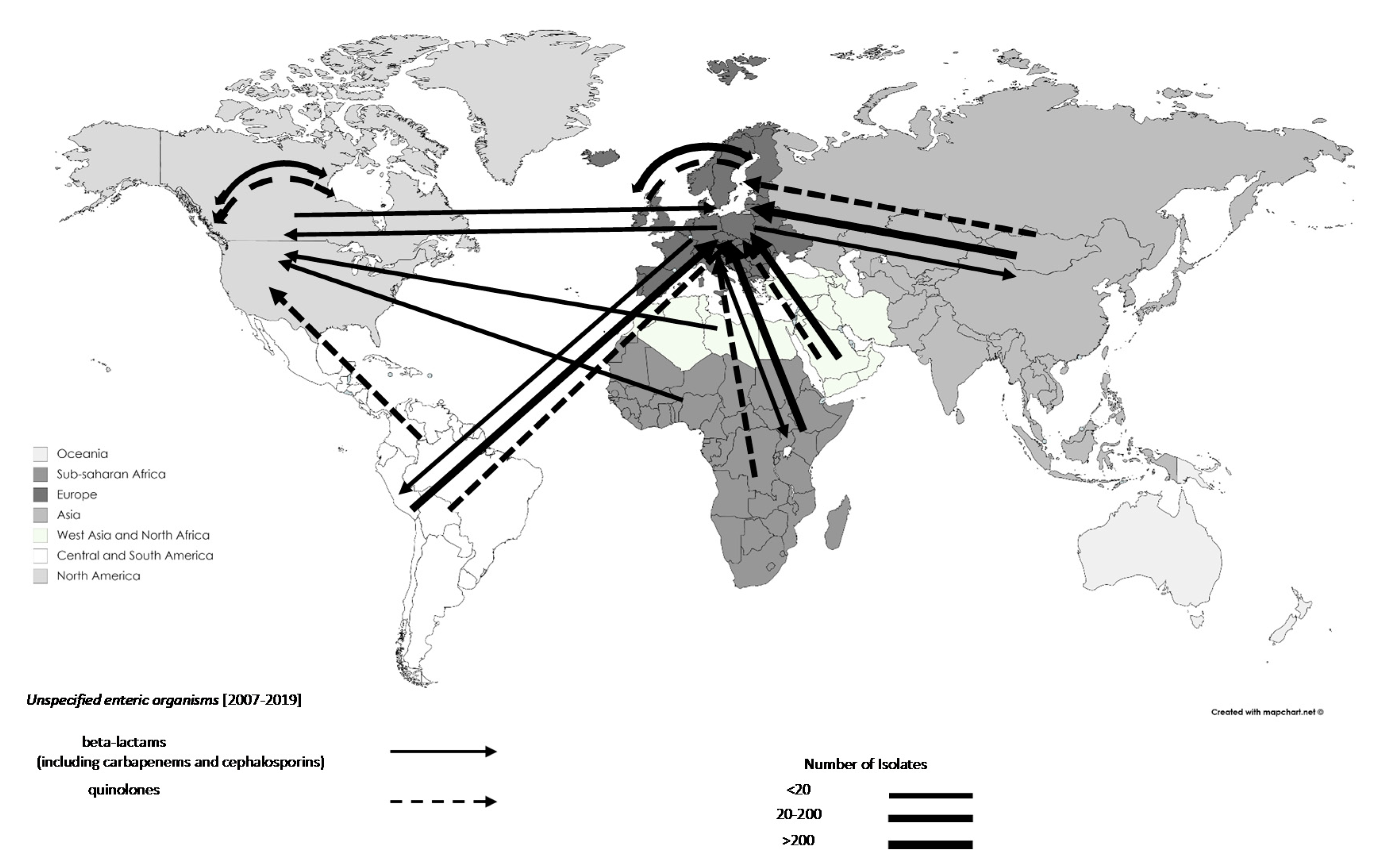
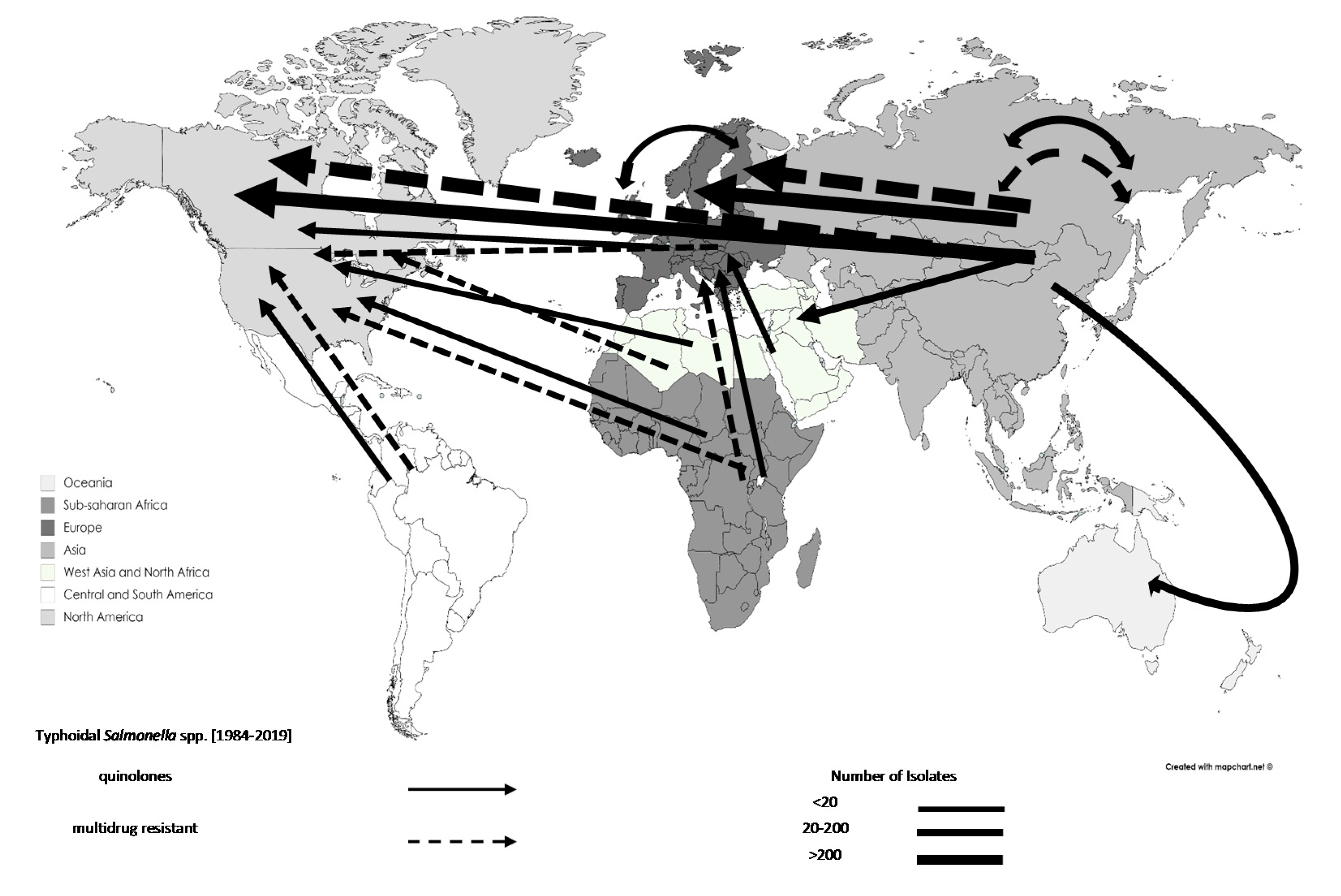

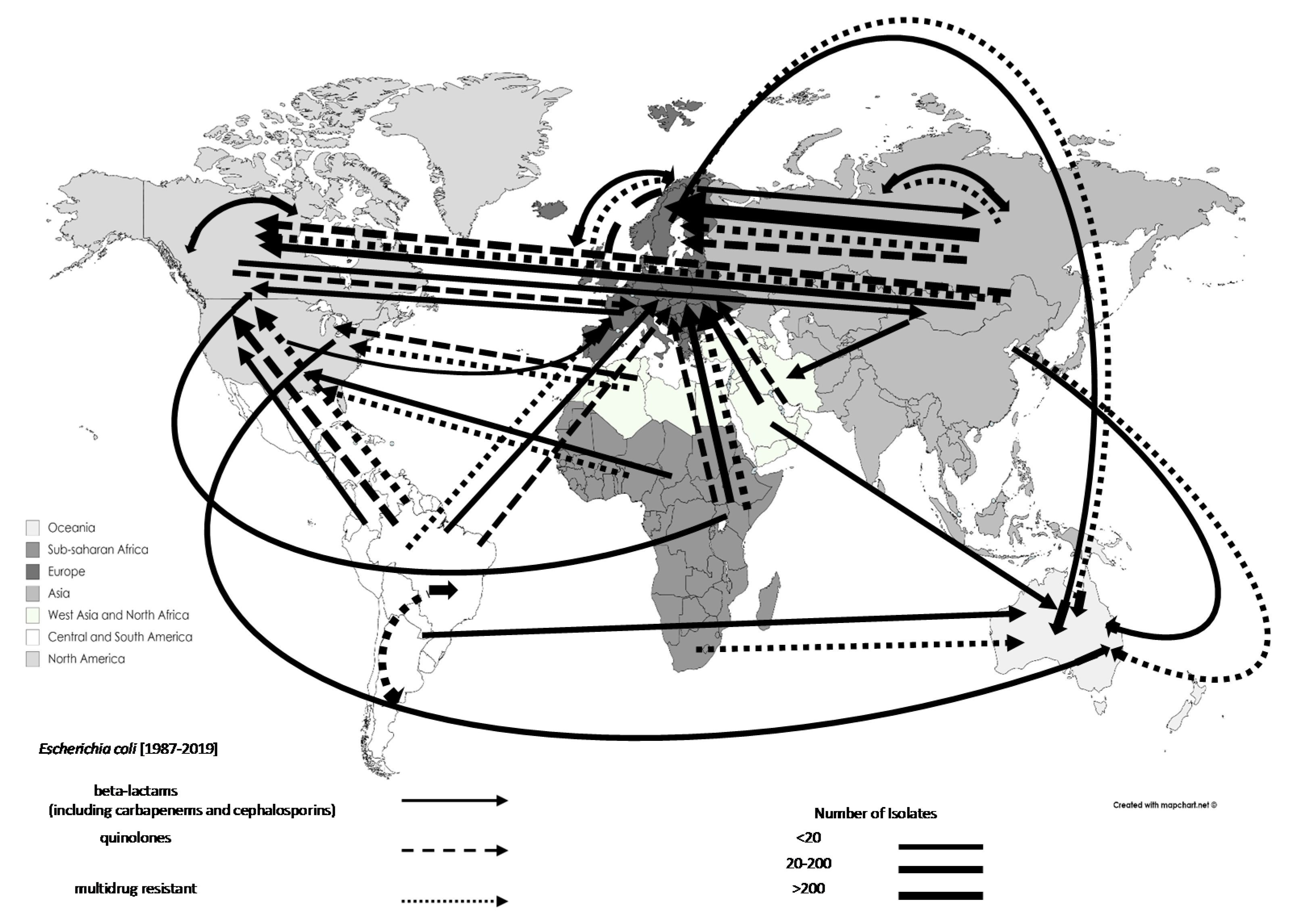

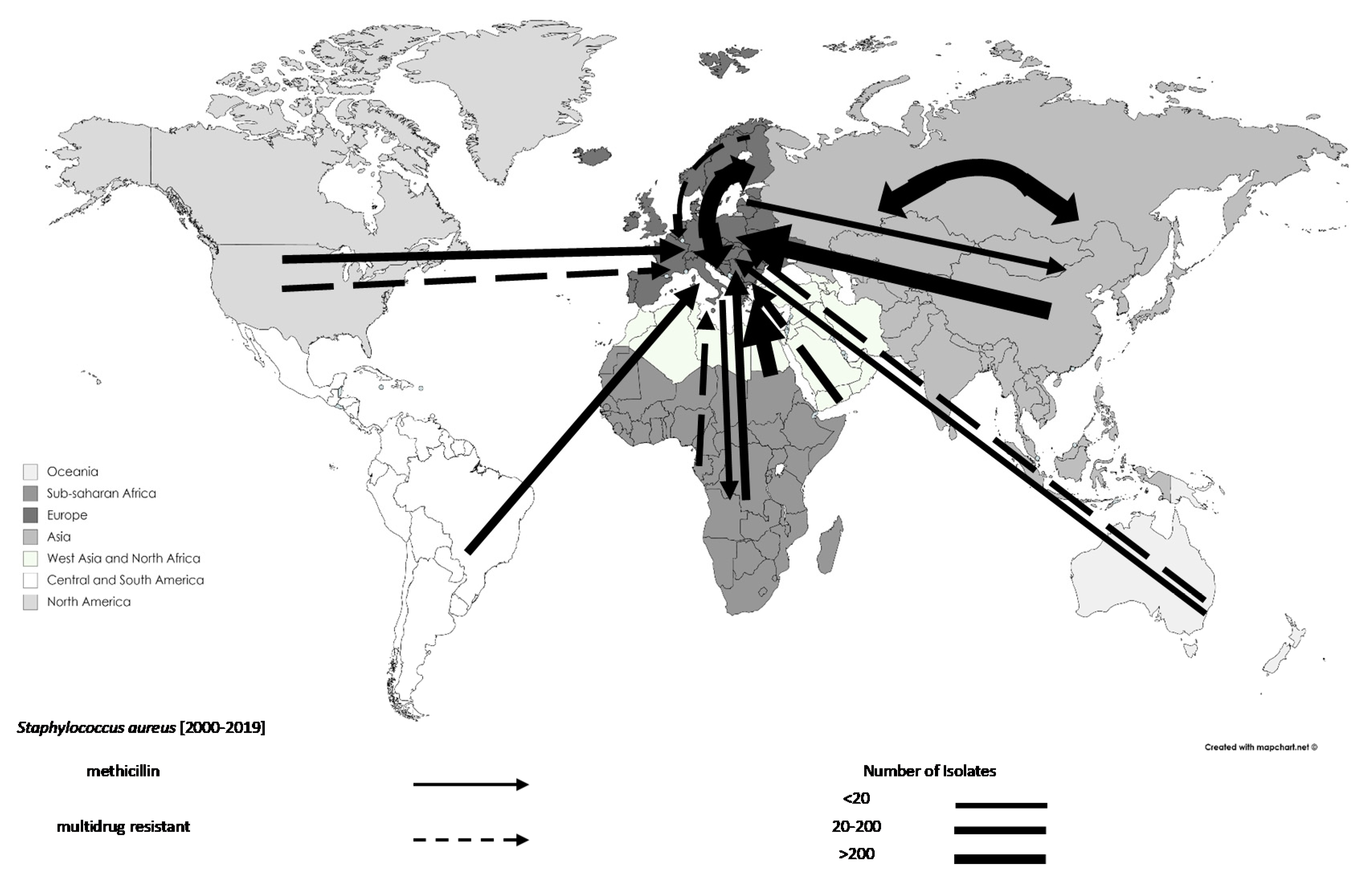
| # | Decade Organism was Isolated | Total Travel-Related AMR Isolates | Total Studies Documenting Travel-Related AMR Isolates 1 | Year of Publication | ||
|---|---|---|---|---|---|---|
| Start | End | from | to | |||
| 1 | Before 1990 | 1074 | 6 | 1989 | 1998 | |
| 2 | 1990 | 1999 | 6570 | 32 | 1993 | 2019 |
| 3 | 2000 | 2009 | 16,688 | 91 | 2001 | 2019 |
| 4 | 2010 | 2019 | 5003 | 126 | 2010 | 2019 |
| Total | Until Jun 2019 (inclusive) | 30,060 2 | 238 2 | 1989 | 2019 | |
| Decade | Before 1990 | 1990–1999 | 2000–2009 | 2010–2019 | Total | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organism | Region | BL/CP | QR | MDR | All AMR | BL/CP | QR | MDR | All AMR | BL/CP | QR | MDR | All AMR | BL/CP | QR | MDR | All AMR | BL/CP | QR | MDR | All AMR |
| Klebsiella pneumoniae | Sub-Saharan Africa | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 4/2 | 0 | 1 | 5 | 4/2 | 0 | 1 | 5 |
| North Africa and West Asia | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 2 | 2 | 7/1 | 0 | 10 | 19 | 7/1 | 0 | 12 | 21 | |
