An Observational Case-Control Study to Determine Human Immunodeficiency Virus and Host Factor Influence on Biomarker Distribution and Serodiagnostic Potential in Adult Pulmonary Tuberculosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Enzyme-Linked Immunoassay (ELISA)
2.3. Statistical Analysis
3. Results
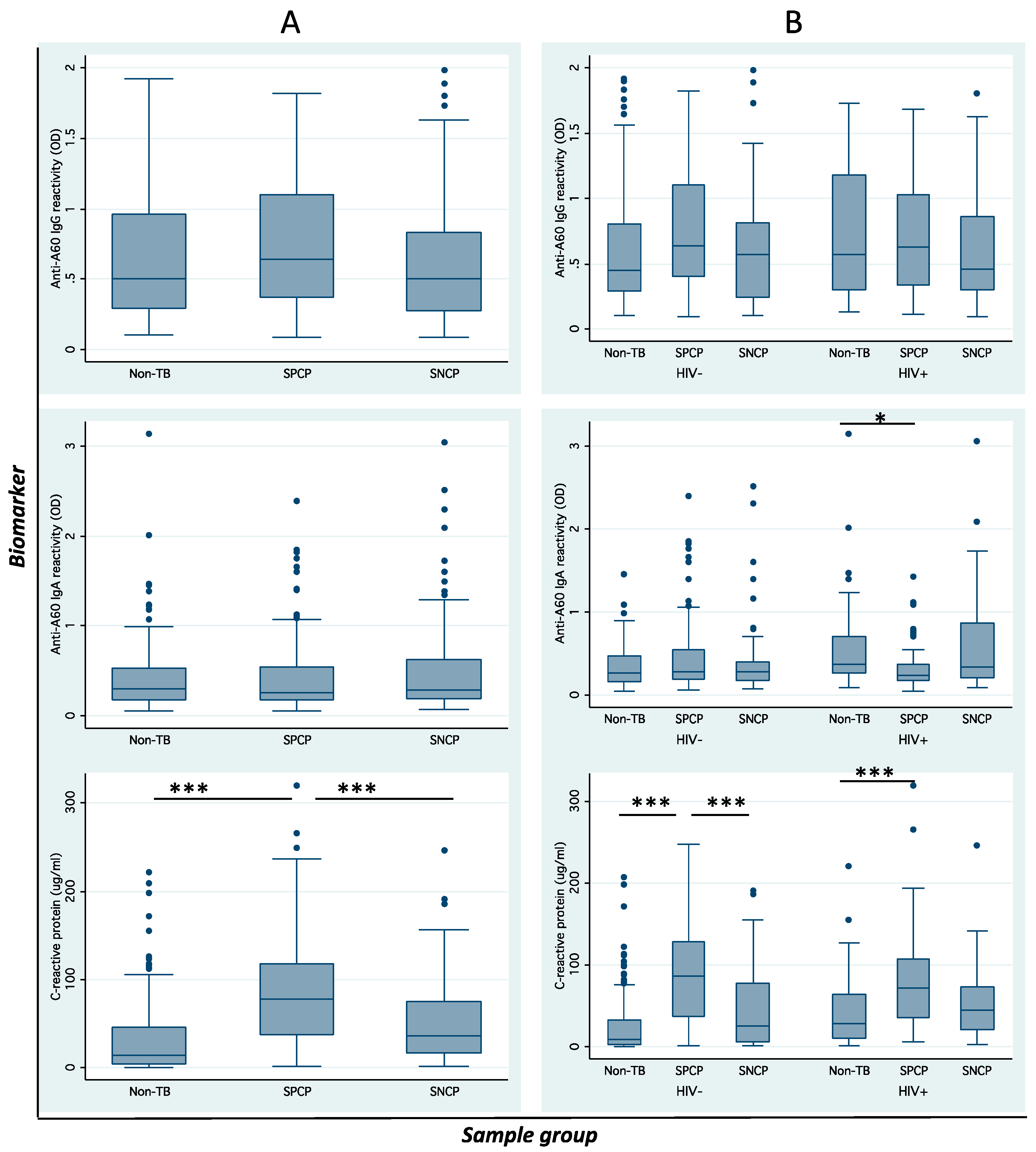
3.1. Host Factors with Diagnostic Status and Biomarker Distribution
