1. Introduction
Highly pathogenic avian influenza (HPAI) H5N1 has caused a large number of outbreaks in poultry in Asia, Europe, and Africa [
1]. Chickens are susceptible to HPAI (H5N1), with high morbidity and a case fatality rate as high as 100% [
2]. Wild birds including shorebirds and gulls and domestic ducks are considered to be the natural reservoir of the virus. These animals may carry and shed HPAI viruses without showing any signs of illness [
3,
4,
5]. Thus they become silent carriers, sustaining and perpetuating H5N1, and transmitting the virus to other susceptible hosts [
3,
6].
A total of 68 countries reported HPAI outbreaks to the World Organization for Animal Health (OIE) between January 2003 and January 2018 [
7]. Globally, more than 15,000 outbreaks were reported in domestic birds from January 2005 to January 2018 [
7]. In 2003, HPAI (H5N1) reemerged in both poultry and humans in Southeast Asian countries [
8,
9]. Domestic poultry from East and Southeast Asian countries have been infected most frequently, and H5N1 became endemic in domestic poultry in these countries. Thus, it has caused severe economic losses. As of December 2006, more than 240 million poultry including chicken, ducks, turkeys, and geese died or had been culled to prevent the spread of H5N1 [
10].
Since 2003, 17 countries have reported a total of 861 human cases ofH5N1 to the World Health Organization (WHO), with most cases arising in Asian countries. The global case fatality rate among all these cases was >50% [
9]. Epidemiological studies have found that most human cases of H5N1 infection involved the subject having a history of poultry exposure such as slaughtering and/or consuming sick poultry and handling infected live and dead poultry [
11,
12,
13,
14].
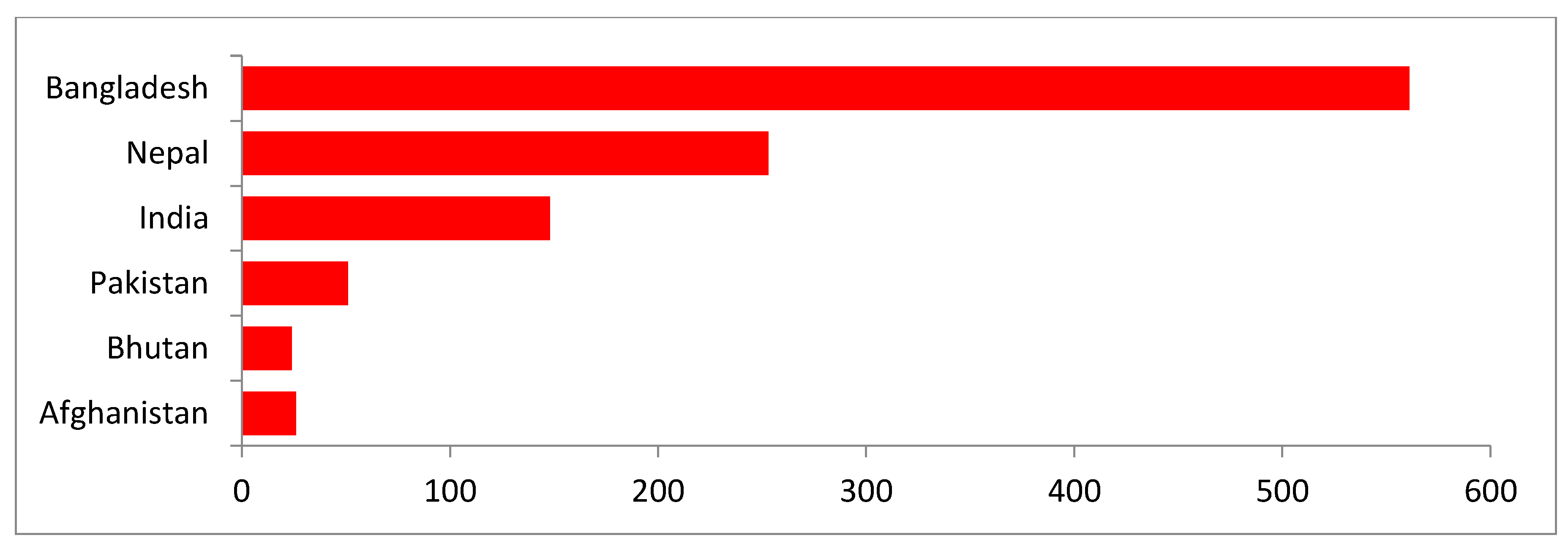
In South Asia, people live very close to their domestic poultry and have limited knowledge about the risk of avian influenza. Among the eight South Asian countries, six countries (Afghanistan, Bangladesh, Bhutan, India, Nepal, and Pakistan) reported HPAI (H5N1) outbreaks in poultry since 2006, and two countries (Bangladesh and Pakistan) reported human cases of H5N1 infection since 2007. The Maldives and Sri Lanka did not report any HPAI outbreaks in poultry or in humans [
1,
9]. Although there have been few human cases in South Asian countries, consistent circulation of H5N1 in poultry and seasonal influenza viruses in humans can promote genetic reassortment and evolution of novel virus strains with the potential for public health importance [
15]. Substantial knowledge about the spatial and temporal distribution of highly pathogenic avian influenza virus in South Asian countries is essential to develop a strategic plan for preventing and controlling future outbreaks of HPAI. In this study, we described the spatial and temporal distribution of HPAI outbreaks in South Asia from January 2006 to June 2019. The findings of this study will help to identify times, places, and hosts most associated with a high risk of H5N1 infection in South Asia.
