Involvement of Hookworm Co-Infection in the Pathogenesis and Progression of Podoconiosis: Possible Immunological Mechanism
Abstract
1. Introduction
2. Immunology of the Pathogenesis of Podoconiosis
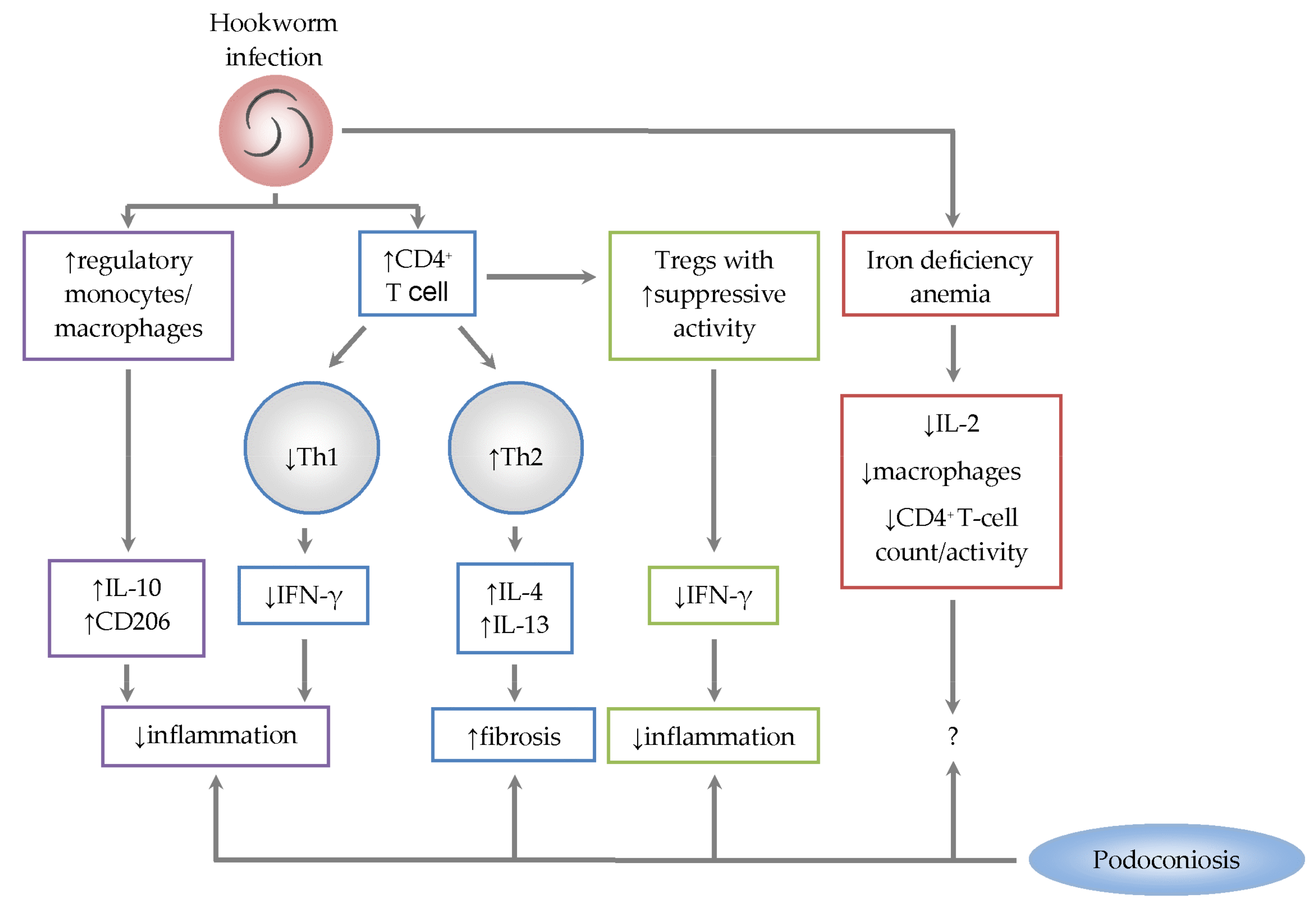
3. Immunological Role of Hookworm Co-Infection in Podoconiosis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Davey, G.; Tekola, F.; Newport, M.J. Podoconiosis: Non-infectious geochemical elephantiasis. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Korevaar, D.A.; Visser, B.J. Podoconiosis, a neglected tropical disease. Neth. J. Med. 2012, 70, 210–214. [Google Scholar] [PubMed]
- Molyneux, D.H. Tropical lymphedemas—Control and prevention. N. Engl. J. Med. 2012, 366, 1169–1171. [Google Scholar] [CrossRef] [PubMed]
- Tora, A.; Franklin, H.; Deribe, K.; Reda, A.A.; Davey, G. Extent of podoconiosis-related stigma in Wolaita Zone, southern Ethiopia: A cross-sectional study. SpringerPlus 2014, 3, 647. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.; Deribe, K.; Tamiru, A.; Amberbir, T.; Medhin, G.; Malik, M.; Hanlon, C.; Davey, G. Depression and disability in people with podoconiosis: A cross-sectional study in rural northern Ethiopia. Int. Health 2016, 8, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Tekola, F.; Mariam, D.H.; Davey, G. Economic costs of endemic non-filarial elephantiasis in Wolaita Zone, Ethiopia. Trop. Med. Int. Health 2006, 11, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Tamiru, A.; Tsegay, G.; Wubie, M.; Gedefaw, M.; Tomczyk, S.; Tekola-Ayele, F. Podoconiosis patients’ willingness to pay for treatment services in northwest Ethiopia: Potential for cost recovery. BMC Public Health 2014, 14, 259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deribe, K.; Cano, J.; Newport, M.J.; Pullan, R.L.; Noor, A.M.; Enquselassie, F.; Murray, C.J.L.; Hay, S.I.; Brooker, S.J.; Davey, G. The global atlas of podoconiosis. Lancet 2017, 5, e477. [Google Scholar] [CrossRef]
- Deribe, K.; Cano, J.; Giorgi, E.; Pigott, D.M.; Golding, N.; Pullan, R.L.; Noor, A.M.; Cromwell, E.A.; Osgood-Zimmerman, A.; Enquselassie, F.; et al. Estimating the number of cases of podoconiosis in Ethiopia using geostatistical methods. Wellcome Open Res. 2017, 2, 78. [Google Scholar] [CrossRef] [PubMed]
- Deribe, K.; Cano, J.; Newport, M.J.; Golding, N.; Pullan, R.L.; Sime, H.; Gebretsadik, A.; Assefa, A.; Kebebe, A.; Hailu, A.; et al. Mapping and modelling the geographical distribution and environmental limits of podoconiosis in Ethiopia. PLoS Negl. Trop. Dis. 2015, 9, e0003946. [Google Scholar] [CrossRef] [PubMed]
