Cryptococcus neoformans: Diagnostic Dilemmas, Electron Microscopy and Capsular Variants
Abstract
:1. Introduction
2. Materials and Methods
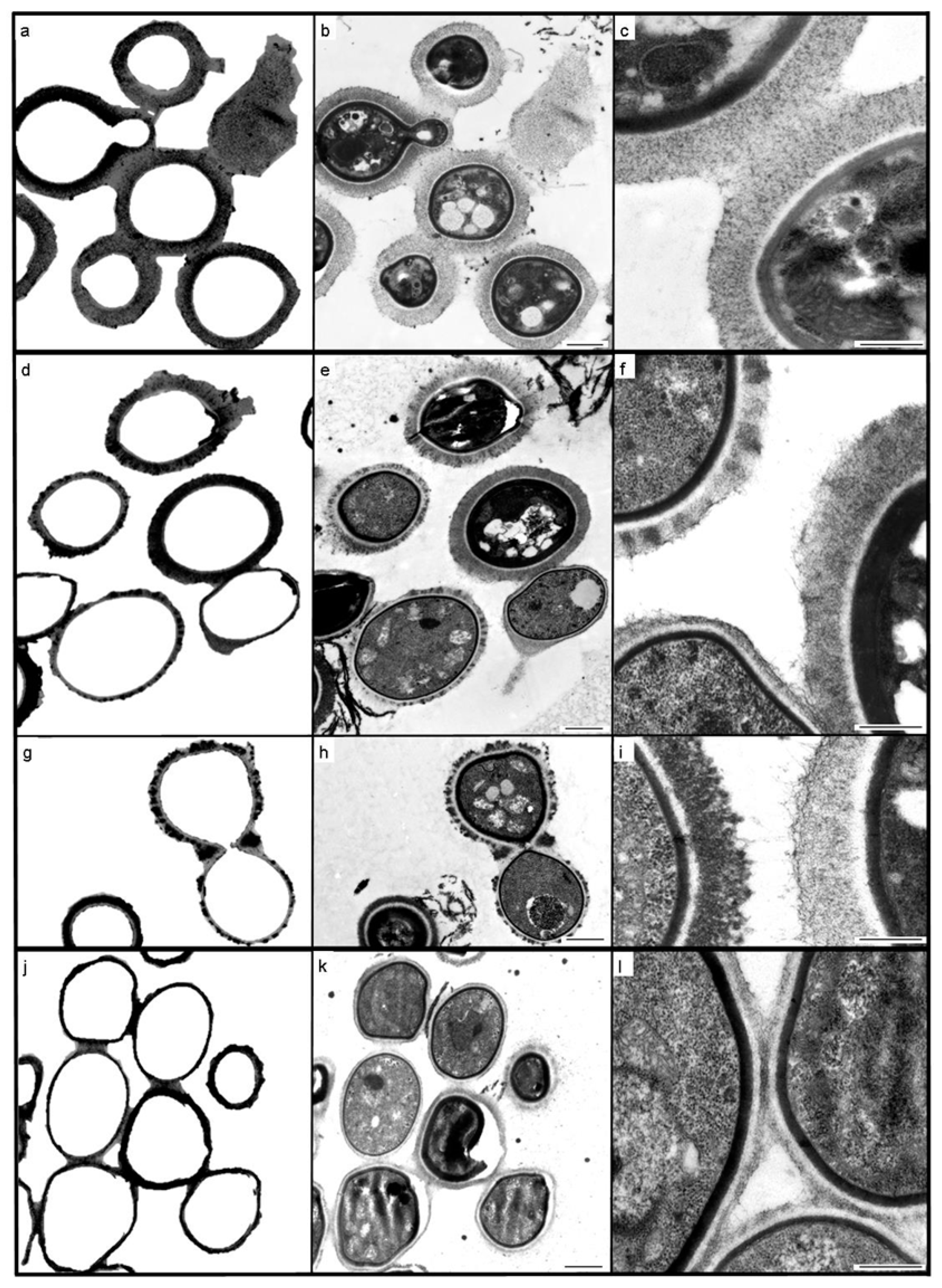
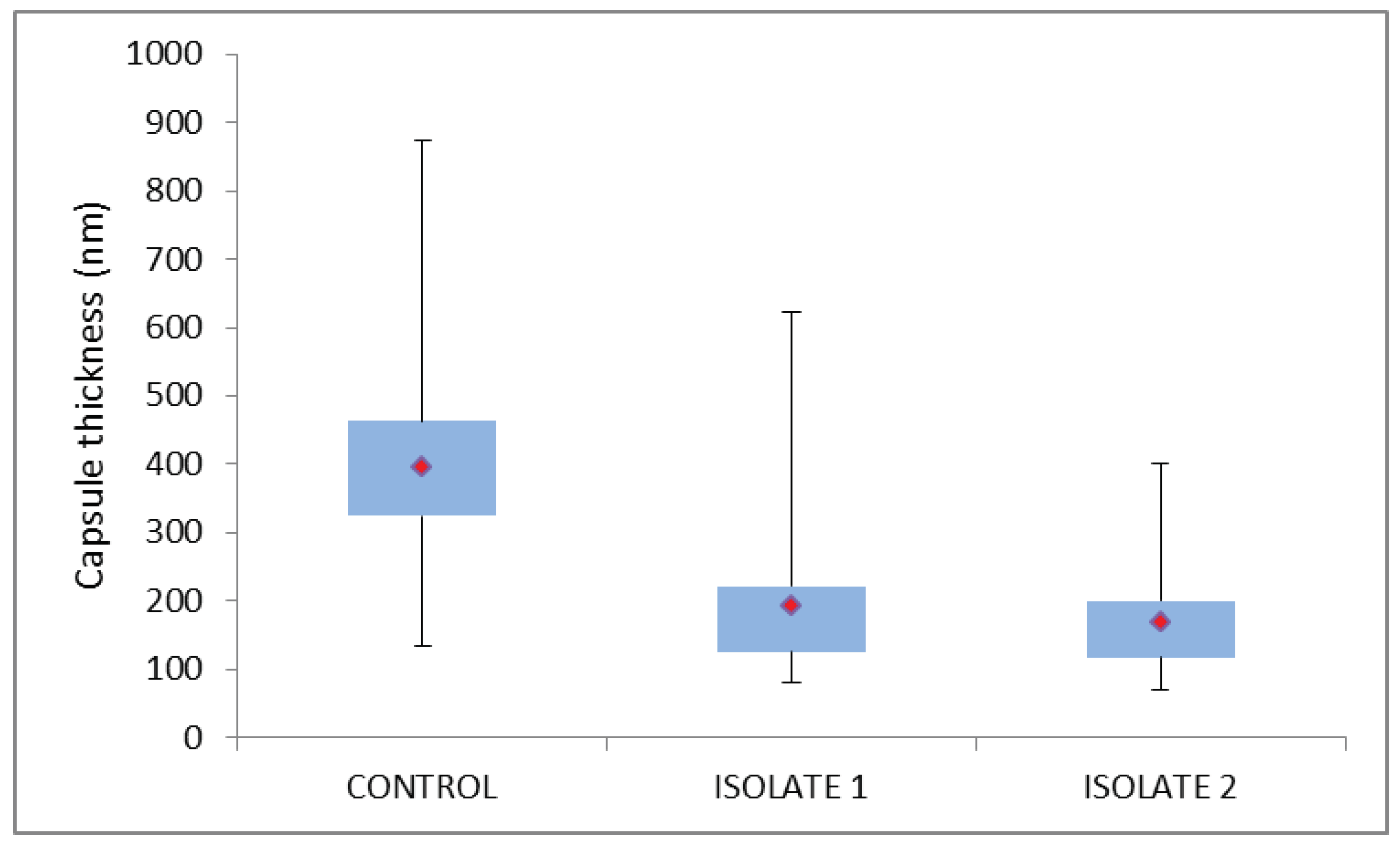
3. Results
3.1. Case 1
3.2. Case 2
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Govender, N.P.; Glencross, D.K. National coverage of reflex cryptococcal antigen screening: A milestone achievement in the care of persons with advanced HIV disease. S. Afr. Med J. 2018, 108, 534–535. [Google Scholar] [CrossRef] [PubMed]
- Larson, B.A.; Rockers, P.C.; Bonawitz, R.; Sriruttan, C.; Glencross, D.K.; Cassim, N. Screening HIV-infected patients with low CD4 counts for cryptococcal antigenemia prior to initiation of antiretroviral therapy: Cost effectiveness of alternative screening strategies in South Africa. PLoS ONE 2016, 11, e0158986. [Google Scholar] [CrossRef] [PubMed]
- Southern African HIV Clinicians Society. Guideline for the prevention, diagnosis and management of cryptococcal meningitis among HIV-infected persons: 2013 update. S. Afr. J. HIV Med. 2013, 14, 76–86. [Google Scholar] [CrossRef] [Green Version]
- Loyse, A.; Burry, J.; Cohn, J.; Ford, N.; Chiller, T.; Riberio, I. Leave no one behind: Response to new evidence and guidelines for the management of cryptococcal meningitis in low-income and middle-income countries. Lancet Infect. Dis. 2018. [Google Scholar] [CrossRef]
- WHO. Guidelines for the Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children: Supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection; World Health Organization: Geneva, Switzerland, 2018; p. 62. ISBN 978-92-4-155027-7. [Google Scholar]
- Oladele, R.O.; Bongomin, F.; Gago, S.; Denning, D.W. HIV-associated cryptococcal disease in resource-limited settings: A case for “Prevention is better than cure”? J. Fungi 2017, 3, 67. [Google Scholar] [CrossRef] [PubMed]
- Mfinanga, S.; Chanda, D.; Kivuyo, S.K.; Guinness, L.; Bottomley, C.; Simms, V. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: An open-label, randomised controlled trial. Lancet 2015, 385, 2173–2182. [Google Scholar] [CrossRef]
