Prognosis of COVID-19 in Patients with Liver and Kidney Diseases: An Early Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Inclusion and Exclusion Criteria
2.2. Data Collection
2.3. Quality Assessment
2.4. Data Extraction and Analysis
3. Results
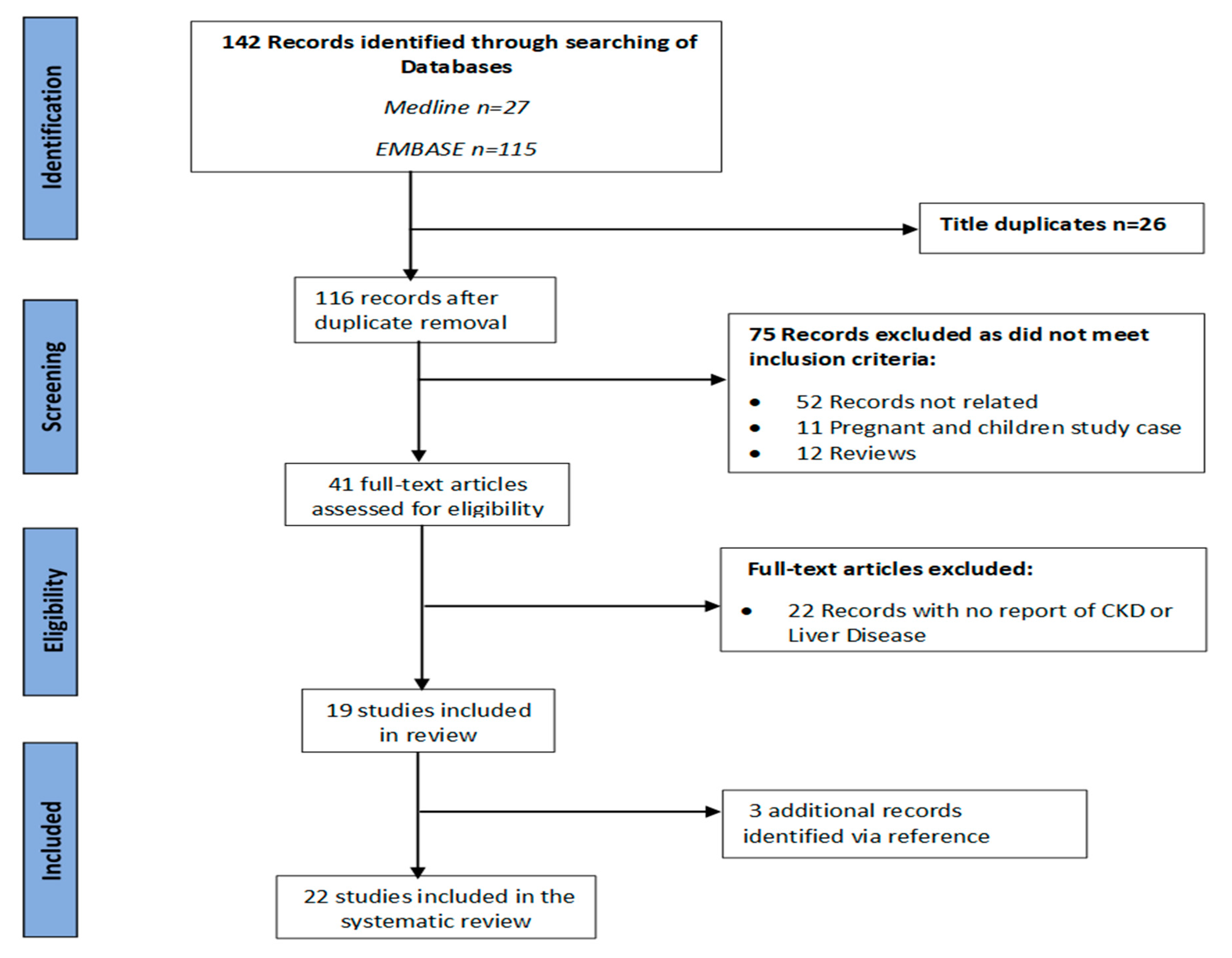
3.1. Description of Included Studies
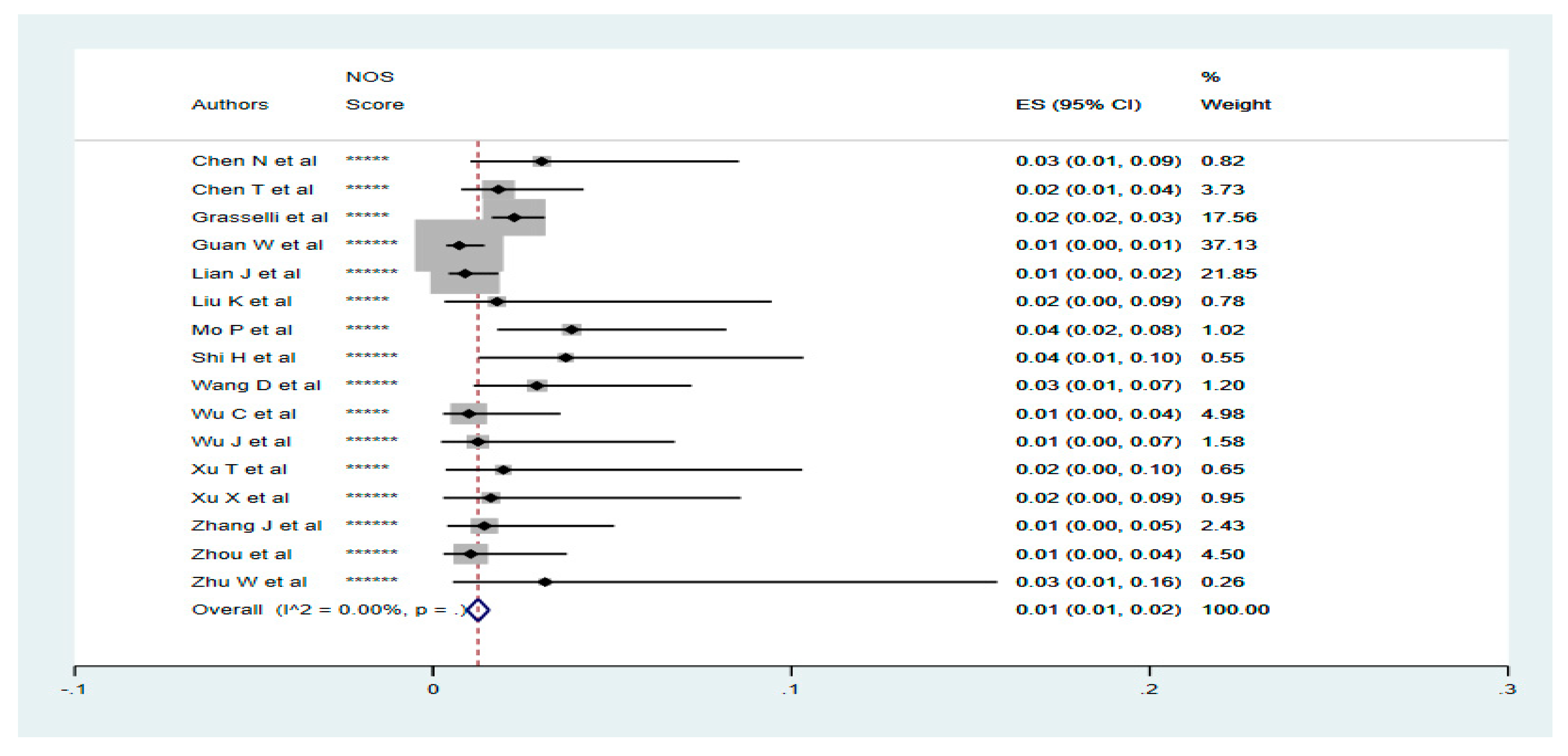
3.2. Prevalence of Renal Diseases in Confirmed COVID-19 Cases
3.3. Disease Outcome for Renal Diseases Patients with COVID-19
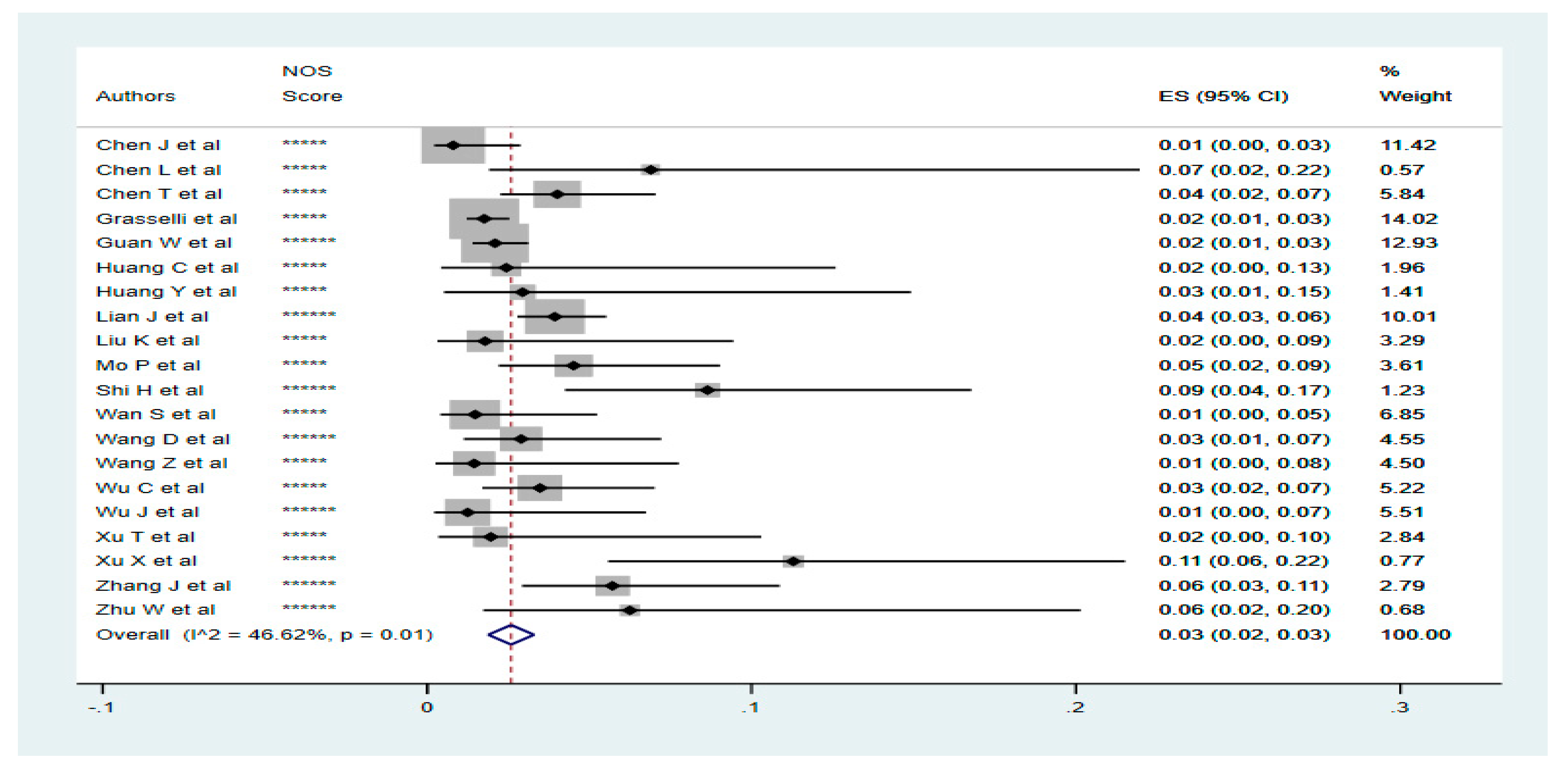
3.4. Prevalence of Liver Diseases in Confirmed COVID-19 Cases
3.5. Disease Outcome for Liver Diseases Patients with COVID-19
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| First Author | SELECTION | ASCERTAINMENT | OUTCOME | OVERALL | |||
|---|---|---|---|---|---|---|---|
| Sample Size Adequate | Population Representative (Multicentre) | Test Adequate | Comorbidity Confirmation Adequate (EMR) | Outcome Reported Adequately (Clinical Staff) | Follow-up Long Enough (≥2 Weeks) | OVERALL (≥4 Stars = Lower Risk of Bias) | |
| Chen J et al. | * | * | * | * | * | ***** | |
| Chen L et al. | * | * | * | * | * | ***** | |
| Chen N et al. | * | * | * | * | * | ***** | |
| Chen T et al. | * | * | * | * | * | ***** | |
| Grasselli et al. | * | * | * | * | * | ***** | |
| Guan W et al. | * | * | * | * | * | * | ****** |
| Huang C et al. | * | * | * | * | * | ***** | |
| Huang Y et al. | * | * | * | * | * | ***** | |
| Lian J et al. | * | * | * | * | * | * | ****** |
| Liu K et al. | * | * | * | * | * | ***** | |
| Mo P et al. | * | * | * | * | * | ***** | |
| Shi H et al. | * | * | * | * | * | * | ****** |
| Wan S et al. | * | * | * | * | * | ***** | |
| Wang D et al. | * | * | * | * | * | * | ****** |
| Wang Z et al. | * | * | * | * | * | ***** | |
| Wu C et al. | * | * | * | * | * | ***** | |
| Wu J et al. | * | * | * | * | * | * | ****** |
| Xu T et al. | * | * | * | * | * | ***** | |
| Xu X et al. | * | * | * | * | * | * | ****** |
| Zhang J et al. | * | * | * | * | * | ***** | |