2. Methods
2.1. Sources of HPAI Outbreak Data
We extracted data for all reported HPAI outbreaks in poultry between January 2006 and June 2019 from the OIE website. We considered the starting date of each outbreak as the onset month of the outbreak [
7]. We organized the extracted data by year and month to estimate the average peak month for HPAI infections in the South Asian countries (Afghanistan, Bangladesh, Bhutan, India, Nepal, Pakistan, Maldives, and Sri Lanka). Data about human cases of H5N1 were obtained from the WHO website [
9].
2.2. Epidemiological and Microbiological Data
We collected data on the epidemiology of H5N1 and on the phylogenetics of virus isolates from published articles. Data from different South Asian countries were compared to determine the similarities and dissimilarities. We performed an internet-based systematic search in Google Scholar and PubMed to collect data from published articles using the following keywords—“avian influenza”, “HPAI”, “H5N1”, “avian influenza in Afghanistan”, “avian influenza in Bangladesh”, “avian influenza in India”, “avian influenza in Pakistan”, “avian influenza in Bhutan”, “avian influenza in Nepal”, “avian influenza in Sri Lanka”, “avian influenza in Maldives”, “risk factors for avian influenza”, “avian influenza in poultry”, “avian influenza in humans”, and “H5N1 clades”.
2.3. Time Periods
In this study, we used H5N1 outbreak data between January 2006 and June 2019. We also collected molecular data on avian influenza viruses from published articles and abstracts. All articles and abstracts reviewed were published between 1st January 2006 and June 2019. We identified a total of 50 articles and abstracts for our analysis that were relevant to our research interest.
2.4. Statistical Analysis
We performed descriptive analyses to show the total numbers of outbreaks, geographic distributions, affected host species, husbandry practices of affected birds, and peak month of H5N1 outbreak detection. For each country, we determined the average month during which the highest number of H5N1 outbreaks were reported to the OIE. We estimated the relative risk (RR) with 95% confidence interval (CI) to identify the association between H5N1 outbreak occurrence and exposure variables using Poisson regression.
