Treading the Path towards Genetic Control of Snail Resistance to Schistosome Infection
Abstract
1. Introduction
2. Biology of Snail Resistance/Susceptibility to Schistosoma Infections–Major Exploits so Far
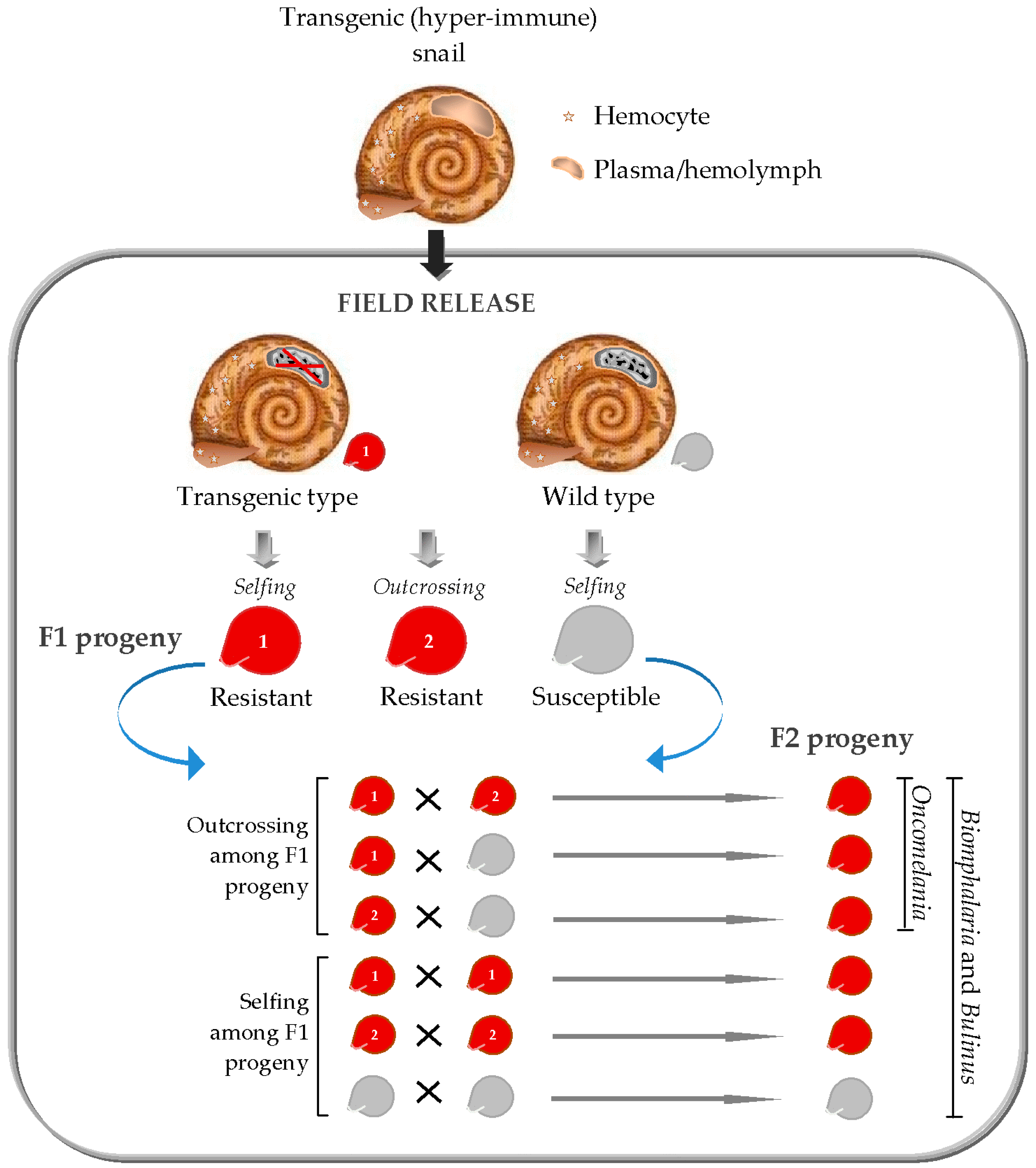
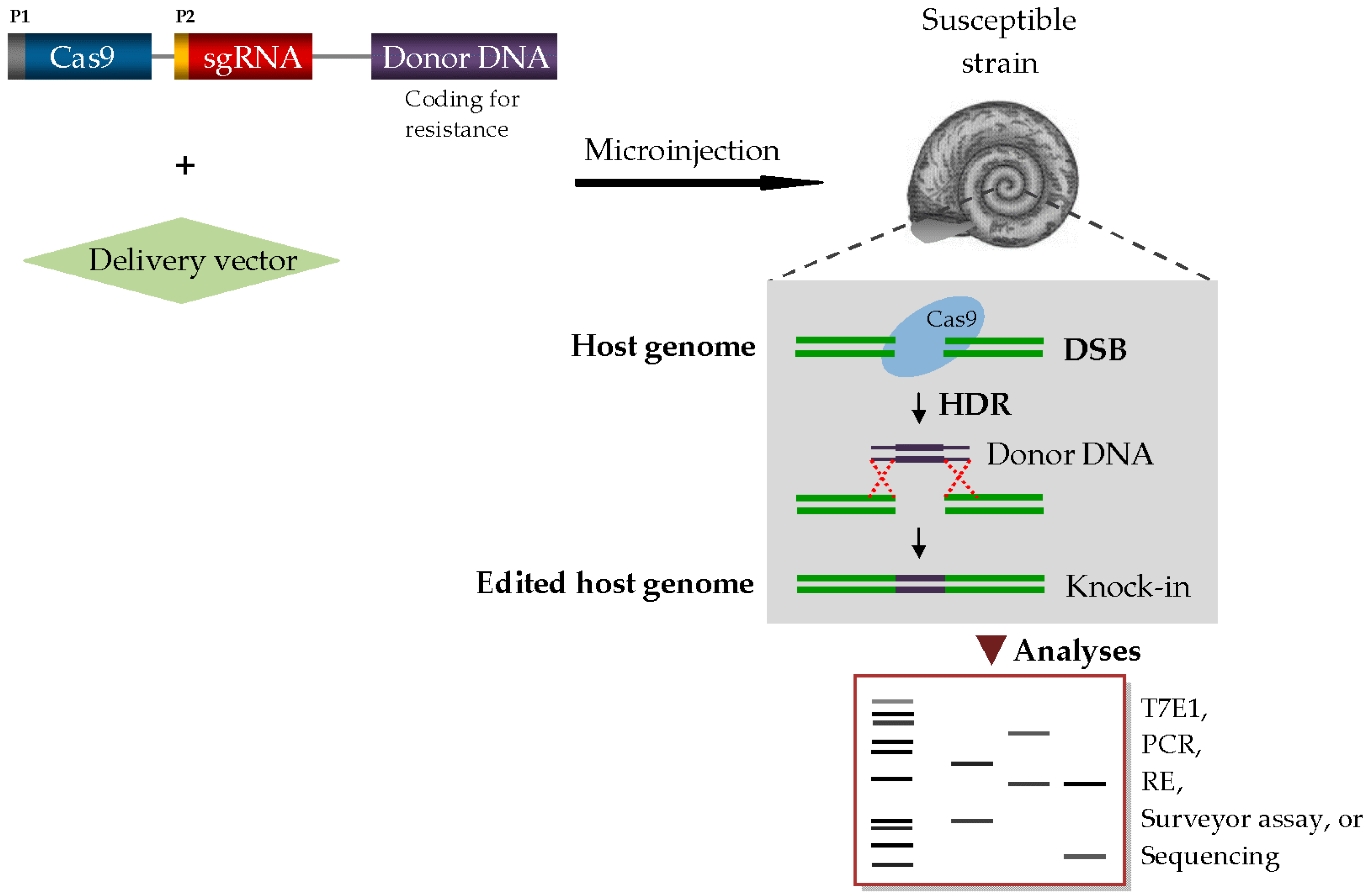
3. Transgenic Snail Methods for Schistosomiasis Control
4. Further Considerations
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Schistosomiasis and soil-transmitted helminthiasis: Number of people treated in 2016. Wkly. Epidemiol. Rec. 2017, 92, 49–60. [Google Scholar]
- World Health Organization. Global Health Estimates Summary Tables. DALYs by Cause, Age and Sex, by WHO Region, 2000–2015; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Muller, R.; Wakelin, D. Worms and Human Disease, 2nd ed.; CABI Publishing: Oxon, UK; New York, NY, USA, 2002; pp. 1–300. [Google Scholar]
- Adenowo, A.B.; Oyinloye, B.E.; Ogunyinka, B.I.; Kappo, A.B. Impact of human schistosomiasis in sub-Saharan Africa. Braz. J. Infect. Dis. 2015, 19, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Famakinde, D.O. Molecular context of Schistosoma mansoni transmission in the molluscan environments: A mini-review. Acta Trop. 2017, 176, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Olveda, D.U.; Li, Y.; Olveda, R.M.; Lam, A.K.; Chau, T.N.P.; Harn, D.A.; Williams, G.M.; Gray, D.J.; Ross, A.G.P. Bilharzia: Pathology, diagnosis, management and control. Trop. Med. Surg. 2013, 1, 135. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Secor, W.E. Immunology of human schistosomiasis. Parasite Immunol. 2014, 36, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.F.; Mital, P.; Kanzaria, H.K.; Olds, G.R.; Kurtis, J.D. Schistosomiasis and pregnancy. Trends Parasitol. 2007, 23, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Salawu, O.T.; Odaibo, A.B. Maternal schistosomiasis: A growing concern in sub-Saharan Africa. Pathog. Glob. Health 2014, 108, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Kjetland, E.F.; Ndhlovu, P.D.; Gomo, E.; Mduluza, T.; Midzi, N.; Gwanzura, L.; Mason, P.R.; Sandvik, L.; Friis, H.; Gunderseen, S.G. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS 2006, 20, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Mbabazi, P.S.; Andan, O.; Fitzgerald, D.W.; Chitsulo, L.; Engels, D.; Downs, J.A. Examining the relationship between urogenital schistosomiasis and HIV infection. PLoS Negl. Trop. Dis. 2011, 5, e1396. [Google Scholar] [CrossRef] [PubMed]
- Bustinduy, A.; King, C.; Scott, J.; Appleton, S.; Sousa-Figueiredo, J.C.; Betson, M.; Stothard, J.R. HIV and schistosomiasis co-infection in African children. Lancet Infect. Dis. 2014, 14, 640–649. [Google Scholar] [CrossRef]
- Tchuenté, L.-A.T.; Rollinson, D.; Stothard, J.R.; Molyneux, D. Moving from control to elimination of schistosomiasis in sub-Saharan Africa: Time to change and adapt strategies. Infect. Dis. Poverty 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- King, C.H.; Sutherland, L.J.; Bertsch, D. Systematic review and meta-analysis of the impact of chemical-based mollusciciding for control of Schistosoma mansoni and S. haematobium transmission. PLoS Negl. Trop. Dis. 2015, 9, e0004290. [Google Scholar] [CrossRef] [PubMed]
- Lo, N.C.; Gurarie, D.; Toon, N.; Coulibaly, J.T.; Bendavid, E.; Andrews, J.R.; King, C.H. Impact and cost-effectiveness of snail control to achieve disease control targets for schistosomiasis. Proc. Natl. Acad. Sci. USA 2018, 115, E584–E591. [Google Scholar] [CrossRef] [PubMed]