| Zhou et al. | * | * | * | * | * | * | ****** |
| Zhu W et al. | * | * | * | * | * | * | ****** |


References
- Mokdad, A.A.; Lopez, A.D.; Shahraz, S.; Lozano, R.; Mokdad, A.H.; Stanaway, J.; Murray, C.J.; Naghavi, M. Liver cirrhosis mortality in 187 countries between 1980 and 2010: A systematic analysis. BMC Med. 2014, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Anthony, P.P.; Ishak, K.G.; Nayak, N.C.; Poulsen, H.E.; Scheuer, P.J.; Sobin, L.H. The morphology of cirrhosis. Recommendations on definition, nomenclature, and classification by a working group sponsored by the World Health Organization. J. Clin. Pathol. 1978, 31, 395–414. [Google Scholar] [CrossRef] [PubMed]
- Fleming, K.M.; Aithal, G.P.; Card, T.R.; West, J. All-cause mortality in people with cirrhosis compared with the general population: A population-based cohort study. Liver Int. 2012, 32, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Sepanlou, S.G.; Safiri, S.; Bisignano, C.; Ikuta, K.S.; Merat, S.; Saberifiroozi, M.; Poustchi, H.; Tsoi, D.; Colombara, D.V.; Abdoli, A.; et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef]
- WHO. Global Health Estimates 2015: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2015; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Levin, A.S.P.; Bilous, R.W.; Coresh, J. Chapter 1: Definition and classification of CKD. Kidney Int. Suppl. 2013, 3, 19–62. [Google Scholar] [CrossRef]
- McClellan, W.M.; Flanders, W.D. Risk factors for progressive chronic kidney disease. J. Am. Soc. Nephrol. 2003, 14, S65–S70. [Google Scholar] [CrossRef]
- Kottgen, A.; Glazer, N.L.; Dehghan, A.; Hwang, S.J.; Katz, R.; Li, M.; Yang, Q.; Gudnason, V.; Launer, L.J.; Harris, T.B.; et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat. Genet. 2009, 41, 712–717. [Google Scholar] [CrossRef]
- Perneger, T.V.; Whelton, P.K.; Klag, M.J. Risk of kidney failure associated with the use of acetaminophen, aspirin, and nonsteroidal antiinflammatory drugs. N. Engl. J. Med. 1994, 331, 1675–1679. [Google Scholar] [CrossRef]