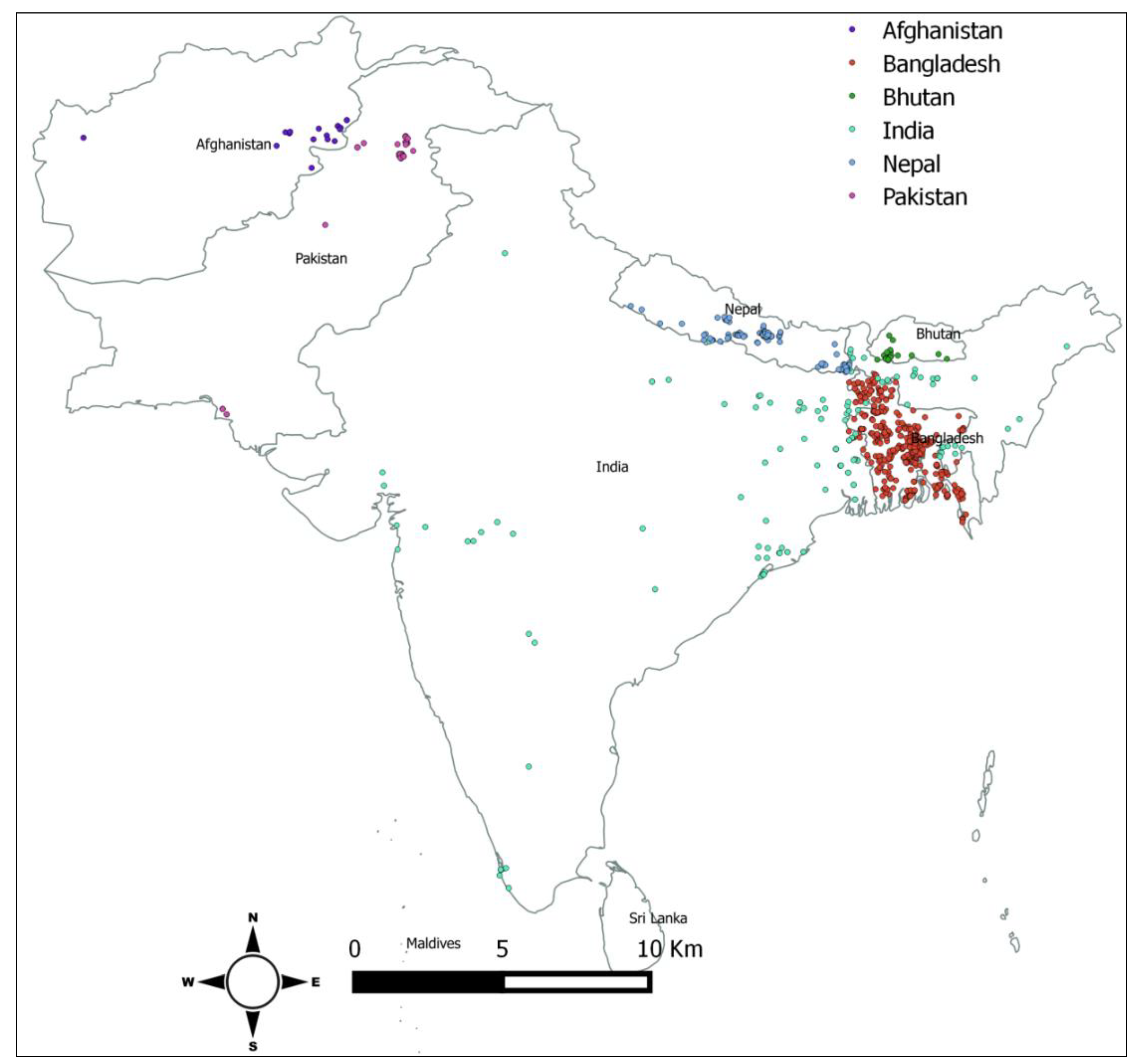
2.5. Mapping the H5N1 Outbreak in Poultry and in Humans
We used latitude and longitude data of reported H5N1 outbreaks in poultry and in humans in the countries of interest using Google Earth software. The latitude and longitude data for each reported H5N1 outbreak were collected from the World Organization for Animal Health (OIE) and the World Health Organization (WHO) websites.
2.6. Phylogenetic Analysis
A total of 46 H5N1 virus isolates were selected from Afghanistan, Bangladesh, Bhutan India, and Nepal for phylogenetic analysis. Nucleotide sequences of the HA (haemagglutinin) gene of these selected H5N1 isolates were downloaded from GenBank. The HA gene sequences of the selected isolates were subjected to ClustalW multiple sequence alignment using the BioEdit 7.2 program [
16]. A phylogenetic tree was constructed with the Kimura 2-parameter model and neighbor-joining methods (with 1000 bootstrap replications) using MEGA 7 [
17].