| Asia (except West Asia) | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 2/2 | 0 | 1 | 3 | 12/11 | 0 | 15 | 28 | 14/13 | 0 | 16 | 31 | |
| Europe | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 6 | 6 | 2/0 | 0 | 11 | 13 | 2/0 | 0 | 17 | 19 | |
| North America | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 1/0 | 0 | 2 | 3 | 1/0 | 0 | 2 | 3 | |
| South and Central America | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 1/0 | 0 | 1 | 2 | 1/0 | 0 | 1 | 2 | |
| Oceania | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | |
| Other | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 1/1 | 0 | 16 | 61 | 12/12 | 12 | 21 | 45 | 13/13 | 12 | 37 | 106 | |
| Campylobacter spp. | Sub-Saharan Africa | 0/0 | 0 | 0 | 0 | 0/0 | 52 | 0 | 98 | 0/0 | 99 | 0 | 140 | 0/0 | 0 | 0 | 0 | 0/0 | 151 | 0 | 238 |
| North Africa and West Asia | 0/0 | 0 | 0 | 0 | 0/0 | 8 | 0 | 8 | 0/0 | 67 | 0 | 90 | 0/0 | 0 | 0 | 0 | 0/0 | 75 | 0 | 98 | |
| Asia (except West Asia) | 0/0 | 0 | 0 | 0 | 0/0 | 227 | 0 | 232 | 0/0 | 383 | 1 | 502 | 0/0 | 17 | 1 | 18 | 0/0 | 627 | 2 | 752 | |
| Europe | 0/0 | 0 | 0 | 0 | 0/0 | 90 | 0 | 90 | 0/0 | 376 | 0 | 402 | 0/0 | 0 | 1 | 1 | 0/0 | 466 | 1 | 493 | |