- Chang, A.; Kramer, H. CKD progression: A risky business. Nephrol. Dial. Transplant. 2012, 27, 2607–2609. [Google Scholar] [CrossRef]
- Iseki, K. Factors influencing the development of end-stage renal disease. Clin. Exp. Nephrol. 2005, 9, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Suleymanlar, G.; Utas, C.; Arinsoy, T.; Ates, K.; Altun, B.; Altiparmak, M.R.; Ecder, T.; Yilmaz, M.E.; Camsari, T.; Basci, A.; et al. A population-based survey of Chronic REnal Disease In Turke—The CREDIT study. Nephrol. Dial. Transplant. 2011, 26, 1862–1871. [Google Scholar] [CrossRef] [PubMed]
- Lackland, D.T.; Egan, B.M.; Fan, Z.J.; Syddall, H.E. Low birth weight contributes to the excess prevalence of end-stage renal disease in African Americans. J. Clin. Hypertens. (Greenwich) 2001, 3, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Bleyer, A.J.; Shemanski, L.R.; Burke, G.L.; Hansen, K.J.; Appel, R.G. Tobacco, hypertension, and vascular disease: Risk factors for renal functional decline in an older population. Kidney Int. 2000, 57, 2072–2079. [Google Scholar] [CrossRef]
- Orth, S.R.; Schroeder, T.; Ritz, E.; Ferrari, P. Effects of smoking on renal function in patients with type 1 and type 2 diabetes mellitus. Nephrol. Dial. Transplant. 2005, 20, 2414–2419. [Google Scholar] [CrossRef]
- Nzerue, C.M.; Demissochew, H.; Tucker, J.K. Race and kidney disease: Role of social and environmental factors. J. Natl. Med. Assoc. 2002, 94, 28s–38s. [Google Scholar]
- Song, E.Y.; McClellan, W.M.; McClellan, A.; Gadi, R.; Hadley, A.C.; Krisher, J.; Clay, M.; Freedman, B.I. Effect of community characteristics on familial clustering of end-stage renal disease. Am. J. Nephrol. 2009, 30, 499–504. [Google Scholar] [CrossRef]
- Gonzalez-Quiroz, M.; Pearce, N.; Caplin, B.; Nitsch, D. What do epidemiological studies tell us about chronic kidney disease of undetermined cause in Meso-America? A systematic review and meta-analysis. Clin. Kidney J. 2018, 11, 496–506. [Google Scholar] [CrossRef]