4. Discussion
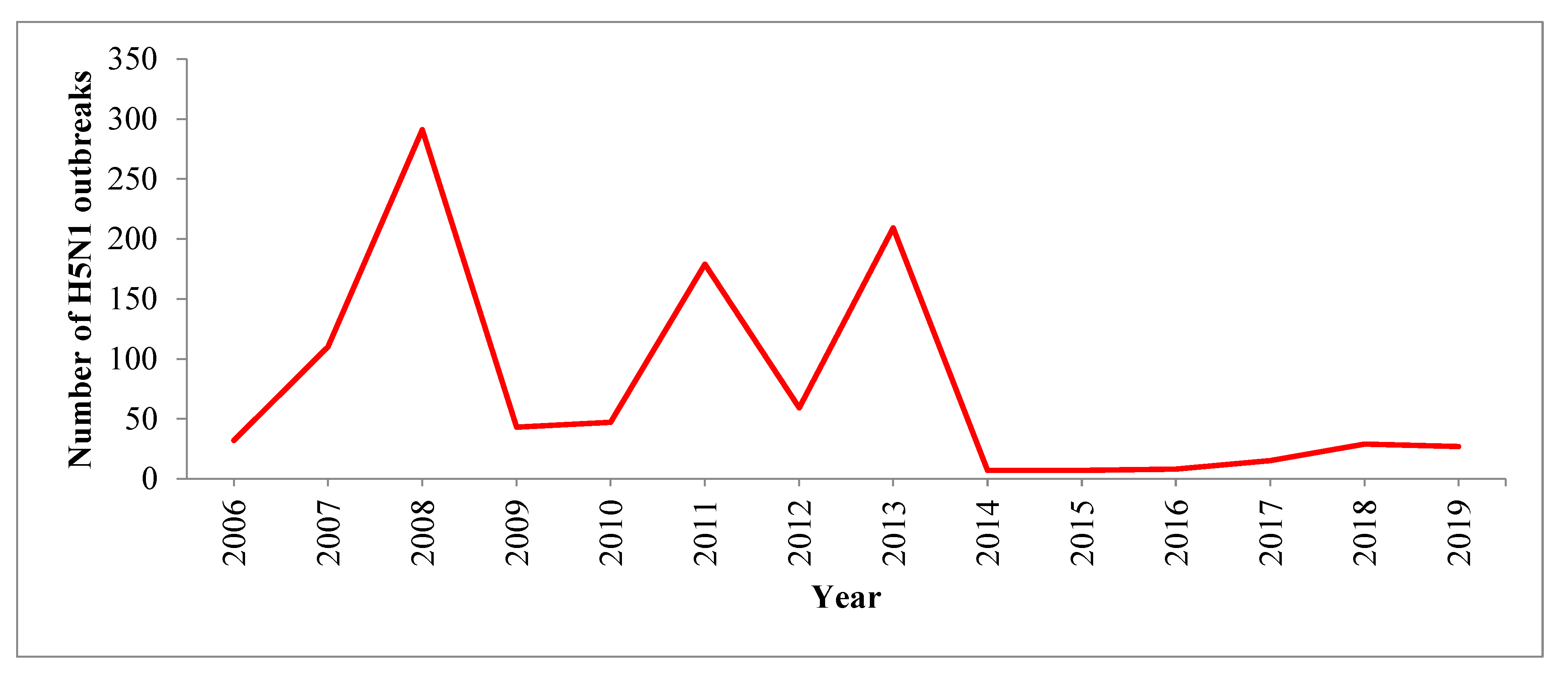
This study provides an update on the state of avian influenza in South Asia. H5N1 outbreaks in this region repeatedly occurred in poultry between January 2006 and June 2019. However, human cases of H5N1 infection were sporadic in nature. According to the OIE, a total of 7122 HPAI outbreaks were reported in domestic birds across 68 countries from January 2013 to August 2018. Asia, Africa and Europe were the regions most affected by these outbreaks [
7]. Between January 2006 and June 2019, South Asian countries reported more than one thousand H5N1 outbreaks to OIE. South Asia detected the first H5N1 outbreak in poultry in 2006, with the highest number of outbreaks in the region reported in 2008. Though few H5N1 outbreaks were reported after 2014, circulation of H5N1 viruses continues. Findings from surveillance and other studies suggest that both HPAI and LPAI viruses continue to circulate among birds in South Asian countries [
20,
21,
27,
30,
33,
35,
37,
42,
43].