- Vidal, J.E.; Boulware, D.R. Lateral flow assay for cryptococcal antigen: An important advance to improve the continuum of HIV care and reduce cryptococcal meningitis-related mortality. Rev. Inst. Med. Trop. Sao Paulo 2015, 57 (Suppl. 19), 38–45. [Google Scholar] [CrossRef]
- Camacho, E.; Casadevall, A. Cryptococcal traits mediating adherence to biotic and abiotic surfaces. J. Fungi 2018, 4, 88. [Google Scholar] [CrossRef]
- Lourens, A.; Jarvis, J.N.; Meintjies, G.; Samuel, C.M. Rapid diagnosis of cryptococcal meningitis using the lateral flow assay on CSF samples: The influence of the high dose ‘hook’ effect. J. Clin. Microbiol. 2014, 52, 4172–4175. [Google Scholar] [CrossRef]
- Lee, G.-H.; Arthur, I.; Leung, M. False-negative serum cryptococcal lateral flow assay result due to the prozone phenomenon. J. Clin. Microbiol. 2018, 56, e01878-17. [Google Scholar] [CrossRef]
- Nyazika, T.K.; Robertson, V.J.; Nherera, B.; Mapondera, P.T.; Meis, J.F.; Hagen, F. Comparison of biotyping methods as alternative identification tools to molecular typing of pathogenic Cryptococcus species in sub-Saharan Africa. Mycoses 2016, 59, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, M.; Govender, N.P.; Mitchell, T.G.; Litvintseva, A.P.; GERMS-SA. Multilocus sequence typing of serially collected isolates of Cryptococcus from HIV-infected patients in South Africa. J. Clin. Microbiol. 2014, 52, 1921–1931. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Application; Innis, M.A., Gelfand, H., Sninsky, J.J., White, T.J., Eds.; Academic Press: London, UK, 1990; pp. 315–322. ISBN 0-12-372181-4. [Google Scholar]
- Meyer, W.; Aanensen, D.M.; Boekhout, T.; Cogliati, M.; Diaz, M.R.; Esposto, M.C. Consensus multi-locus sequence typing scheme for Cryptococcus neoformans and Cryptococcus gattii. Med. Mycol. 2009, 47, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Birkhead, M.; Ganesh, K.; Ndlangisa, K.M.; Koornhof, H.J. Transmission electron microscopy protocols for capsule visualisation in pathogenic respiratory and meningeal bacteria. In Microscopy and Imaging Science: Practical Approaches to Applied Research and Education; Mendez-Vilas, A., Ed.; Formatex Research Centre: Badajoz, Spain, 2017; pp. 628–639. ISBN 978-84-942134-9-6. [Google Scholar]