- Goldstein, S.L.; Devarajan, P. Acute kidney injury in childhood: Should we be worried about progression to CKD? Pediatric Nephrol. 2011, 26, 509–522. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Cockwell, P.; Fisher, L.-A. The global burden of chronic kidney disease. Lancet 2020, 395. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Chen, H.; Yan, S.; Li, D.; Li, Y.; Gong, Z. Coronavirus Disease 19 Infection Does Not Result in Acute Kidney Injury: An Analysis of 116 Hospitalized Patients from Wuhan, China. Am. J. Nephrol. 2020, 51, 343–348. [Google Scholar] [CrossRef]
- WHO. Coronavirus Disease (COVID-2019) Situation Reports; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Chan, J.F.; Yuan, S.; Kok, K.H.; To, K.K.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.; Poon, R.W.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- WHO. Coronavirus Disease 2019 (COVID-19), Situation Report—73; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Guan, W.J.; Liang, W.H.; Zhao, Y.; Liang, H.R.; Chen, Z.S.; Li, Y.M.; Liu, X.Q.; Chen, R.C.; Tang, C.L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: A Nationwide Analysis. Eur. Respir. J. 2020. [Google Scholar] [CrossRef]
- Alqahtani, J.S.; Oyelade, T.; Aldhahir, A.M.; Alghamdi, S.M.; Almehmadi, M.; Alqahtani, A.S.; Quaderi, S.; Mandal, S.; Hurst, J.R. Prevalence, Severity and Mortality associated with COPD and Smoking in patients with COVID-19: A Rapid Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0233147. [Google Scholar] [CrossRef]
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ Evid. Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef]
- World Health Organization. Laboratory Testing for Coronavirus Disease ( COVID-19) in Suspected Human Cases: Interim Guidance, 19 March 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Chen, J.; Qi, T.; Liu, L.; Ling, Y.; Qian, Z.; Li, T.; Li, F.; Xu, Q.; Zhang, Y.; Xu, S.; et al. Clinical progression of patients with COVID-19 in Shanghai, China. J. Infect. 2020. [Google Scholar] [CrossRef]