- Sokolow, S.H.; Wood, C.L.; Jones, I.J.; Lafferty, K.D.; Kuris, A.M.; Hseih, M.H.; De Loe, G.A. To reduce the global burden of human schistosomiasis, use ‘old fashioned’ snail control. Trends Parasitol. 2018, 34, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Sokolow, S.H.; Wood, C.L.; Jones, I.J.; Swartz, S.J.; Lopez, M.; Hseih, M.H.; Lafferty, K.D.; Kuris, A.M.; Rickards, C.; De Leo, G.A. Global assessment of schistosomiasis control over the past century shows targeting the snail intermediate host works best. PLoS. Negl. Trop. Dis. 2016, 10, e0004794. [Google Scholar] [CrossRef] [PubMed]
- Alsaqabi, S.M.; Lofty, W.M. Praziquantel: A review. J. Vet. Sci. Technol. 2014, 5, 1000200. [Google Scholar] [CrossRef]
- Cioli, D.; Pica-Mattoccia, L.; Basso, A.; Guidi, A. Schistosomiasis control: Praziquantel forever? Mol. Biochem. Parasitol. 2014, 195, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Tebeje, B.M.; Harvie, M.; You, H.; Loukas, A.; McManus, D.P. Schistosomiasis vaccines: Where do we stand? Parasit. Vectors 2016, 9, 528. [Google Scholar] [CrossRef] [PubMed]
- Ebisawa, I. Epidemiology and eradication of schistosomiasis japonica in Japan. J. Travel Med. 1998, 5, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.M.R. Epidemiology of schistosomiasis in Egypt: Travel through time: Review. J. Adv. Res. 2013, 4, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Secor, W.E. Water-based interventions for schistosomiasis control. Pathog. Glob. Health 2014, 108, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Tlamçani, Z.; Er-Rami, M. Schistosomiasis control: Moroccan experience compared to other endemic countries. Asian Pac. J. Trop. Dis. 2014, 4, 329–332. [Google Scholar] [CrossRef]
- King, C.H.; Bertsch, D. Historical perspective: Snail control to prevent schistosomiasis. PLoS Negl. Trop. Dis. 2015, 9, e0003657. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.P.; Wang, W.; Hong, Q.B.; Li, S.Z.; Liang, Y.S.; Yang, H.T.; Zhou, X.N. Approaches being used in the national schistosomiasis elimination programme in China: A review. Infect. Dis. Poverty 2017, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Lardans, V.; Dissous, C. Snail control strategies for reduction of schistosomiasis transmission. Parasitol. Today 1998, 14, 413–417. [Google Scholar] [CrossRef]
- Huang, D.; Zhen, J.; Quan, S.; Liu, M.; Liu, L. Risk assessment for niclosamide residues in water and sediments from Nan Ji Shan Island within Poyang Lake region, China. Adv. Mat. Res. 2013, 721, 608–612. [Google Scholar] [CrossRef]
- World Health Organization. Field Use of Molluscicides in Schistosomiasis Control Programmes: An Operational Manual for Programme Managers; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Assembly. Elimination of Schistosomiasis. In Sixty-fifth World Health Assembly: Resolutions and Decisions; World Health Organization: Geneva, Switzerland, 2012; pp. 36–37. [Google Scholar]
- Miyairi, K.; Suzuki, M. On the development of Schistosoma japonicum. Tokyo Med. J. 1913, 1836, 1961–1965. [Google Scholar]
- Tanaka, H.; Tsuji, M. From discovery to eradication of schistosomiasis in Japan: 1847–1996. Int. J. Parasitol. 1997, 27, 1465–1480. [Google Scholar] [CrossRef]
- Leiper, R.T. On the relation between the terminal-spined and lateral-spined eggs of bilharzia. Br. Med. J. 1916, 1, 411. [Google Scholar] [CrossRef] [PubMed]
- Leiper, R.T. Report on the results of the bilharzias mission in Egypt, 1915. J. R. Army Med. Corps 1918, 30, 235–260. [Google Scholar]
- Newton, W.L. The comparative tissue reaction of two strains of Australorbis glabratus to infection with Schistosoma mansoni. J. Parasitol. 1952, 38, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Newton, W.L. The inheritance of susceptibility to infection with Schistosoma mansoni in Australorbis glabratus. Exp. Parasitol. 1953, 2, 242–257. [Google Scholar] [CrossRef]
- Richards, C.S. Genetic studies of a molluscan vector of schistosomiasis. Nature 1970, 227, 231–241. [Google Scholar] [CrossRef]