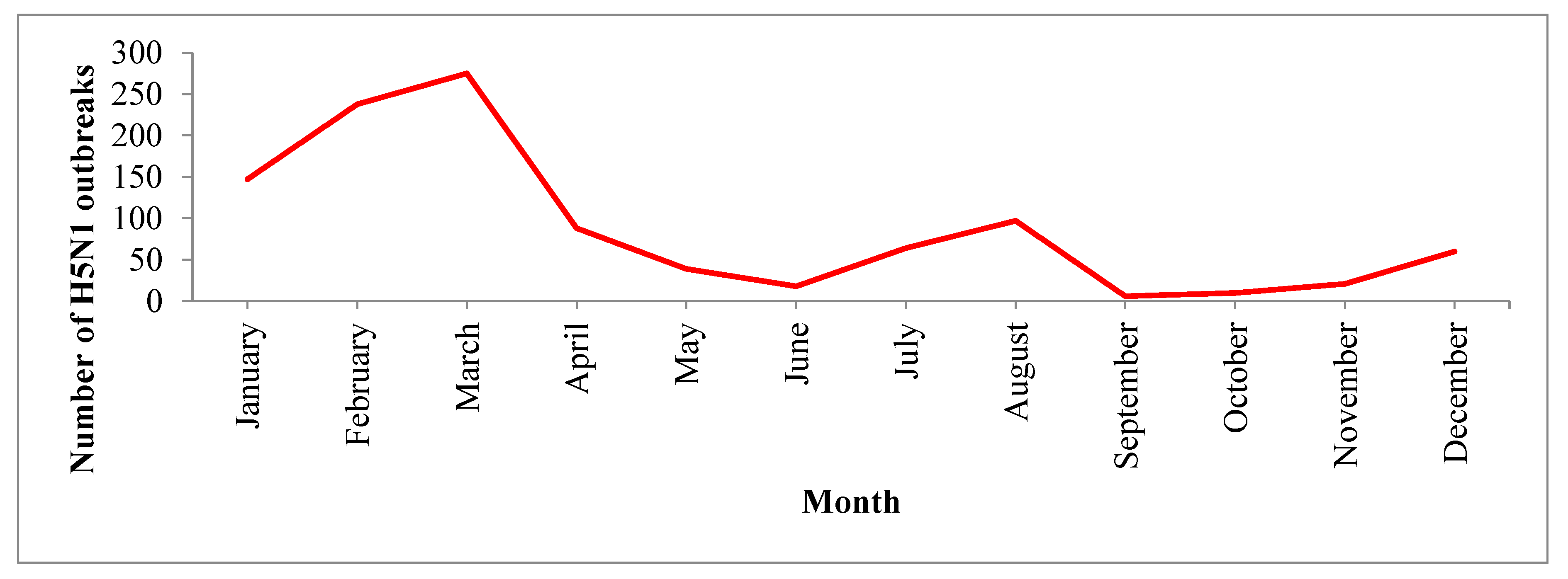
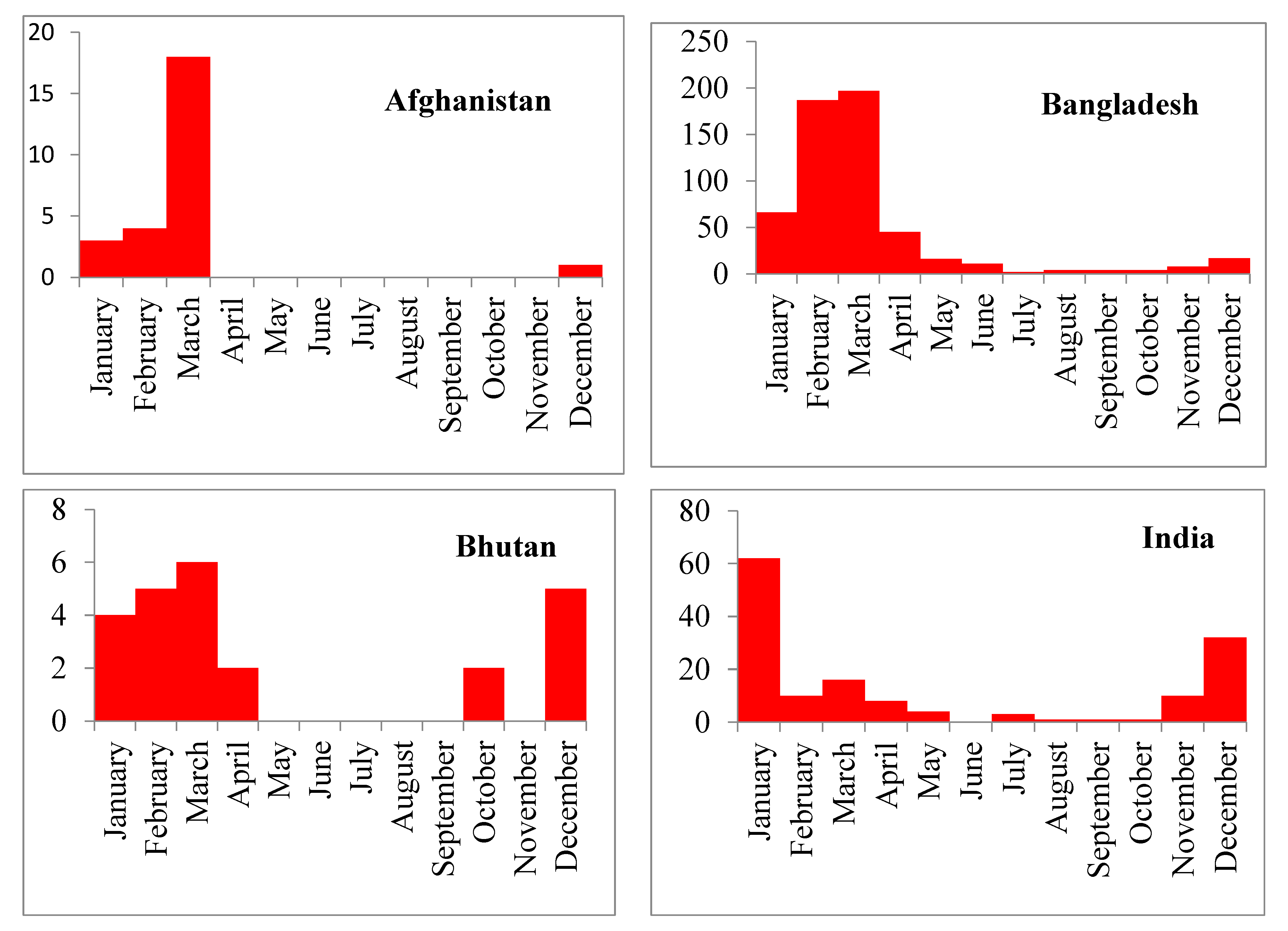
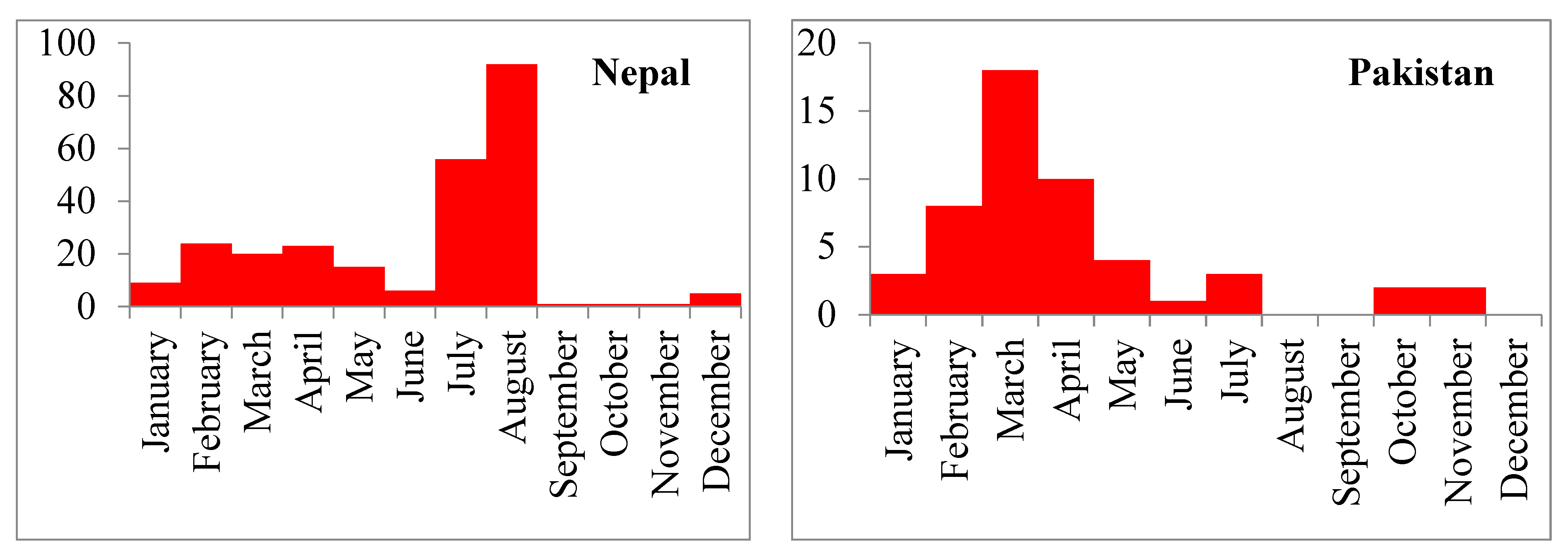
In this analysis, we found that HPAI (H5N1) outbreaks in the region occurred most frequently in the winter season (January to March), with the largest number of outbreaks occurring specifically in March. This finding is similar to the results from Southeast Asian countries where H5N1 outbreaks were frequently detected during winter in poultry [
44]. HPAI (H5N1) outbreaks globally showed a clear seasonal pattern in poultry, humans and wild birds; most human cases (50%) were reported during January to March [
15]. This seasonality could be due to the lower ambient temperature (average minimum temperature 13.9 °C). Lower temperatures are suitable for the survival of avian influenza viruses. Avian influenza viruses persist in cold water for a long time and this may be associated with a higher chance of transmission [
45]. One study suggested that lower ambient temperatures may cause decreased immunity in poultry and thus make the birds more susceptible to infection with the H5N1 virus [