| North America | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 0/0 | 3 | 0 | 4 | 0/0 | 0 | 0 | 0 | 0/0 | 3 | 0 | 4 | |
| South and Central America | 0/0 | 0 | 0 | 0 | 0/0 | 42 | 0 | 76 | 0/0 | 274 | 0 | 299 | 0/0 | 0 | 0 | 0 | 0/0 | 316 | 0 | 375 | |
| Oceania | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | |
| Other | 0/0 | 0 | 0 | 19 | 0/0 | 0 | 0 | 1050 | 0/0 | 715 | 0 | 240 | 0/0 | 218 | 0 | 0 | 0/0 | 933 | 0 | 1309 | |
| Shigella spp. | Sub-Saharan Africa | 0/0 | 0 | 0 | 0 | 0/0 | 4 | 187 | 337 | 0/0 | 1 | 181 | 426 | 0/0 | 0 | 34 | 42 | 0/0 | 5 | 402 | 805 |
| North Africa and West Asia | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 1 | 60 | 0/0 | 3 | 8 | 66 | 1/0 | 0 | 48 | 70 | 1/0 | 3 | 57 | 196 | |
| Asia (except West Asia) | 0/0 | 0 | 0 | 0 | 0/0 | 99 | 251 | 434 | 0/0 | 143 | 262 | 1023 | 8/0 | 48 | 127 | 240 | 8/0 | 280 | 640 | 1697 | |
| Europe | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 100 | 100 | 0/0 | 0 | 62 | 67 | 0/0 | 1 | 3 | 10 | 0/0 | 1 | 165 | 177 | |
| North America | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 0/0 | 6 | 0 | 180 | 0/0 | 0 | 3 | 4 | 0/0 | 6 | 3 | 184 | |