- Richards, C.S. Genetic factors in susceptibility of Biomphalaria glabrata for different strains of Schistosoma mansoni. Parasitology 1975, 70, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.S. Schistosoma mansoni: susceptibility reversal with age in the snail host. Exp. Parasitol. 1977, 42, 165–168. [Google Scholar] [CrossRef]
- Richards, C.S.; Knight, M.; Lewis, F.A. Genetics of Biomphalaria glabrata and its effects on the outcome of Schistosoma mansoni infection. Parasitol. Today 1992, 8, 171–174. [Google Scholar] [CrossRef]
- Abou-El-Naga, I.F.; Eissa, M.M.; Mossallam, S.F.; El-Halim, S.I.A. Inheritance of Schistosoma mansoni infection incompatibility in Biomphalaria alexandrina snails. Mem. do Inst. Oswaldo Cruz 2010, 105, 149–154. [Google Scholar] [CrossRef]
- Dos Santos, M.B.L.; Freitas, J.R.; Correia, M.C.R.; Coelho, P.M.Z. Susceptibility of Biomphalaria tenagophila hybrids to Schistosoma mansoni: Crossing between strains from Taim (RS), Cabo Frio (RJ), and Belo Horizonte (MG), Brasil. Rev. Inst. de Med. Trop. São Paulo 1979, 21, 281–286. [Google Scholar]
- Knight, M.; Miller, A.N.; Patterson, C.N.; Rowe, C.G.; Michaels, G.; Carr, D.; Richards, C.S.; Lewis, F.A. The identification of markers segregating with resistance to Schistosoma mansoni infection in the snail Biomphalaria glabrata. Proc. Natl. Acad. Sci. USA 1999, 96, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Rosa, F.M.; Godard, A.L.B.; Azevedo, V.; Coelho, P.M.Z. Biomphalaria tenagophila: dominant character of the resistance to Schistosoma mansoni in descendants of crossbreedings between resistant (Taim, RS) and susceptible (Joinville, SC) strains. Mem. do Inst. Oswaldo Cruz 2005, 100, 19–23. [Google Scholar] [CrossRef]
- Haggag, S.H.; El-Sherbiny, M. Molecular markers associated with resistance to Schistosoma mansoni infection in the Biomphalaria glabrata snails. Biotechnology 2006, 5, 404–412. [Google Scholar]
- Ittiprasert, W.; Miller, A.; Myers, J.; Nene, V.; El-Sayed, N.M.; Knight, M. Identification of immediate response genes dominantly expressed in juvenile resistant and susceptible Biomphalaria glabrata snails upon exposure to Schistosoma mansoni. Mol. Biochem. Parasitol. 2010, 169, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Paraense, W.L.; Correa, L.R. Variation in susceptibility of populations of Australorbis glabratus to a strain of Schistosoma mansoni. Rev. Inst. Med. Trop. São Paulo 1963, 5, 15–22. [Google Scholar] [PubMed]
- Richards, C.S. Susceptibility of adult Biomphalaria glabrata to Schistosoma mansoni infection. Am. J. Trop. Med. Hyg. 1973, 22, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Goodall, C.P.; Bender, R.C.; Brooks, J.K.; Bayne, C.J. Biomphalaria glabrata cytosolic copper/zinc superoxide dismutase (SOD1) gene: Association of SOD1 alleles with resistance/susceptibility to Schistosoma mansoni. Mol. Biochem. Parasitol. 2006, 147, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Newton, W.L. The establishment of a strain of Australorbis glabratus which combines albinism and high susceptibility to infection with Schistosoma mansoni. J. Parasitol. 1955, 41, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Adema, C.M.; Luo, M.Z.; Hanelt, B.; Hertel, L.A.; Marshall, J.J.; Zhang, S.M.; DeJong, R.J.; Kim, H.R.; Kudrna, D.; Wing, R.A.; et al. A bacterial artificial chromosome library for Biomphalaria glabrata, intermediate snail host of Schistosoma mansoni. Mem. Inst. Oswaldo Cruz 2006, 101, S167–S177. [Google Scholar] [CrossRef]
- Ittiprasert, W.; Myers, J.; Odoemelam, E.C.; Raghavan, N.; Lewis, F.; Bridger, J.M.; Knight, M. Advances in the genomics and proteomics of the freshwater intermediate snail host of Schistosoma mansoni, Biomphalaria glabrata. In Biomphalaria Snails and Larval Trematodes; Toledo, R., Fried, B., Eds.; Springer: New York, NY, USA, 2011; pp. 191–213. [Google Scholar]