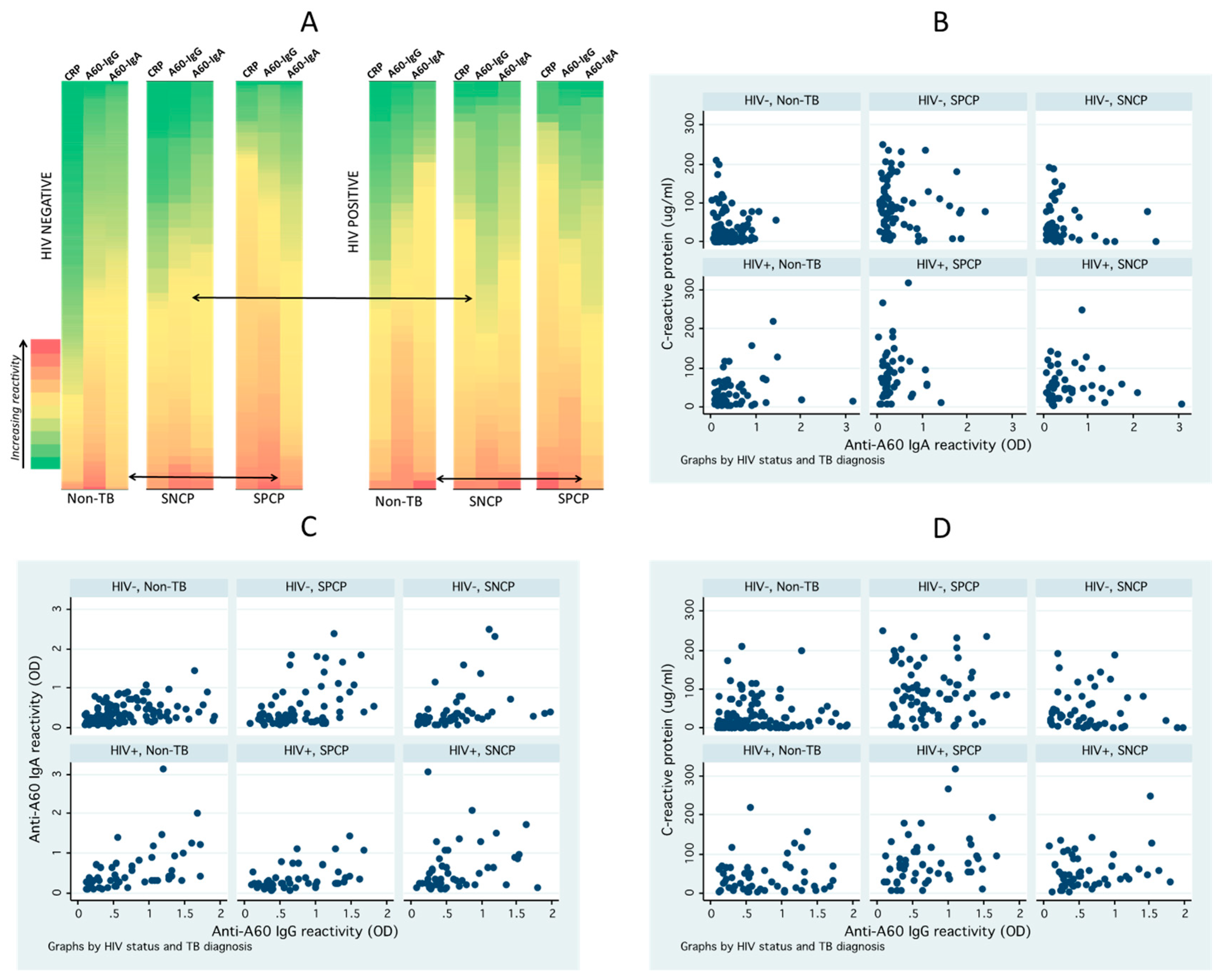
3.2. Distribution and Correlation of Seroimmunological Biomarkers
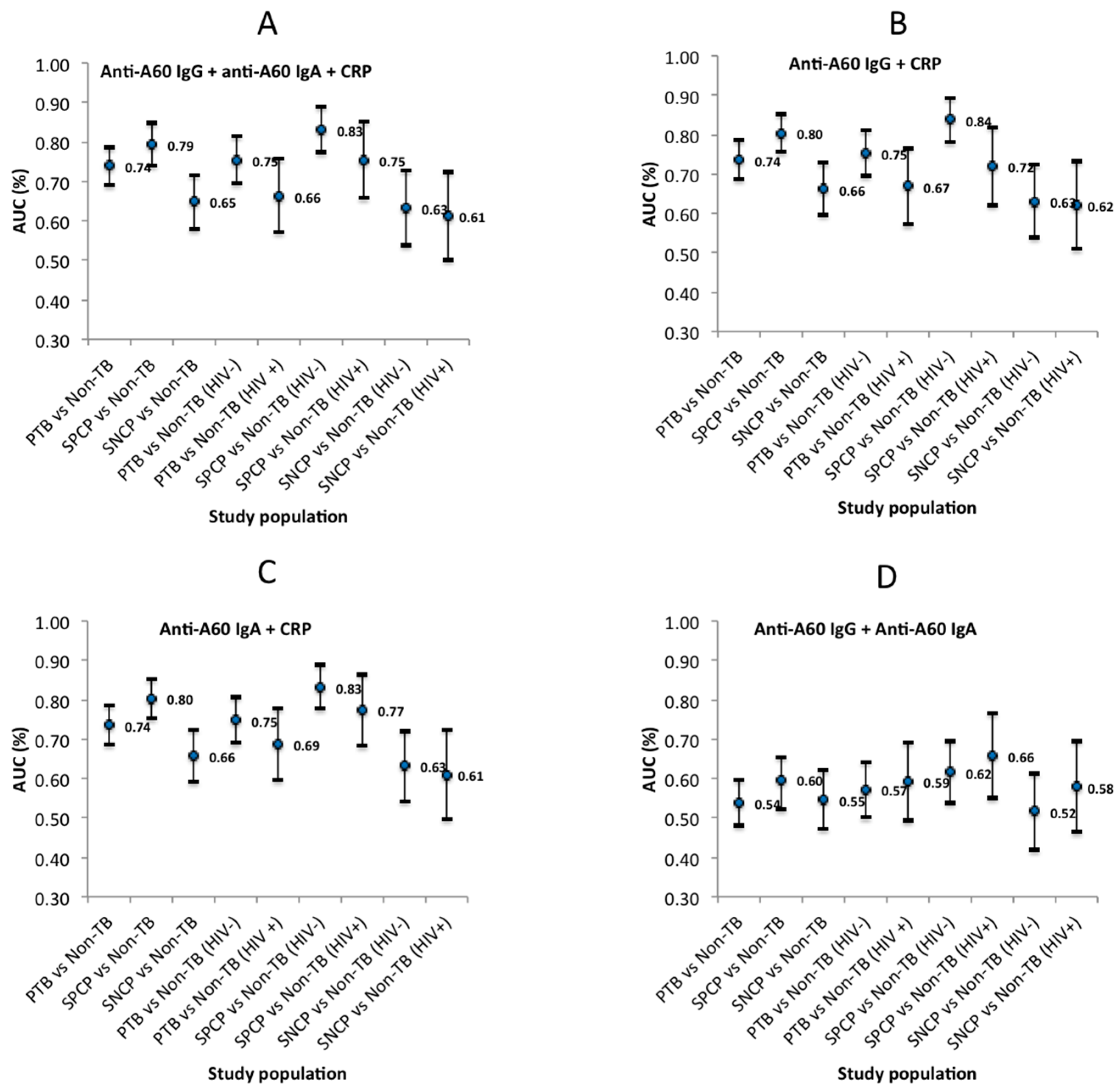
3.3. Biomarker Diagnostic Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A60 | Antigen 60 |
| AUC | Area-under receiver operating characteristic curves |
| CRP | C-reactive protein |
| CFU | Colony forming unit |
| CI | Confidence interval |
| ELISA | Enzyme-linked immunosorbent assays |
| HIV | Human immunodeficiency |
| Ig | Immunoglobulin |
| OR | Odds ratio |
| OD | Optical density |
| POC | Point-of-care |
| SPCP/SNCP | Smear positive/negative culture positive |
| Sp | Specificity |
| SD | Standard deviation |
| TB | Tuberculosis |
| ULN | Upper limit of normal |
References
- Global Tuberculosis Report 2017; World Health Organization: Geneva, Switzerland, 2017; p. 262.