- Chen, L.; Liu, H.G.; Liu, W.; Liu, J.; Liu, K.; Shang, J.; Deng, Y.; Wei, S. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi 2020, 43, E005. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Huang, Y.; Tu, M.; Wang, S.; Chen, S.; Zhou, W.; Chen, D.; Zhou, L.; Wang, M.; Zhao, Y.; Zeng, W.; et al. Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: A retrospective single center analysis. Travel Med. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Lian, J.; Jin, X.; Hao, S.; Cai, H.; Zhang, S.; Zheng, L.; Jia, H.; Hu, J.; Gao, J.; Zhang, Y.; et al. Analysis of Epidemiological and Clinical features in older patients with Corona Virus Disease 2019 (COVID-19) out of Wuhan. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Y.; Lin, R.; Han, K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J. Infect. 2020. [Google Scholar] [CrossRef]
- Mo, P.; Xing, Y.; Xiao, Y.; Deng, L.; Zhao, Q.; Wang, H.; Xiong, Y.; Cheng, Z.; Gao, S.; Liang, K.; et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Shi, H.; Han, X.; Jiang, N.; Cao, Y.; Alwalid, O.; Gu, J.; Fan, Y.; Zheng, C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect. Dis. 2020, 20, 425–434. [Google Scholar] [CrossRef]
- Wan, S.; Xiang, Y.; Fang, W.; Zheng, Y.; Li, B.; Hu, Y.; Lang, C.; Huang, D.; Sun, Q.; Xiong, Y.; et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B.; Li, Q.; Wen, L.; Zhang, R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Zhao, X.; Liu, C.; Wang, W.; Wang, D.; Xu, W.; Zhang, C.; Yu, J.; Jiang, B.; et al. Clinical Characteristics of Imported Cases of COVID-19 in Jiangsu Province: A Multicenter Descriptive Study. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Xu, T.; Chen, C.; Zhu, Z.; Cui, M.; Chen, C.; Dai, H.; Xue, Y. Clinical features and dynamics of viral load in imported and non-imported patients with COVID-19. Int. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Xu, X.W.; Wu, X.X.; Jiang, X.G.; Xu, K.J.; Ying, L.J.; Ma, C.L.; Li, S.B.; Wang, H.Y.; Zhang, S.; Gao, H.N.; et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: Retrospective case series. BMJ 2020, 368, m792. [Google Scholar] [CrossRef]