- Pinaud, S.; Portela, J.; Duval, D.; Nowacki, F.C.; Olive, M.A.; Allienne, J.F.; Galinier, R.; Dheilly, N.M.; Kieffer-Jaquinod, S.; Mitta, G.; et al. A shift from cellular to humoral responses contributes to innate immune memory in the vector snail Biomphalaria glabrata. PLoS Pathog. 2016, 12, e1005361. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.P.; Coustau, C. Immunobiology of Biomphalaria–trematode interactions. In Biomphalaria Snails and Larval Trematodes; Toledo, R., Fried, B., Eds.; Springer: New York, NY, USA, 2011; pp. 159–189. [Google Scholar]
- Pila, E.A.; Li, H.; Hambrook, J.R.; Wu, X.; Hanington, P. Schistosomiasis from a snail’s perspective: Advances in snail immunity. Trends Parasitol. 2017, 33, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.L. A cell line from embryos of Biomphalaria glabrata (Pulmonata): Establishment and characteristics. In Invertebrate Tissue Culture: Research Applications; Maramorosch, K., Ed.; Academic Press: New York, NY, USA, 1976; pp. 75–97. [Google Scholar]
- Adema, C.M.; Hillier, L.D.W.; Jones, C.S.; Loker, E.S.; Knight, M.; Minx, P.; Oliveira, G.; Raghavan, N.; Shedlock, A.; do Amaral, L.R.; et al. Whole genome analysis of a schistosomiasis-transmitting freshwater snail. Nat. Comm. 2017, 8, 15451. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, Z.; Lockyer, A.E.; Davies, A.J.; Kirk, R.S.; Emery, A.M.; Rollinson, D.; Jones, C.S.; Noble, L.R.; Walker, A.J. Differences in the gene expression profiles of haemocytes from schistosome-susceptible and -resistant Biomphalaria glabrata exposed to Schistosoma mansoni excretory secretory products. PLoS ONE 2014, 9, e93215. [Google Scholar] [CrossRef] [PubMed]
- Larson, M.K.; Bender, R.C.; Bayne, C.J. Resistance of Biomphalaria glabrata 13–16-R1 snails to Schistosoma mansoni PR1 is a function of haemocyte abundance and constitutive levels of specific transcripts in haemocytes. Int. J. Parasitol. 2014, 44, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Pila, E.A.; Gordy, M.A.; Phillips, V.K.; Kabore, A.L.; Rudkom, S.P.; Hanington, P.C. Endogenous growth factor stimulation of hemocyte proliferation induces resistance to Schistosoma mansoni challenge in the snail host. Proc. Natl. Acad. Sci. USA 2016, 113, 5305–5310. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.B.; Pierce, R.J.; Gourbal, B.; Werkmeister, E.; Colinet, D.; Reichhart, J.; Dissous, C.; Coustau, C. Involvement of the cytokine MIF in the snail host immune response to the parasite Schistosoma mansoni. PLoS Pathog. 2010, 6, e1001115. [Google Scholar]
- Pila, E.A.; Tarrabain, M.; Kabore, A.L.; Hanington, P.C. A novel toll-like receptor (TLR) influences compatibility between the gastropod Biomphalaria glabrata, and the digenean trematode Schistosoma mansoni. PLoS Pathog. 2016, 12, e1005513. [Google Scholar] [CrossRef] [PubMed]
- Galinier, R.; Portela, J.; Mone, Y.; Allienne, J.F.; Henri, H.; Delbecq, S.; Mitta, G.; Gourbal, B.; Duval, D. Biomphalysin, a new β pore-forming toxin involved in Biomphalaria glabrata immune defense against Schistosoma mansoni. PLoS Pathog. 2013, 9, e1003216. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Ittiprasert, N.; Raghavan, N.; Miller, A.; Knight, M. Differences in cysteine protease activity in Schistosoma mansoni-resistant and -susceptible Biomphalaria glabrata and characterization of the hepatopancreas cathepsin B fulllength cDNA. J. Parasitol. 2008, 94, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, A.E.; Spinks, J.; Kane, R.A.; Hoffmann, K.F.; Fitzpatrick, J.M.; Rollinson, D.; Noble, L.R.; Jones, C.S. Biomphalaria glabrata transcriptome: cDNA microarray profiling identifies resistant- and susceptible-specific gene expression in haemocytes from snail strains exposed to Schistosoma mansoni. BMC Genomics 2008, 9, 634. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Dinguirard, N.; Sabat, G.; Lui, H.; Gonzalez, L.; Gehring, M.; Bickham-Wright, U.; Yoshino, T.P. Proteomic analysis of Biomphalaria glabrata plasma proteins with binding affinity to those expressed by early developing larval Schistosoma mansoni. PLoS Pathog. 2017, 13, e1006081. [Google Scholar] [CrossRef] [PubMed]