- Mahtab, S.; Coetzee, D. Influence of HIV and other risk factors on tuberculosis. S. Afr. Med. J. 2017, 107, 428–434. [Google Scholar] [CrossRef] [PubMed]
- The Stop TB Strategy: Building on and Enhancing DOTS to Meet the TB-Related Millenium Development Goals; Stop TB Partnership, World Health Organization: Geneva, Switzerland, 2006.
- Pai, M. Innovations in Tuberculosis Diagnostics: Progress and Translational Challenges. EBioMedicine 2015, 2, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Syed, J. The Tuberculosis Diagnostic Pipeline; Treatment Action Group: New York, NY, USA, 2011. [Google Scholar]
- MacPherson, P.; Dimairo, M.; Bandason, T.; Zezai, A.; Munyati, S.S.; Butterworth, A.E.; Mungofa, S.; Rusakaniko, S.; Fielding, K.; Mason, P.R.; et al. Risk factors for mortality in smear-negative tuberculosis suspects: A cohort study in Harare, Zimbabwe. Int. J. Tuberc. Lung Dis. 2011, 15, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- LaCourse, S.M.; Cranmer, L.M.; Matemo, D.; Kinuthia, J.; Richardson, B.A.; John-Stewart, G.; Horne, D.J. Tuberculosis case finding in HIV-infected pregnant women in Kenya reveals poor performance of symptom screening and rapid diagnostic tests. J. Acquir. Immune Defic. Syndr. 2016, 71, 219. [Google Scholar] [CrossRef] [PubMed]
- Kik, S.V.; Denkinger, C.M.; Casenghi, M.; Vadnais, C.; Pai, M. Tuberculosis diagnostics: Which target product profiles should be prioritised? Eur. Respir. J. 2014, 44, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Nicol, M.P.; Gnanashanmugam, D.; Browning, R.; Click, E.S.; Cuevas, L.E.; Detjen, A.; Graham, S.M.; Levin, M.; Makhene, M.; Nahid, P.; et al. A Blueprint to Address Research Gaps in the Development of Biomarkers for Pediatric Tuberculosis. Clin. Infect. Dis. 2015, 61, S164–S172. [Google Scholar] [CrossRef] [PubMed]
- Mohd Hanafiah, K.; Garcia, M.; Anderson, D. Point-of-care testing and the control of infectious diseases. Biomark. Med. 2013, 7, 333–347. [Google Scholar] [CrossRef]
- Pai, M.; Nicol, M.P.; Boehme, C.C. Tuberculosis Diagnostics: State of the Art and Future Directions. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Rrimers 2016, 2, 16076. [Google Scholar] [CrossRef]
- Ben-selma, W.; Harizi, H.; Marzouk, M.; Kahla, I.B.; Lazreg, F.B.; Ferjeni, A.; Boukadida, J. Evaluation of the diagnostic value of measuring IgG, IgM, and IgA antibodies to mycobacterial A60 antigen in active tuberculosis. Diagn. Microbiol. Infect. Dis. 2010, 68, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Maes, R. Comparison of A60 and three glycolipid antigens in an ELISA test for tuberculosis. Clin. Microbiol. Infect. 1997, 3, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Steingart, K.R.; Henry, M.; Laal, S.; Hopewell, P.C.; Ramsay, A.; Menzies, D.; Cunningham, J.; Weldingh, K.; Pai, M. Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: A systematic review. PLoS Med. 2007, 4, 1041–1060. [Google Scholar] [CrossRef]
- Drain, P.K.; Mayeza, L.; Bartman, P.; Hurtado, R.; Moodley, P.; Varghese, S.; Maartens, G.; Alvarez, G.G.; Wilson, D. Diagnostic accuracy and clinical role of rapid C-reactive protein testing in HIV-infected individuals with presumed tuberculosis in South Africa. Int. J. Tuberc. Lung Dis. 2014, 18, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Badri, M.; Maartens, G. Performance of serum C-reactive protein as a screening test for smear-negative tuberculosis in an ambulatory high HIV prevalence population. PLoS ONE 2011, 6, e15248. [Google Scholar] [CrossRef]