- Zhang, J.J.; Dong, X.; Cao, Y.Y.; Yuan, Y.D.; Yang, Y.B.; Yan, Y.Q.; Akdis, C.A.; Gao, Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Zhu, W.; Xie, K.; Lu, H.; Xu, L.; Zhou, S.; Fang, S. Initial clinical features of suspected coronavirus disease 2019 in two emergency departments outside of Hubei, China. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Chai, X.; Hu, L.; Zhang, Y.; Han, W.; Lu, Z.; Ke, A.; Zhou, J.; Shi, G.; Fang, N.; Fan, J.; et al. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. BioRxiv 2020. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, L.; Wang, F.S. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol. Hepatol. 2020, 5, 428–430. [Google Scholar] [CrossRef]
- Imig, J.D.; Ryan, M.J. Immune and inflammatory role in renal disease. Compr. Physiol. 2013, 3, 957–976. [Google Scholar] [CrossRef]
- Lely, A.T.; Hamming, I.; van Goor, H.; Navis, G.J. Renal ACE2 expression in human kidney disease. J. Pathol. 2004, 204, 587–593. [Google Scholar] [CrossRef]
- Fan, C.; Li, K.; Ding, Y.; Lu, W.L.; Wang, J. ACE2 Expression in Kidney and Testis May Cause Kidney and Testis Damage After 2019-nCoV Infection. MedRxiv 2020. [Google Scholar] [CrossRef]
| Authors | Country | Study Type | Sample Size | Female (%) | Mortality (%) | Follow-up Time (Days) | Liver Patients (%) | Liver Severity (%) | Liver Deaths (%) | Renal Patients (%) | Renal Severity (%) | Renal Death (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen J et al. [33] | China-Shanghai | Retrospective Analysis | 249 | 123/249 (49.40) | 2/249 (0.80) | 16 | 2/249 (0.80) | - | - | - | - | - |
| Chen L et al. [34] | China-Wuhan | Retrospective Analysis | 29 | 8/29 (27.59) | 26/29 (89.66) | 15 | 2/29 (6.90) | - | - | - | - | - |
| Chen N et al. [35] | China-Wuhan | Descriptive Analysis | 99 | 32/99 (32.32) | 11/99 (11.11) | 20 | - | - | - | 3/99 (3.03) | - | - |