- Bonner, K.M.; Bayne, C.J.; Larson, M.K.; Blouin, M.S. Effects of Cu/Zn superoxide dismutase (sod1) genotype and genetic background on growth, reproduction and defense in Biomphalaria glabrata. PLoS Negl. Trop. Dis. 2012, 6, e1701. [Google Scholar] [CrossRef] [PubMed]
- Tennessen, J.A.; Bonner, K.M.; Bollmann, S.R.; Johnstun, J.A.; Yeh, J.Y.; Marine, M.; Tavalire, H.F.; Bayne, C.J.; Blouin, M.S. Genome-wide scan and test of candidate genes in the snail Biomphalaria glabrata reveal new locus influencing resistance to Schistosoma mansoni. PLoSNegl. Trop. Dis. 2015, 9, e0004077. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, A.E.; Emery, A.M.; Kane, R.A.; Walker, A.J.; Mayer, C.D.; Mitta, G.; Coustau, C.; Adema, C.M.; Hanelt, B.; Rollinson, D.; et al. Early differential gene expression in haemocytes from resistant and susceptible Biomphalaria glabrata strains in response to Schistosoma mansoni. PLoS ONE 2012, 7, e51102. [Google Scholar] [CrossRef] [PubMed]
- Nowak, T.S.; Woodards, A.C.; Jung, Y.; Adema, C.M.; Loker, E.S. Identification of transcripts generated during the response of resistant Biomphalaria glabrata to Schistosoma mansoni infection using suppression subtractive hybridization. J. Parasitol. 2004, 90, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Hanington, P.C.; Forys, M.A.; Dragoo, J.W.; Zhang, S.; Adema, C.M.; Loker, E.S. Role for a somatically diversified lectin in resistance of an invertebrate to parasite infection. Proc. Natl. Acad. Sci. USA 2010, 107, 21087–21092. [Google Scholar] [CrossRef] [PubMed]
- Hanington, P.C.; Forys, M.A.; Loker, E.S. A somatically diversified defense factor, FREP3, is a determinant of snail resistance to schistosome infection. PLoS Negl. Trop. Dis. 2012, 6, e1591. [Google Scholar] [CrossRef] [PubMed]
- Martins-Souza, R.L.; Pereira, C.A.J.; Rodrigues, L.; Araújo, E.S.; Coelho, P.M.Z.; Corrêa, A., Jr.; Negrão-Corrêa, D. Participation of N-acetyl-D-glucosamine carbohydrate moieties in the recognition of Schistosoma mansoni sporocysts by haemocytes of Biomphalaria tenagophila. Mem. Inst. Oswaldo Cruz 2011, 106, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Allan, E.R.O.; Tennessen, J.A.; Bollmann, S.R.; Hanington, P.C.; Bayne, C.J.; Blouin, M.S. Schistosome infectivity in the snail, Biomphalaria glabrata, is partially dependent on the expression of Grctm6, a Guadeloupe Resistance Complex protein. PLoS Negl. Trop. Dis. 2017, 11, e0005362. [Google Scholar] [CrossRef] [PubMed]
- Ittiprasert, W.; Knight, M. Reversing the resistance phenotype of the Biomphalaria glabrata snail host Schistosoma mansoni infection by temperature modulation. PLoS Pathog. 2012, 8, e1002677. [Google Scholar] [CrossRef] [PubMed]
- Ittiprasert, W.; Nene, R.; Miller, A.; Raghavan, N.; Lewis, F.; Hodgson, J.; Knight, M. Schistosoma mansoni infection of juvenile Biomphalaria glabrata induces a differential stress response between resistant and susceptible snails. Exp. Parasitol. 2009, 123, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.; Elhelu, O.; Smith, M.; Haugen, B.; Miller, A.; Raghavan, N.; Wellman, C.; Cousin, C.; Dixon, F.; Mann, V.; et al. Susceptibility of snails to infection with schistosomes is influenced by temperature and expression of heat shock proteins. Epidemiology 2015, 5, 1–18. [Google Scholar]