| South and Central America | 0/0 | 0 | 31 | 150 | 0/0 | 1 | 57 | 201 | 0/0 | 8 | 53 | 824 | 2/0 | 2 | 28 | 67 | 2/0 | 11 | 169 | 1242 | |
| Oceania | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 11 | 1/0 | 0 | 0 | 9 | 1/0 | 0 | 4 | 20 | |
| Other | 0/0 | 18 | 37 | 491 | 0/0 | 0 | 27 | 170 | 0/0 | 22 | 104 | 1929 | 1/0 | 1 | 11 | 20 | 1/0 | 41 | 179 | 2610 | |
| Salmonella spp. | Sub-Saharan Africa | 0/0 | 0 | 0 | 0 | 0/0 | 4 | 3 | 14 | 0/0 | 39 | 27 | 96 | 0/0 | 1 | 3 | 5 | 0/0 | 44 | 33 | 115 |
| North Africa and West Asia | 0/0 | 0 | 0 | 0 | 0/0 | 23 | 1 | 25 | 0/0 | 282 | 1 | 287 | 0/0 | 9 | 8 | 20 | 0/0 | 314 | 10 | 332 | |
| Asia (except West Asia) | 0/0 | 0 | 16 | 16 | 0/0 | 220 | 154 | 403 | 0/0 | 1905 | 354 | 2344 | 0/0 | 50 | 35 | 91 | 0/0 | 2290 2 | 1169 3 | 3579 2,3 | |
| Europe | 0/0 | 0 | 0 | 0 | 0/0 | 24 | 2 | 332 | 0/0 | 70 | 0 | 74 | 0/0 | 4 | 0 | 4 | 0/0 | 98 | 2 | 410 | |
| North America | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 2 | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 2 | |
| South and Central America | 0/0 | 0 | 1 | 1 | 0/0 | 3 | 2 | 6 | 0/0 | 14 | 5 | 35 | 0/0 | 1 | 14 | 15 | 0/0 | 18 | 22 | 57 | |