- Bothamley, G.H.; Beck, J.S.; Schreuder, G.M.T.; D’Amaro, J.; de Vries, R.R.; Kardjito, T.; Ivanyi, J. Association of tuberculosis and M. tuberculosis-specific antibody levels with HLA. J. Infect. Dis. 1989, 159, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Kunnath-Velayudhan, S.; Davidow, A.L.; Wang, H.Y.; Molina, D.M.; Huynh, V.T.; Salamon, H.; Pine, R.; Michel, G.; Perkins, M.D.; Xiaowu, L.; et al. Proteome-scale antibody responses and outcome of Mycobacterium tuberculosis infection in nonhuman primates and in tuberculosis patients. J. Infect. Dis. 2012, 206, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Ivanyi, J.; Bothamley, G.H.; Jackett, P.S. Immunodiagnostic assays for tuberculosis and leprosy. Br. Med. Bull. 1988, 44, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Yang, X.; Xiu, B.; Qie, S.; Dai, Z.; Chen, K.; Zhao, P.; Zhang, L.; Nicholson, R.A.; Wang, G.; et al. IgG, IgM and IgA antibodies against the novel polyprotein in active tuberculosis. BMC Infect. Dis. 2014, 14, 336. [Google Scholar] [CrossRef] [PubMed]
- Baumann, R.; Kaempfer, S.; Chegou, N.N.; Oehlmann, W.; Loxton, A.G.; Kaufmann, S.H.; van Helden, P.D.; Black, G.F.; Singh, M.; Walzl, G. Serologic diagnosis of tuberculosis by combining Ig classes against selected mycobacterial targets. J. Infect. 2014, 69, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Lyashchenko, K.; Colangeli, R.; Houde, M.; Al Jahdali, H.; Menzies, D.; Gennaro, M.L. Heterogeneous antibody responses in tuberculosis. Infect. Immun. 1998, 66, 3936–3940. [Google Scholar] [PubMed]
- Mohd, H.; Liu, J.J.; Lieschke, K.; Barnes, N.C.; Garcia, M.L.; Anderson, D.A. Serological biomarker screening and host factor analysis elucidating immune response heterogeneity in active pulmonary tuberculosis. Trop. Biomed. 2017, 34, 556–569. [Google Scholar]
- FIND’s Standard Specimen Banking 2015. Available online: http://www.finddx.org/specimen-banks/ (accessed on 25 May 2018).
- Nathanson, C.M.; Cuevas, L.E.; Cunningham, J.; Perkins, M.D.; Peeling, R.W.; Guillerm, M.; Moussy, F.; Ramsay, A. The TDR Tuberculosis Specimen Bank: A resource for diagnostic test developers. Int. J. Tuberc. Lung Dis. 2010, 14, 1461–1467. [Google Scholar] [PubMed]
- Hajian-Tilaki, K. Sample size estimation in diagnostic test studies of biomedical informatics. J. Biomed. Inform. 2014, 48, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Pouthier, F.; Perriëns, J.H.; Mukadi, Y.; Kayembe, T.; St, M.L.; Brown, C.; Prignot, J. Anti-A60 immunoglobulin G in the serodiagnosis of tuberculosis in HIV-seropositive and seronegative patients. AIDS 1994, 8, 1277–1280. [Google Scholar] [CrossRef] [PubMed]
- Steingart, K.R.; Dendukuri, N.; Henry, M.; Schiller, I.; Nahid, P.; Hopewell, P.C.; Ramsay, A.; Pai, M.; Laal, S. Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: A meta-analysis. Clin. Vaccine Immunol. 2009, 16, 260–276. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, P.A.; Black, K.P.; Shen, L.; Jackson, S. High prevalence of serum IgA HIV-1 infection-enhancing antibodies in HIV-infected persons. Masking by IgG. J. Immunol. 1995, 154, 6163–6173. [Google Scholar]