15]. The circulation of avian influenza viruses is also related to the migration of wild birds, particularly during the winter season, as reported by some global studies [
18,
46,
47]
.This analysis noted that the high incidence of H5N1 outbreak in commercial poultry farms in Bangladesh, Nepal and Pakistan. India, Afghanistan and Bhutan reported more outbreaks in backyard poultry flock. This geographical diversity for H5N1 outbreaks within South Asia could be due to the variation in farm biosecurity system, proximity to the migratory bird flyway, wild bird-domestic poultry interaction, animal health infrastructure and strength of outbreak reporting system.
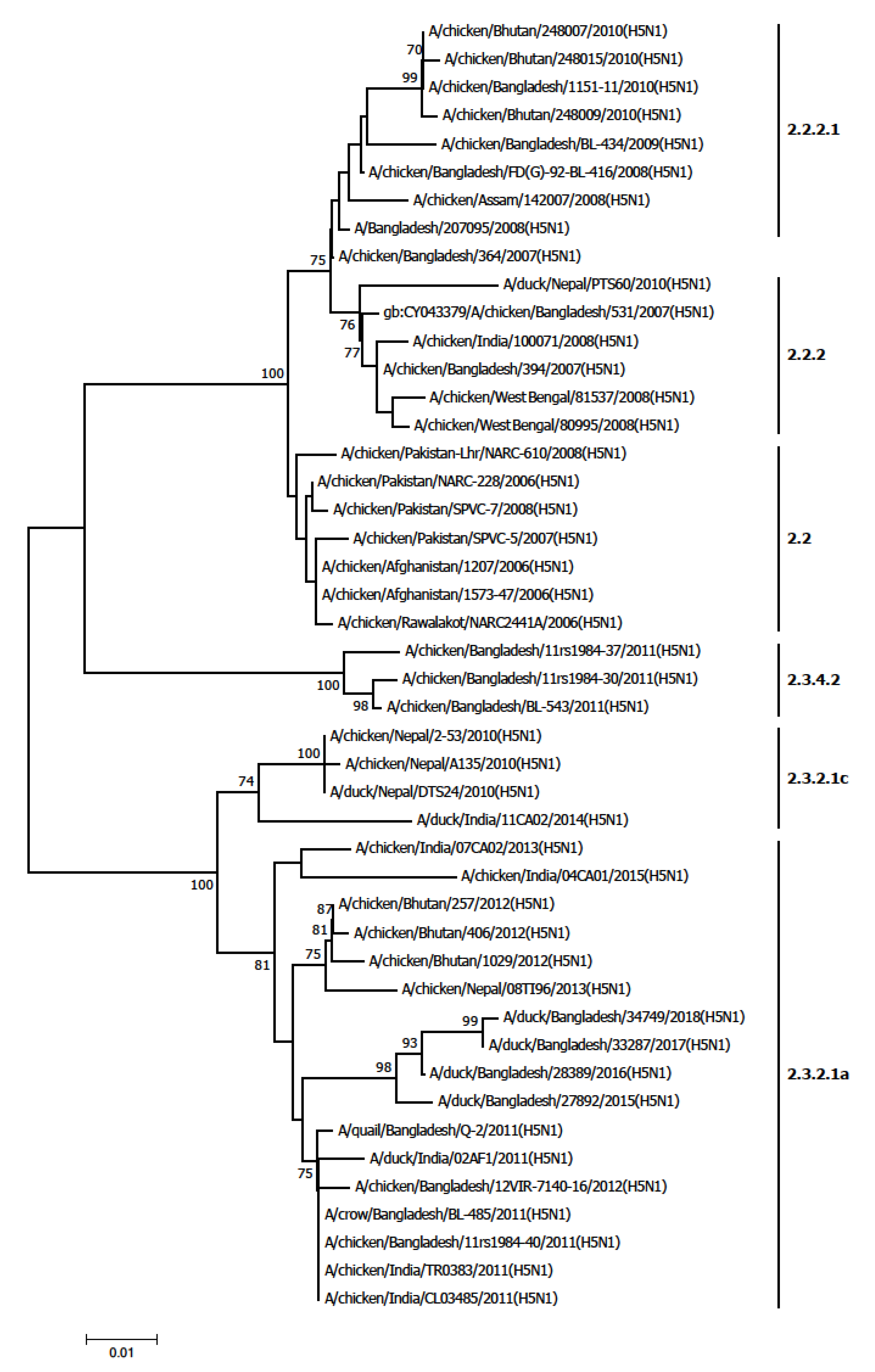
Avian influenza viruses continue to evolve and infect a wide range of poultry species and humans. In South Asia, multiple clades of the H5N1 virus were detected in poultry from 2006 to 2019. The most commonly detected clade in this region was 2.2. Clade 2.3.2.1a was also common and was identified in India, Bangladesh, Nepal and Bhutan [