| Oceania | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 1 | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 1 | |
| Other | 0/0 | 0 | 0 | 51 | 0/0 | 9 | 167 | 773 | 0/0 | 200 | 30 | 710 | 0/0 | 1 | 1 | 2 | 0/0 | 210 | 198 | 1536 | |
| Escherichia coli | Sub-Saharan Africa | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 4 | 5 | 46/0 | 0 | 9 | 55 | 17/0 | 1 | 29 | 49 | 63/0 | 1 | 42 | 109 |
| North Africa and West Asia | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 13 | 14 | 70/0 | 15 | 9 | 178 | 3/0 | 23 | 3 | 34 | 73/0 | 38 | 25 | 226 | |
| Asia (except West Asia) | 0/0 | 0 | 0 | 0 | 0/0 | 6 | 7 | 14 | 352/0 | 134 | 41 | 950 | 106/8 | 16 | 68 | 205 | 458/8 | 156 | 116 | 1169 | |
| Europe | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 19/0 | 0 | 0 | 24 | 71/1 | 23 | 4 | 108 | 90/1 | 23 | 4 | 132 | |
| North America | 0/0 | 0 | 0 | 25 | 0/0 | 0 | 0 | 0 | 35/0 | 0 | 0 | 38 | 4/0 | 3 | 0 | 7 | 39/0 | 3 | 0 | 70 | |
| South and Central America | 0/0 | 0 | 25 | 203 | 0/0 | 1 | 12 | 75 | 28/0 | 171 | 13 | 1090 | 5/0 | 7 | 12 | 24 | 33/0 | 179 | 62 | 1392 | |
| Oceania | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | 0/0 | 0 | 0 | 0 | |