- Second Edition of National Neglected Tropical Diseases Master Plan; Federal Ministry of Health: Addis Ababa, Ethiopia, 2016.
- Deribe, K.; Brooker, S.J.; Pullan, R.L.; Sime, H.; Gebretsadik, A.; Assefa, A.; Kebede, A.; Hailu, A.; Rebollo, M.P.; Shafi, O.; et al. Epidemiology and individual, household and geographical risk factors of podoconiosis in Ethiopia: Results from the first nationwide mapping. Am. J. Trop. Med. Hyg. 2015, 92, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Price, E.W.; Henderson, W.J. The elemental content of lymphatic tissues of barefooted people in Ethiopia, with reference to endemic elephantiasis of the lower legs. Trans. R. Soc. Trop. Med. Hyg. 1978, 72, 132–136. [Google Scholar] [CrossRef]
- Molla, Y.B.; Wardrop, N.A.; Le Blonde, J.S.; Baxter, P.; Newport, M.J.; Atkinson, P.M.; Davey, G. Modelling environmental factors correlated with podoconiosis: A geospatial study of non-filarial elephantiasis. Int. J. Health Geogr. 2014, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Patial, R.K.; Nazim, S.; Patial, T.; Rathor, P.K.; Mohan, N. Occupational podoconiosis. J. Assoc. Physicians India 2013, 61, 680. [Google Scholar] [PubMed]
- Spooner, N.T.; Davies, J.E. The possible role of soil particles in the aetiology of non-filarial (endemic) elephantiasis: A macrophage cytotoxicity assay. Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 222–225. [Google Scholar] [CrossRef]
- Ayele, F.T.; Adeyemo, A.; Finan, C.; Hailu, E.; Sinnott, P.; Burlinson, N.D.; Aseffa, A.; Rotimi, C.N.; Newport, M.J.; Davey, G. HLA class II locus and susceptibility to podoconiosis. N. Engl. J. Med. 2012, 366, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Tekola, F.; Ayele, Z.; HalleMariam, D.; Fuller, C.; Davey, G. Development of a de novo clinical staging system for podoconiosis (endemic non-filarial elephantiasis). Trop. Med. Int. Health 2008, 13, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Brooker, S.; Bethony, J.M.; Bottazzi, M.E.; Loukas, A.; Xiao, S. Hookworm infection. N. Engl. J. Med. 2004, 351, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.K.; Mawson, A.R. Neglected tropical diseases: Epidemiology and global burden. Trop. Med. Infect. Dis. 2017, 2, 36. [Google Scholar] [CrossRef]
- Ngui, R.; Lim, Y.A.L.; Traub, R.; Mahmud, R.; Mistam, M.S. Epidemiological and genetic data supporting the transmission of Ancylostoma ceylanicum among human and domestic animals. PLoS Negl. Trop. Dis. 2012, 6, e1522. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Hookworm. 2017. Available online: https://www.cdc.gov/dpdx/hookworm/index.html (accessed on 10 March 2018).