- Granath, W.O., Jr.; Connors, V.A.; Tarleton, R.L. Interleukin 1 activity haemolymph from strains of the snail Biomphalaria glabrata varying in susceptibility to the human blood fluke, Schistosoma mansoni: Presence, differential expression, and biological function. Cytokine 1994, 6, 21–27. [Google Scholar] [CrossRef]
- Zhang, S.M.; Coultas, K.A. Identification and characterization of five transcription factors that are associated with evolutionarily conserved immune signaling pathways in the schistosome-transmitting snail Biomphalaria glabrata. Mol. Immunol. 2011, 48, 1868–1881. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.; Raghavan, N.; Goodal, C.; Cousin, C.; Ittiprasert, W.; Sayed, A.; Miller, A.; Williams, D.L.; Bayne, C. Biomphalaria glabrata peroxiredoxin: effect of Schistosoma mansoni infection on differential gene regulation. Mol. Biochem. Parasitol. 2009, 167, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Ouwe-Missi-Oukem-Boyer, O.; Porchet, E.; Capron, A.; Dissous, C. Characterization of immunoreactive TNF-α molecules in the gastropod Biomphalaria glabrata. Dev. Comp. Immunol. 1994, 18, 211–218. [Google Scholar] [CrossRef]
- Alphey, L. Genetic control of mosquitoes. Annu. Rev. Emtomol. 2014, 59, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Macias, V.M.; Ohm, J.R.; Rasgon, J.L. Gene drive for mosquito control: Where did it come from and where are we headed? Int. J. Environ. Res. Public Health 2017, 14, 1006. [Google Scholar] [CrossRef] [PubMed]
- Hubendick, B. A possible method of schistosome-vector control by competition between resistant and susceptible strains. Bull. World Health Organ. 1958, 18, 1113–1116. [Google Scholar] [PubMed]
- Rozendaal, J.A.; World Health Organization. Freshwater snails. In Vector Control: Methods for Use by Individuals and Communities; World Health Organization: Geneva, Switzerland, 1997; pp. 337–356. [Google Scholar]
- Claveria, F.G.; Etges, F.J. Differential susceptibility of male and female Oncomelania hupensis quadrasi infected with Schistosoma japonicum. Int. J. Parasitol. 1987, 17, 1273–1277. [Google Scholar] [CrossRef]
- Moose, J.W.; Williams, J.E. Infection rates of Schistosoma japonicum in experimentally exposed female and male oncomelanid snails. Jan. J. Med. Sci. Biol. 1964, 17, 333–334. [Google Scholar] [CrossRef]
- Sun, D.; Gou, Z.; Liu, Y.; Zhang, Y. Progress and prospects of CRISPR/Cas systems in insects and other arthropods. Front. Physiol. 2017, 8, 608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Y.; Gao, F.; Han, S.; Cheah, K.S.; Tse, H.F.; Lian, Q. CRISPR/Cas9 genome-editing system in human stem cells: Current status and future prospects. Mol. Ther. Nucleic Acids 2017, 9, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Braddick, D.; Dhar, P.K. Exploring the potential of genome editing CRISPR-Cas9 technology. Gene 2017, 599, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, S.; Xu, J.; Suin, C.; Wei, J. Application of CRISPR/Cas9 in plant biology. Acta Pharm. Sin. B 2017, 7, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Fisher, A.L.; Huang, H.; Xie, Z. CRISR-mediated genome editing and human diseases. Genes Dis. 2016, 3, 244–251. [Google Scholar] [CrossRef]
- Mouahid, G.; Rognon, A.; de Carvalho-Augusto, R.; Driguez, P.; Geyer, K.; Karinshak, S.; Luviano, N.; Mann, V.; Quack, T.; Rawlinson, K.; et al. Transplantation of schistosome sporocysts between host snails: A video guide. Wellcome Open Res. 2018, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Tennessen, J.A.; Théron, A.; Marine, M.; Yeh, J.Y.; Rognon, A.; Blouin, M.S. Hyperdiverse gene cluster in snail host conveys resistance to human schistosome parasites. PLoS Genet. 2015, 11, e1005067. [Google Scholar] [CrossRef] [PubMed]