- Kunnath-Velayudhan, S.; Salamon, H.; Wang, H.Y.; Davidow, A.L.; Molina, D.M.; Huynh, V.T.; Cirillo, D.M.; Michel, G.; Talbot, E.A.; Perkins, M.D.; et al. Dynamic antibody responses to the Mycobacterium tuberculosis proteome. Proc. Natl. Acad. Sci. USA 2010, 107, 14703–14708. [Google Scholar] [CrossRef]
- De Groote, M.A.; Sterling, D.G.; Hraha, T.; Russell, T.M.; Green, L.S.; Wall, K.; Kraemer, S.; Ostroff, R.; Janjic, N.; Ochsner, U.A. Discovery and Validation of a Six-Marker Serum Protein Signature for the Diagnosis of Active Pulmonary Tuberculosis. J. Clin. Microbiol. 2017, 55, 3057–3071. [Google Scholar] [CrossRef] [PubMed]
- Achkar, J.M.; Cortes, L.; Croteau, P.; Yanofsky, C.; Mentinova, M.; Rajotte, I.; Schirm, M.; Zhou, Y.; Junqueira-Kipnis, A.P.; Kasprowicz, V.O.; et al. Host Protein Biomarkers Identify Active Tuberculosis in HIV Uninfected and Co-infected Individuals. EBioMedicine 2015, 2, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Den Boon, S.; Verver, S.; Lombard, C.J.; Bateman, E.D.; Irusen, E.M.; Enarson, D.A.; Borgdorff, M.W.; Beyers, N. Comparison of symptoms and treatment outcomes between actively and passively detected tuberculosis cases: The additional value of active case finding. Epidemiol. Infect. 2008, 136, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.C.; Rocha, M.V.; Willers, D.M.; Silva, D.R. Characteristics of Patients with Smear-Negative Pulmonary Tuberculosis (TB) in a Region with High TB and HIV Prevalence. PLoS ONE 2016, 11, e0147933. [Google Scholar] [CrossRef] [PubMed]
- Paton, N.I.; Ng, Y.M. Body composition studies in patients with wasting associated with tuberculosis. Nutrition 2006, 22, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.; Lambert, M.L.; Walley, J. Clinical diagnosis of smear-negative pulmonary tuberculosis in low-income countries: The current evidence. Lancet Infect. Dis. 2003, 3, 288–296. [Google Scholar] [CrossRef]
- Mendez-Samperio, P. Diagnosis of tuberculosis in HIV co-infected individuals: Current status, challenges, and opportunities for the future. Scand. J. Immunol. 2017, 86. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Clark, K.; Smith, C.; Hopwood, J.; Lynard, O.; Toolan, M.; Creer, D.; Barker, J.; Breen, R.; Brown, T.; Cropley, I. Variation in C—Reactive protein response according to host and mycobacterial characteristics in active tuberculosis. BMC Infect. Dis. 2016, 16, 265. [Google Scholar] [CrossRef] [PubMed]
- French, M.A. Lymphadenopathy and selective IgA deficiency. Br. Med. J. 1984, 289, 646–647. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Z.; Zhang, X.; Suzuki, Y.; Chagan-Yasutan, H.; Chen, H.; Wan, Y.; Xu, J.; Ashino, Y.; Hattori, T. Evaluation of Anti-TBGL Antibody in the Diagnosis of Tuberculosis Patients in China. J. Immunol. Res. 2015, 2015, 834749. [Google Scholar] [CrossRef] [PubMed]
- Fiske, C.T.; Hamilton, C.D.; Stout, J.E. Alcohol use and clinical manifestations of tuberculosis. J. Infect. 2009, 58, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Barr, T.; Girke, T.; Sureshchandra, S.; Nguyen, C.; Grant, K.; Messaoudi, I. Alcohol Consumption Modulates Host Defense in Rhesus Macaques by Altering Gene Expression in Circulating Leukocytes. J. Immunol. 2016, 196, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Lenaerts, A.; Barry, C.E., 3rd; Dartois, V. Heterogeneity in tuberculosis pathology, microenvironments and therapeutic responses. Immunol. Rev. 2015, 264, 288–307. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Rehn, A.; Rahman, J.; Andersson, J.; Svensson, M.; Brighenti, S. Pulmonary tuberculosis patients with a vitamin D deficiency demonstrate low local expression of the antimicrobial peptide LL-37 but enhanced FoxP3+ regulatory T cells and IgG-secreting cells. Clin. Immunol. 2015, 156, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Schnappinger, D.; Ehrt, S. A broader spectrum of tuberculosis. Nat. Med. 2016, 22, 1076–1077. [Google Scholar] [CrossRef] [PubMed]