- Pearson, M.S.; Tribolet, L.; Cantacessi, C.; Periago, M.V.; Valerio, M.A.; Jariwala, A.R.; Hotez, P.; Dimert, D.; Loukas, A.; Bethony, J. Molecular mechanisms of hookworm disease: Stealth, virulence, and vaccines. J. Allergy Clin. Immunol. 2012, 130, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.; Wakelin, D. Worms and Human Disease, 2nd ed.; CABI Publishing: Oxon, UK; New York, NY, USA, 2002; pp. 1–300. [Google Scholar]
- Bartsch, S.M.; Hotez, P.J.; Asti, L.; Zapf, K.M.; Bottazzi, M.E.; Diemert, D.J.; Lee, B.Y. The global economic and health burden of human hookworm infection. PLoS Negl. Trop. Dis. 2016, 10, e0004922. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Estimates Summary Tables. DALYs by Cause, Age and Sex, by WHO Region, 2000–2015; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Taye, B.; Alemayehu, B.; Birhanu, A.; Desta, K.; Addisu, S.; Petros, B.; Davey, G.; Tsegaye, A. Podoconiosis and soil-transmitted helminths (STHs): double burden of neglected tropical diseases in Wolaita zone, rural southern Ethiopia. PLoS Negl. Trop. Dis. 2013, 7, e2128. [Google Scholar]
- Amenu, D. Health impact of intestinal helminth infections among podoconiosis patients. Trends Bacteriol. 2014, 1, 2. [Google Scholar] [CrossRef]
- World Health Organization. Worldwide Prevalence of Anaemia 1993–2005, WHO Global Database on Anaemia; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Short, M.W.; Domagalski, J.E. Iron deficiency anemia: Evaluation and management. Am. Fam. Physician 2013, 87, 98–104. [Google Scholar] [PubMed]
- Navarro, S.; Ferreira, I.; Loukas, A. The hookworm pharmacopoeia for inflammatory diseases. Int. J. Parasitol. 2013, 43, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Gaze, S.T. Experimental human hookworm infection: therapeutic potential. Rep. Parasitol. 2016, 5, 35–41. [Google Scholar] [CrossRef]
- Deribe, K.; Wanji, S.; Shafi, O.; Tukahebwa, E.M.; Umulisa, I.; Molyneux, D.H.; Davey, G. The feasibility of eliminating podoconiosis. Bull. World Health Organ. 2015, 93, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Tarique, A.A.; Logan, J.; Thomas, E.; Holt, P.G.; Sly, P.D.; Fantino, E. Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. Am. J. Respir. Cell Mol. Biol. 2015, 53, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Alisi, A.; Carpino, G.; Oliveira, F.L.; Panera, N.; Nobili, V.; Gaudio, E. The role of tissue macrophage-mediated inflammation on NAFLD pathogenesis and its clinical implications. Mediat. Inflamm. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H. A mechanistic review of silica-induced inhalation toxicity. Inhal. Toxicol. 2015, 27, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.I.; Waksman, J.; Curtis, J. Silicosis: A review. Dis. Mon. 2007, 53, 394–416. [Google Scholar] [CrossRef] [PubMed]
- Kolahian, S.; Fernandez, I.E.; Eickelberg, O.; Hertl, D. Immune mechanisms in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2016, 55, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Price, E.W. The site of lymphatic blockage in endemic (non-filarial) elephantiasis of the lower legs. J. Trop. Med. Hyg. 1977, 80, 230–237. [Google Scholar] [PubMed]
- Price, E.W. The pathology of non-filarial elephantiasis of the lower leg. Trans. R. Soc. Trop. Med. Hyg. 1972, 66, 150–156. [Google Scholar] [CrossRef]
- Wendemagegn, E.; Tirumalae, R.; Boer-Auer, A. Histopathological and immunohistochemical features of nodular podoconiosis. J. Cutan. Path. 2015, 42, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Addisu, S.; El-Metwally, T.H.; Davey, G.; Worku, Y.; Titheradge, M.A. The role of transforming growth factor-β1 and oxidative stress in podoconiosis pathogenesis. Br. J. Dermatol. 2010, 162, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Fibrotic disease and the TH1/TH2 paradigm. Nat. Rev. Immunol. 2004, 4, 5835–5894. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Hesse, M.; Sandler, N.G.; Kaviratne, M.; Hoffmann, K.F.; Chiaramonte, M.G.; Reiman, R.; Cheever, A.W.; Sypek, J.P.; Mentink-Kane, M.M. P-selectin suppresses hepatic inflammation and fibrosis in mice by regulating interferon γ and the IL-13 decoy receptor. Hepatology 2004, 39, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, D.D.; Whitters, M.J.; Fitz, L.J.; Neben, T.Y.; Finnerty, H.; Henderson, S.L.; O’Hara, R.M., Jr.; Beier, D.R.; Turner, K.J.; Wood, C.R.; et al. The murine IL-13 receptor α2: Molecular cloning, characterization, and comparison with murine IL-13 receptor α11. J. Immunol. 1998, 161, 2317–2324. [Google Scholar] [PubMed]