- Perry, K.J.; Henry, J.Q. CRISPR/Cas9-mediated genome modification in the mollusc, Crepidula fornicata. Genesis 2015, 53, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Bortesi, L.; Zhu, C.; Zischewski, J.; Perez, L.; Bassié, L.; Nadi, R.; Forni, G.; Lade, S.B.; Soto, E.; Jin, X.; et al. Patterns of CRISPR/Cas9 activity in plants, animals and microbes. Plant Biotechnol. J. 2016, 14, 2203–2216. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, F.; Dang, L.; Liang, D.; Wang, C.; He, B.; Liu, J.; Li, D.; Wu, X.; Xu, X.; et al. In vivo delivery systems for therapeutic genome editing. Int. J. Mol. Sci. 2016, 17, 626. [Google Scholar] [CrossRef] [PubMed]
- Jarne, P.; Pointier, J.-P.; David, P. Biosystematics of Biomphalaria spp. with an emphasis on Biomphalaria glabrata. In Biomphalaria Snails and Larval Trematodes; Toledo, R., Fried, B., Eds.; Springer: New York, NY, USA, 2011; pp. 1–32. [Google Scholar]
- Blouin, M.S.; Bonner, K.M.; Cooper, B.; Amarasinghe, V.; O’Donnell, R.P.; Bayne, C.J. Three genes involved in the oxidative burst are closely linked in the genome of the snail, Biomphalaria glabrata. Int. J. Parasitol. 2013, 43, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Berg, C.O. Biological control of snail-borne diseases: A review. Exp. Parasitol. 1973, 33, 318–330. [Google Scholar] [CrossRef]
- Galinier, R.; Roger, E.; Moné, Y.; Duval, D.; Portet, A.; Pinaud, S.; Chaparro, C.; Grunau, C.; Genthon, C.; Dubois, E.; et al. A multistrain approach to studying the mechanisms underlying compatibility in the interaction between Biomphalaria glabrata and Schistosoma mansoni. PLoS Negl. Trop. Dis. 2017, 11, e0005398. [Google Scholar] [CrossRef] [PubMed]
- Mitta, G.; Adema, C.M.; Gourbal, B.; Loker, E.S.; Theron, A. Compatibility polymorphism in snail/schistosome interactions: From field to theory to molecular mechanisms. Dev. Comp. Immunol. 2012, 37, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.S.; Wang, A.Y.; Zhao, H.B.; Chen, Y.H. Transcriptome sequencing and differential gene expression analysis of the schistosome-transmitting snail Oncomelania hupensis inhabiting hilly and marshland regions. Sci. Rep. 2017, 7, 15809. [Google Scholar] [CrossRef] [PubMed]
- Esvelt, K.M.; Smidler, A.L.; Catteruccia, F.; Church, G.M. Concerning RNA-guided gene drives for the alteration of wild population. eLife 2014, 3, e03401. [Google Scholar] [CrossRef] [PubMed]
| Resistance Factor | Snail spp. | Schistosoma spp. | Function | Reference(s) |
|---|---|---|---|---|
| 40S ribosomal protein S9 | B. glabrata | S. mansoni | Protein translation in hemocytes. | [59] |
| BgAIF | B. glabrata | S. mansoni | Modulates hemocyte activation. | [60] |
| BgGRN | B. glabrata | S. mansoni | Production of adherent hemocytes. | [61] |
| BgMIF | B. glabrata | S. mansoni | Induces hemocyte proliferation. | [62] |
| BgTLR | B. glabrata | S. mansoni | Parasite recognition and activation of effector functions. | [63] |
| Biomphalysin | B. glabrata | S. mansoni | Binds to the sporocyst surface and lyses it. | [54,64] |
| Cathepsin B | B. glabrata | S. mansoni | Lysis of encapsulated sporocyst. | [65] |
| Cathepsin L | B. glabrata | S. mansoni | Lysis of encapsulated sporocyst. | [66] |
| Copine 1 | B. glabrata | S. mansoni | Involves in signaling processes. | [66] |
| CREPs | B. glabrata | S. mansoni | Pattern recognition receptors/adhesion proteins. | [67] |
| Cu/Zn SOD (SOD1) | B. glabrata | S. mansoni | Catalyzes the production of H2O2 which is cytotoxic to sporocyst. | [50,68,69] |
| Cystatin 2 | B. glabrata | S. mansoni | Protease inhibitor. | [70,71] |
| Cytidine deaminase | B. glabrata | S. mansoni | Nucleobase, nucleoside, nucleotide, and nucleic acid metabolism. | [47] |