| Chen T et al. [36] | China-Wuhan | Retrospective Analysis | 274 | 103/274 (37.59) | 113/274 (41.24) | 30 | 11/274 (4.02) | - | 5/11 (45.46) | 5/274 (1.83) | - | 4/5 (80.00) |
| Grasselli et al. [37] | Italy-Multicentre | Retrospective Analysis | 1591 | 287/1591 (18.04) | 405/1591 (25.46) | 34 | 28/1591 (1.76) | 28/28 (100.00) | - | 36/1591 (2.26) | 36/36 (100.00) | - |
| Guan W et al. [38] | China-Multicentre | Retrospective Analysis | 1099 | 459/1099 (41.77) | 15/1099 (1.37) | 49 | 23/1099 (2.09) | 1/23 (4.35) | 1/23 (4.35) | 8/1099 (0.73) | 3/8 (37.50) | 2/8 (25.00) |
| Huang C et al. [25] | China-Wuhan | Prospective Analysis | 41 | 11/41 (26.83) | - | 32 | 1/41 (2.44) | - | - | - | - | - |
| Huang Y et al. [39] | China-Wuhan | Retrospective Analysis | 34 | 20/34 (58.82) | - | 39 | 1/34 (2.94) | - | - | - | - | - |
| Lian J et al. [40] | China-Zhejiang | Retrospective Analysis | 788 | 381/788 (48.35) | - | 26 | 31/788 (3.93) | - | - | 7/788 (0.89) | - | - |
| Liu K et al. [41] | China-Hainan | Retrospective Analysis | 56 | 25/56 (44.64) | 3/56 (5.36) | 46 | 1/56 (1.79) | - | - | 1/56 (1.79) | - | - |
| Mo P et al. [42] | China-Wuhan | Retrospective Analysis | 155 | 69//155 (44.52) | 22/155 (14.19) | 36 | 7/155 (4.52) | 5/7 (71.43) | - | 6/155 (3.87) | 4/6 (66.67) | - |
| Shi H et al. [43] | China-Wuhan | Descriptive Analysis | 81 | 39/81 (48.15) | 3/81 (3.70) | 47 | 7/81 (8.64) | - | - | 3/81 (3.70) | - | - |
| Wan S et al. [44] | China-Chongqing | Retrospective Analysis | 135 | 63/135 (46.67) | 1/135 (0.74) | 16 | 2/135 (1.48) | 1/2 (50.00) | - | - | - | - |
| Wang D et al. [45] | China-Wuhan | Retrospective Analysis | 138 | 63/138 (45.65) | 6/138 (4.35) | 34 | 4/138 (2.90) | - | - | 4/138 (2.90) | 2/4 (50.00) | - |
| Wang Z et al. [46] | China-Wuhan | Retrospective Analysis | 69 | 37/69 (53.62) | 5/69 (7.25) | 19 | 1/69 (1.45) | - | - | - | - | - |
| Wu C et al. [47] | China-Wuhan | Retrospective Analysis | 201 | 73/201 (36.32) | 44/201 (21.89) | 63 | 7/201 (3.48) | - | - | 2/201 (1.00) | - | - |
| Wu J et al. [48] | China-Jiangsu | Retrospective Analysis | 80 | 41/80 (51.25) | - | 23 | 1/80 (1.25) | - | - | 1/80 (1.25) | - | - |