| Other | 0/0 | 0 | 0 | 88 | 0/0 | 13 | 0 | 483 | 1/0 | 60 | 108 | 1446 | 84/2 | 32 | 101 | 346 | 85/2 | 105 | 209 | 2363 | |
| Staphylococcus aureus | MRSA | MRSA | MRSA | MRSA | MRSA | ||||||||||||||||
| Sub-Saharan Africa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 57 | 0 | 1 | 58 | 7 | 2 | 2 | 118 | 64 | 2 | 3 | 176 | |
| North Africa and West Asia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 139 | 0 | 0 | 141 | 16 | 0 | 0 | 16 | 155 | 0 | 0 | 157 | |
| Asia (except West Asia) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 705 | 0 | 31 | 737 | 56 | 20 | 23 | 201 | 761 | 20 | 54 | 938 | |
| Europe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 426 | 0 | 1 | 431 | 26 | 0 | 2 | 39 | 452 | 0 | 3 | 470 | |
| North America | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 84 | 0 | 1 | 90 | 5 | 0 | 0 | 5 | 89 | 0 | 1 | 95 | |
| South and Central America | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 87 | 0 | 0 | 88 | 30 | 3 | 0 | 57 | 117 | 3 | 0 | 145 | |
| Oceania | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 1 | 27 | 1 | 1 | 0 | 6 | 26 | 1 | 1 | 33 | |