| Cytochrome b | B. glabrata | S. mansoni | Mitochondrial respiration. | [70] |
| Cytochrome C oxidase subunits | B. glabrata | S. mansoni | Mitochondrial respiration. | [70,71] |
| Dermatopontin2 | B. glabrata | S. mansoni | Participates in hemocyte adhesion and encapsulation responses. | [59,67,70] |
| Elastase2 | B. glabrata | S. mansoni | Lysis of encapsulated sporocyst. | [66,70] |
| Elongation factors 1α & 2 | B. glabrata | S. mansoni | Transcription enzymes (bind t-RNA to ribosomes). | [59,67] |
| Endo-1,4-β-glucanase | B. glabrata | S. mansoni | Carbohydrate metabolism. | [70] |
| Ferritin | B. glabrata | S. mansoni | Stores and transport iron in non-toxic form. | [70,71] |
| FREP1, 2, 3 & 12 | B. glabrata | S. mansoni | Pattern recognition receptors/adhesion proteins. | [67,70,72,73] |
| Fribillin | B. glabrata | S. mansoni | Participates in hemocyte adhesion and encapsulation responses. | [70] |
| GlcNAc ↓ | B. tenagophila | S. mansoni | Increases hemocyte binding to sporocyst. | [74] |
| GPCR kinase 2 | B. glabrata | S. mansoni | Signal transduction. | [70] |
| Grctm6 | B. glabrata | S. mansoni | Modulates cercarial shedding. | [75] |
| GREPs | B. glabrata | S. mansoni | Pattern recognition receptors/adhesion proteins. | [67] |
| GSTs | B. glabrata | S. mansoni | Prevent cellular damage to the hemocytes. | [70] |
| Hsp40, 60 & 70 # | B. glabrata | S. mansoni | Housekeeping cell repair activities. | [66,67,70,76,77,78] |
| Importin 7 | B. glabrata | S. mansoni | Involves in signaling processes. | [66] |
| Inferred phagocyte oxidase | B. glabrata | S. mansoni | Production of superoxide anions. | [60] |
| Interleukin 1 | B. glabrata | S. mansoni | Stimulates hemocyte defense response. | [79] |
| LPS-binding protein | B. glabrata | S. mansoni | Adhesion protein. | [67] |
| Matrilin | B. glabrata | S. mansoni | Participates in hemocyte adhesion and encapsulation responses. | [59,70] |
| Metalloproteases | B. glabrata | S. mansoni | Tissue morphogenesis/remodeling. | [67] |
| MPEG 1 | B. glabrata | S. mansoni | Participates in hemocyte defense responses. | [47] |
| Neo-calmodulin | B. glabrata | S. mansoni | Cacium signaling and homeostasis. | [67] |
| NF-kB | B. glabrata | S. mansoni | Downstream transcription in the TLR pathway. | [59,63,70,80] |
| NADH dehydrogenase subunis | B. glabrata | S. mansoni | Mitochondrial respiration. | [70] |
| Peroxiredoxines 1 & 4 | B. glabrata | S. mansoni | Neutralize ROS and RNS that can damage cellular functions. | [60,81] |
| PGRP 1 | B. glabrata | S. mansoni | Pattern recognition receptor. | [70] |
| PKC receptor | B. glabrata | S. mansoni | Signal transduction. | [47] |
| TEPs | B. glabrata | S. mansoni | Pattern recognition receptors/adhesion proteins. | [67] |
| TNF-α | B. glabrata | S. mansoni | Stimulates hemocyte defense response. | [82] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Famakinde, D.O. Treading the Path towards Genetic Control of Snail Resistance to Schistosome Infection. Trop. Med. Infect. Dis. 2018, 3, 86. https://doi.org/10.3390/tropicalmed3030086
Famakinde DO. Treading the Path towards Genetic Control of Snail Resistance to Schistosome Infection. Tropical Medicine and Infectious Disease. 2018; 3(3):86. https://doi.org/10.3390/tropicalmed3030086
Chicago/Turabian StyleFamakinde, Damilare O. 2018. "Treading the Path towards Genetic Control of Snail Resistance to Schistosome Infection" Tropical Medicine and Infectious Disease 3, no. 3: 86. https://doi.org/10.3390/tropicalmed3030086
APA StyleFamakinde, D. O. (2018). Treading the Path towards Genetic Control of Snail Resistance to Schistosome Infection. Tropical Medicine and Infectious Disease, 3(3), 86. https://doi.org/10.3390/tropicalmed3030086