- Percival, A.; Thorkildson, P.; Kozel, T.R. Monoclonal antibodies specific for immunorecessive epitopes of glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans, reduce serotype bias in an immunoassay for cryptococcal antigen. Clin. Vaccine Immunol. 2011, 18, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Denham, S.T.; Brown, J.C.S. Mechanisms of pulmonary escape and dissemination by Cryptococcus neoformans. J. Fungi 2018, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, K.R.; Roberts, A.L.; Curtis, M.T.; Fortuna, D.; Dharia, R.; Sheehan, L. Diagnostic challenges of Cryptococcus neoformans in an immunocompetent individual masquerading as chronic hydrocephalus. Case Rep. Neurol. Med. 2016, 2016, e7381943. [Google Scholar] [CrossRef]
- Grossman, N.T.; Casadevall, A. Physiological differences in Cryptococcus neoformans strains in vitro versus in vivo and their effects on antifungal susceptibility. Antimicrob. Agents Chemother. 2017, 61, e021088-16. [Google Scholar] [CrossRef]
- Fernandes, K.E.; Brockway, A.; Haverkamp, M.; Cuomo, C.A.; van Ogtop, F.; Perfect, J.R. Phenotypic variability correlates with clinical outcome in Cryptococcus isolates obtained from Botswana HIV/AIDS patients. mBio 2018, 9, e02016-18. [Google Scholar] [CrossRef]
- Gish, S.R.; Maier, E.J.; Haynes, B.C.; Santiago-Tirado, F.H.; Srikanta, D.L.; Ma, C.Z. Computational analysis reveals a key regulator of cryptococcal virulence and determinant of host response. mBio 2016, 7, e00313-16. [Google Scholar] [CrossRef]
- Cruikshank, J.G.; Cavill, R.; Jelbert, M. Cryptococcus neoformans of unusual morphology. Appl. Microbiol. 1973, 25, 309–312. [Google Scholar]
- Grijpsta, J.; Gerwig, G.J.; Wosten, H.; Kamerling, J.; de Cock, H. Production of extracellular polysaccharides by CAP mutants of Cryptococcus neoformans. Eukaryot. Cell 2009, 8, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
| Laboratory Testing | Case 1 | Case 2 | Normal Range | |||
|---|---|---|---|---|---|---|
| CSF PARAMETER | Day 3 | Day 1 | Day 6 | Day 13 | Day 43 | |
| Chloride (mmol/L) | 111 | 96 | 104 | 127 | 129 | 118–132 |
| Glucose(mmol/L) | 3.6 | 0.1 | 1.3 | 2 | 2.6 | 2.5–4.4 |
| Protein (g/L) | 0.22 | 3.57 | 2.56 | 0.88 | 0.65 | 0.15–0.45 |
| Adenosine deaminase (U/L) | * | 10.7 | 17.9 | 9.2 | 0 | <6 |
| Polymorphonucleocytes (cells/µL) | 0 | 2 | 4 | 0 | 0 | <5 |