| Characteristic Frequency (%) | Non-TB (n = 182) | SPCP (n = 112) | SNCP (n = 100) | p Value |
|---|---|---|---|---|
| Age group | 0.016 | |||
| 18–24 years | 22 (14) | 35 (31) | 19 (19) | |
| 25–35 years | 43 (28) | 25 (22) | 24 (24) | |
| 36–45 years | 39 (25) | 29 (26) | 32 (32) | |
| 46 years and older | 52 (33) | 23 (21) | 25 (25) | |
| Age in years: Mean (SD) | 40 (15) | 34 (11) | 37 (37) | 0.005 |
| Male | 79 (51) | 87 (74) | 68 (68) | <0.001 * |
| Cigarette Smoker | 42 (27) | 61 (54) | 35 (35) | <0.001 * |
| Country ** | ||||
| Viet Nam | 72 (40) | 71 (58) | 40 (40) | |
| South Africa | 110 (60) | 51 (42) | 60 (60) | |
| Alcohol consumption | 0.008 | |||
| No | 88 (72) | 50 (47) | 51 (61) | |
| Less | 25 (20) | 41 (38) | 19 (23) | |
| Weekly | 6 (5) | 10 (9) | 12 (14) | |
| Daily | 3 (2) | 6 (6) | 2 (2) | |
| Frequent to intoxication | 2 (2) | 2 (2) | 1 (1) | |
| HIV positive ** | 50 (27) | 49 (40) | 50 (50) | |
| Clinical symptoms and Medical History | ||||
| Cough duration | <0.001 * | |||
| 1–4 weeks | 97 (78) | 52 (48) | 55 (71) | |
| 5–10 weeks | 17 (14) | 33 (30) | 15 (19) | |
| 11 weeks or longer | 10 (8) | 24 (22) | 7 (9) | |
| Cough duration (weeks): Mean (SD) | 7 (28) | 10 (18) | 5 (5) | <0.001 * |
| Expectoration | 93 (98) | 66 (97) | 51 (94) | 0.512 |
| Haemoptysis | 19 (12) | 16 (14) | 15 (15) | 0.773 |
| Dsypnea | 62 (40) | 44 (39) | 53 (53) | 0.068 |
| Night sweats | 88 (56) | 70 (63) | 68 (69) | 0.141 |
| Weight loss | 95 (61) | 78 (70) | 70 (71) | 0.178 |
| Fever above 38 °C | 76 (49) | 74 (66) | 67 (68) | 0.002 * |
| Chest pain | 79 (51) | 65 (58) | 66 (67) | 0.041 |
| Malaise | 95 (61) | 73 (65) | 70 (71) | 0.277 |
| Wasting | 12 (8) | 21 (19) | 28 (28) | <0.001 * |
| Enlarged lymph nodes | 1 (1) | 5 (5) | 5 (6) | 0.107 |
| Abnormal chest x-ray | 96 (64) | 116 (98) | 83 (92) | <0.001 * |
| Chest x-ray interpretation | 0.011 | |||
| Infiltrate or consolidated | 70 (76) | 74 (65) | 52 (63) | |
| Pleural effusion | 1 (1) | 2 (2) | 4 (5) | |
| Cavitary lesion | 2 (2) | 20 (18) | 9 (11) | |
| Tuberculoma | 0 (0) | 0 (0) | 1 (1) | |
| Mediastinal/hilar lymphoadenopathy | 5 (5) | 3 (3) | 5 (6) | |
| Micronodules (Miliar) | 0 (0) | 5 (4) | 3 (4) | |
| Other | 14 (15) | 9 (8) | 8 (10) | |
| Chest x-ray conclusion | <0.001 * | |||
| TB likely | 15 (16) | 114 (98) | 64 (77) | |
| Pneumonia or atypical TB | 35 (36) | 1 (1) | 16 (19) | |
| Pneumonia (TB unlikely) | 27 (28) | 0 (0) | 0 (0) | |
| Other | 19 (20) | 1 (1) | 3 (4) | |
| Contact with active TB case | 27 (17) | 21 (19) | 27 (27) | 0.142 |
| BCG vaccinated | 81 (63) | 63 (67) | 62 (67) | 0.757 |
| Previous TB | 36 (23) | 13 (11) | 21 (22) | 0.029 |
| General appearance | 0.004 | |||
| Not ill | 41 (26) | 13 (12) | 13 (13) | |
| Mildly ill | 68 (44) | 47 (42) | 51 (51) | |
| Moderately/gravely ill | 47 (30) | 52 (46) | 36 (36) |