| Other | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 12 | 39 | 1 | 1 | 42 | 78 | 14 | 16 | 94 | 117 | 15 | 26 | 148 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokhary, H.; Pangesti, K.N.A.; Rashid, H.; Abd El Ghany, M.; Hill-Cawthorne, G.A. Travel-Related Antimicrobial Resistance: A Systematic Review. Trop. Med. Infect. Dis. 2021, 6, 11. https://doi.org/10.3390/tropicalmed6010011
Bokhary H, Pangesti KNA, Rashid H, Abd El Ghany M, Hill-Cawthorne GA. Travel-Related Antimicrobial Resistance: A Systematic Review. Tropical Medicine and Infectious Disease. 2021; 6(1):11. https://doi.org/10.3390/tropicalmed6010011
Chicago/Turabian StyleBokhary, Hamid, Krisna N. A. Pangesti, Harunor Rashid, Moataz Abd El Ghany, and Grant A. Hill-Cawthorne. 2021. "Travel-Related Antimicrobial Resistance: A Systematic Review" Tropical Medicine and Infectious Disease 6, no. 1: 11. https://doi.org/10.3390/tropicalmed6010011
APA StyleBokhary, H., Pangesti, K. N. A., Rashid, H., Abd El Ghany, M., & Hill-Cawthorne, G. A. (2021). Travel-Related Antimicrobial Resistance: A Systematic Review. Tropical Medicine and Infectious Disease, 6(1), 11. https://doi.org/10.3390/tropicalmed6010011