| Lymphocytes (cells/µL) | 0 | 163 | 463 | 647 | 6 | <5 |
| Erythrocytes (cells/µL) | 20 | 19 | 0 | >10,000 | 125 | 0 |
| India ink | Negative | Negative | Negative | Negative | Negative | Negative |
| CrAg LFA ** | Negative | Negative | Negative | Negative | Negative | Negative |
| Culture | C. neoformans | C. neoformans | No growth | No growth | No growth | No growth |
| SERUM CHEMISTRY | Day 1 | Day 1 | Day 6 | Day 13 | Day 43 | |
| Sodium (mmol/L) | 126 | * | 125 | 129 | * | 136–145 |
| Potassium (mmol/L) | 3.3 | * | 3.8 | haemolysed | * | 3.5–5.1 |
| Urea | 4.4 | * | 11 | 9.5 | * | 2.1–7.1 |
| Creatinine (µmol/L) | 70 | * | 124 | 388 | * | 64–104 |
| C–reactive protein (mg/L) | 92 | * | * | 336 | * | <10 |
| Total protein (g/L) | 82 | * | * | 55 | * | 60–78 |
| Albumin (g/L) | 22 | * | * | 16 | * | 35–52 |
| Total bilirubin (µmol/L) | 16 | * | * | 58 | * | 5–21 |
| Conjugated bilirubin (µmol/L) | 5 | * | * | 40 | * | 0–3 |
| Alanine transaminase (U/)L | 36 | * | * | 38 | * | 7–35 |
| Aspartate transaminase (U/L) | 51 | * | * | 181 | * | 13–35 |
| Alkaline phosphatase (U/L) | 380 | * | * | 237 | * | 42–98 |
| Gamma–glutamyl transferase (U/L) | 279 | * | * | 218 | * | <40 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birkhead, M.; Naicker, S.D.; Blasich, N.P.; Rukasha, I.; Thomas, J.; Sriruttan, C.; Abrahams, S.; Mavuso, G.S.; Govender, N.P. Cryptococcus neoformans: Diagnostic Dilemmas, Electron Microscopy and Capsular Variants. Trop. Med. Infect. Dis. 2019, 4, 1. https://doi.org/10.3390/tropicalmed4010001
Birkhead M, Naicker SD, Blasich NP, Rukasha I, Thomas J, Sriruttan C, Abrahams S, Mavuso GS, Govender NP. Cryptococcus neoformans: Diagnostic Dilemmas, Electron Microscopy and Capsular Variants. Tropical Medicine and Infectious Disease. 2019; 4(1):1. https://doi.org/10.3390/tropicalmed4010001
Chicago/Turabian StyleBirkhead, Monica, Serisha D. Naicker, Nozuko P. Blasich, Ivy Rukasha, Juno Thomas, Charlotte Sriruttan, Shareef Abrahams, Grisselda S. Mavuso, and Nelesh P. Govender. 2019. "Cryptococcus neoformans: Diagnostic Dilemmas, Electron Microscopy and Capsular Variants" Tropical Medicine and Infectious Disease 4, no. 1: 1. https://doi.org/10.3390/tropicalmed4010001