| Xu T et al. [49] | China-Changzhou | Retrospective Analysis | 51 | 26/51 (50.98) | - | 35 | 1/51 (1.96) | - | - | 1/51 (1.96) | - | - |
| Xu X et al. [50] | China-Zhejiang | Retrospective Analysis | 62 | 27/62 (43.55) | - | 16 | 7/62 (11.29) | 4/7 (57.14) | - | 1/62 (1.61) | - | - |
| Zhang J et al. [51] | China-Wuhan | Retrospective Analysis | 140 | 69/140 (49.29) | - | 34 | 8/140 (5.71) | 4/8 (50.00) | - | 2/140 (1.43) | 2/2 (100.00) | - |
| Zhou et al. [52] | China-Wuhan | Retrospective Analysis | 191 | 72/191 (37.70) | 54/191 (28.27) | 15 | - | - | - | 2/191 (1.05) | - | 2/2 (100.00) |
| Zhu W et al. [53] | China-Anhui | Retrospective Analysis | 32 | 17/32 (53.13) | - | 27 | 2/32 (6.3) | - | - | 1/32 (3.13) | - | - |
| Total | 5595 | 2045/5595 (36.55) | 710/4367 (16.26) | 672 | 147/5305 (2.77) | 43/75 (57.33) | 6/34 (17.65) | 83/5038 (1.65) | 47/56 (83.93) | 8/15 (53.33) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyelade, T.; Alqahtani, J.; Canciani, G. Prognosis of COVID-19 in Patients with Liver and Kidney Diseases: An Early Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2020, 5, 80. https://doi.org/10.3390/tropicalmed5020080
Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID-19 in Patients with Liver and Kidney Diseases: An Early Systematic Review and Meta-Analysis. Tropical Medicine and Infectious Disease. 2020; 5(2):80. https://doi.org/10.3390/tropicalmed5020080
Chicago/Turabian StyleOyelade, Tope, Jaber Alqahtani, and Gabriele Canciani. 2020. "Prognosis of COVID-19 in Patients with Liver and Kidney Diseases: An Early Systematic Review and Meta-Analysis" Tropical Medicine and Infectious Disease 5, no. 2: 80. https://doi.org/10.3390/tropicalmed5020080
APA StyleOyelade, T., Alqahtani, J., & Canciani, G. (2020). Prognosis of COVID-19 in Patients with Liver and Kidney Diseases: An Early Systematic Review and Meta-Analysis. Tropical Medicine and Infectious Disease, 5(2), 80. https://doi.org/10.3390/tropicalmed5020080