18,
19,
20]. Clade 2.3.2.1a was detected in India, Bangladesh, Nepal and Bhutan which shared common ancestry [
23]. India identified clade 2.2 during outbreaks in 2006–2007, clade 2.2.2.1 in 2008–2010, clade 2.3.2.1a in 2011-2015 and clade 2.3.2.1c during in 2014–2015 [
21,
22,
23]. Bangladesh identified clade 2.2 during outbreaks in 2007–2010, clade 2.3.4 in 2011, clade 2.3.2 in 2011, clade 2.3.2.1 in 2011 and clade 2.3.2.1a in 2014 [
18,
24]. The co-detection of genetically related avian influenza viruses in human and poultry during the same period suggested that viruses were transmitted from poultry to human in Bangladesh Pakistan and Nepal.
Multiple subtypes of avian influenza viruses were identified in South Asian countries between January 2006 and June 2019, including: H5N1, H5N6, H5N2, H5N3, H9N2, H1N1, H1N2, H1N3, H1N4, H2N3, H2N4, H3N1, H3N2, H3N5, H3N6, H3N8, H4N1, H4N2, H4N6, H5N8, H6N1, H6N2, H7N3, H6N7, H7N9, H10N7, H11N1, H11N2, H11N6, H11N9 and H11N3 [
23,
29,
34,
35,
36,
38,
39,
40,
41,
42,
43]. Avian influenza strain diversity may increase the probability of genetic reassortment between influenza subtypes which may facilitate the evolution of a future novel pathogenic strain of animal and public health importance. Similar viral diversity was also observed in other Asian countries [
48,
49].
Human cases of H5N1 were few despite high numbers of avian influenza outbreaks among birds in South Asia. This finding was unusual compared to other avian influenza affected countries like China, Egypt, Indonesia, Thailand and Vietnam, which reported a large number of human cases to the WHO during the period of interest [
9]. The mortality rate for human H5N1 infections in South Asia (25%) was lower than that observed globally (>50%) [
9]. The majority of human cases in the region had a history of poultry exposure [
50,
51]. Though human cases of HPAI were rare, continuous circulation of the H5N1 virus in the poultry population continues to provide potential opportunities for transmission to humans.