| Study Population | Biomarker (n) | Model Predictors | F Statistic | Significant Predictors | β Coefficient | LL | UL | p Value |
|---|---|---|---|---|---|---|---|---|
| Total | CRP (217) | Expectoration, chestpain, wasting, smear count, culture count, TB status | F(13, 203) = 9.45 | Expectoration | 3.39 | 1.23 | 9.39 | 0.019 |
| Chest pain | 1.55 | 1.07 | 2.23 | 0.019 | ||||
| Wasting | 3.42 | 1.90 | 6.23 | <0.001 | ||||
| Culture count | 2.92 | 1.31 | 6.49 | 0.009 | ||||
| anti-A60 IgG (367) | Night sweats, smear count, TB status | F(9, 158) = 2.31 | Smear count | 0.46 | 0.06 | 0.86 | 0.023 | |
| anti-A60 IgA (168) | Expectoration, Enlarged lymph node, HIV status, smear count, TB status | F(9, 158) = 2.31 | Expectoration | −1.12 | −1.77 | −0.49 | 0.001 | |
| Enlarged lymph node | −0.68 | −1.2 | −0.16 | 0.011 | ||||
| Non-TB | CRP (123) | Enlarged lymphnode, wasting, HIV status | F(3, 119) = 5.87 | Wasting | 3.82 | 1.30 | 11.25 | 0.015 |
| HIV | 1.86 | 1.06 | 3.25 | 0.03 | ||||
| Anti-A60 IgG (124) | Malaise, Alcohol consumption | Alcohol consumption | 0.9 | 0.32 | 1.49 | 0.003 | ||
| SPCP | CRP (92) | Weight loss, BCG, HIV status, Alcohol consumption | F(7,84) = 2.95 | Weight loss | 1.84 | 1.17 | 2.89 | 0.009 |
| BCG | 1.62 | 1.11 | 2.34 | 0.013 | ||||
| HIV | 0.63 | 0.44 | 0.90 | 0.014 | ||||
| Anti-A60 IgG (109) | Weight loss, Abnormal chest x-ray, HIV status, Alcohol consumption | F(5, 118) = 3.00 | Weight loss | 0.28 | 0.09 | 0.48 | 0.004 | |
| Abnormal chest x-ray | −0.68 | −1.29 | −0.07 | 0.03 | ||||
| SNCP | CRP (85) | Fever, Alcohol consumption, Culture count | F(9, 75) = 2.92 | Fever | 2.89 | 1.62 | 5.21 | 0.001 |
| Alcohol consumption | 10.18 | 1.01 | 102.51 | 0.049 | ||||
| Anti-A60 IgA (71) | Sex, malaise, TB history, BCG, Smoking, Abnormal chest x-ray, Age, Culture count, Alcohol consumption | F(17, 53) = 2.85 | BCG | 0.44 | 0.14 | 0.73 | 0.004 | |
| Alcohol consumption | 1.8 | 0.59 | 3 | 0.004 | ||||
| Culture count | −0.57 | −0.98 | −0.15 | 0.009 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Hanafiah, K.; Garcia, M.L.; Anderson, D.A. An Observational Case-Control Study to Determine Human Immunodeficiency Virus and Host Factor Influence on Biomarker Distribution and Serodiagnostic Potential in Adult Pulmonary Tuberculosis. Trop. Med. Infect. Dis. 2019, 4, 57. https://doi.org/10.3390/tropicalmed4020057
Mohd Hanafiah K, Garcia ML, Anderson DA. An Observational Case-Control Study to Determine Human Immunodeficiency Virus and Host Factor Influence on Biomarker Distribution and Serodiagnostic Potential in Adult Pulmonary Tuberculosis. Tropical Medicine and Infectious Disease. 2019; 4(2):57. https://doi.org/10.3390/tropicalmed4020057
Chicago/Turabian StyleMohd Hanafiah, Khayriyyah, Mary Louise Garcia, and David Andrew Anderson. 2019. "An Observational Case-Control Study to Determine Human Immunodeficiency Virus and Host Factor Influence on Biomarker Distribution and Serodiagnostic Potential in Adult Pulmonary Tuberculosis" Tropical Medicine and Infectious Disease 4, no. 2: 57. https://doi.org/10.3390/tropicalmed4020057
APA StyleMohd Hanafiah, K., Garcia, M. L., & Anderson, D. A. (2019). An Observational Case-Control Study to Determine Human Immunodeficiency Virus and Host Factor Influence on Biomarker Distribution and Serodiagnostic Potential in Adult Pulmonary Tuberculosis. Tropical Medicine and Infectious Disease, 4(2), 57. https://doi.org/10.3390/tropicalmed4020057