- Chiaramonte, M.G.; Donaldson, D.D.; Cheever, A.W.; Wynn, T.A. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J. Clin. Investig. 1999, 104, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Chiaramonte, M.G.; Mentink-Kane, M.; Jacobson, B.A.; Cheever, A.W.; Whitters, M.J.; Goad, M.E.P.; Wong, A.; Collins, M.; Donaldson, D.D.; Grusby, M.J.; et al. Regulation and function of the interleukin 13 receptor α2 during a T helper cell type 2-dominant immune response. J. Exp. Med. 2003, 197, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Chiaramonte, M.G.; Cheever, A.W.; Malley, J.D.; Donaldson, D.D.; Wynn, T.A. Studies of murine schistosomiasis reveal interleukin-13 blockade as a treatment for established and progressive liver fibrosis. Hepatology 2001, 34, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Taube, C.; Duez, C.; Cui, Z.H.; Takeda, K.; Rha, Y.H.; Park, J.W.; Balhorn, A.; Donaldson, D.D.; Dakhama, A.; Gelfand, E.W. The role of IL-13 in established allergic airway disease. J. Immunol. 2002, 169, 6482–6489. [Google Scholar] [CrossRef] [PubMed]
- Verrecchia, F.; Mauviel, A. Transforming growth factor-β and fibrosis. World J. Gastroenterol. 2007, 13, 3056–3062. [Google Scholar] [CrossRef] [PubMed]
- Borish, L.C.; Steinke, J.W. Cytokines and chemokines. J. Allergy Clin. Immunol. 2003, 111, 460–475. [Google Scholar] [CrossRef]
- Liu, F.; Dai, W.; Li, C.; Lu, X.; Chen, Y.; Weng, D.; Chen, J. Role of IL-10-producing regulatory B cells in modulating T-cell immune responses during silica-induced lung inflammation and fibrosis. Sci. Rep. 2016, 6, 28911. [Google Scholar] [CrossRef] [PubMed]
- Dondji, B.; Sun, T.; Bungiro, R.D.; Vermeire, J.J.; Harrison, M.L.; Bifulco, C.; Cappello, M. CD4+ T cells mediate mucosal and systemic immune responses to experimental hookworm infection. Parasite Immunol. 2010, 32, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Quinnell, R.J.; Pritchard, D.I.; Raiko, A.; Brown, A.P.; Shaw, M. Immune responses in human necatoriasis: Association between interleukin-5 responses and resistance to reinfection. J. Infect. Dis. 2004, 190, 430–438. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wright, V.; Bickle, Q. Immune responses following experimental human hookworm infection. Clin. Exp. Immunol. 2005, 142, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Gaze, S.; McSorley, H.J.; Daveson, J.; Jones, D.; Bethony, J.M.; Oliveira, L.M.; Speare, R.; McCarthy, J.S.; Engwerda, C.R.; Croese, J.; et al. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog. 2012, 8, e1002520. [Google Scholar] [CrossRef] [PubMed]
- Gaze, S.; Bethony, J.M.; Periago, M.W. Immunology of experimental and natural human hookworm infection. Parasite Immunol. 2014, 36, 358–366. [Google Scholar] [CrossRef] [PubMed]
- McSorley, H.J.; Loukas, A. The immunology of human hookworm infections. Parasite Immunol. 2010, 32, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Passos, L.S.A.; Gazzinelli-Guimaraes, P.H.; Mendes, T.A.O.; Guimarães, A.C.G.; Lemos, D.S.; Ricci, N.D.; Gonçalves, R.; Bartholomeu, D.C.; Fujiwara, R.T.; Bueno, L.L. Regulatory monocytes in helminth infections: Insights from the modulation during human hookworm infection. BMC Infect. Dis. 2017, 17, 253. [Google Scholar] [CrossRef] [PubMed]
- Wammes, L.J.; Hamid, F.; Wiria, A.E.; De Gier, B.; Sartono, E.; Maizels, R.M.; Luty, A.J.; Fillié, Y.; Brice, G.T.; Supali, T.; et al. Regulatory T cells in human geohelminth infection suppress immune responses to BCG and Plasmodium falciparum. Eur. J. Immunol. 2010, 40, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Rőszer, T. Understanding the mysterious M2 macrophage throughactivation markers and effector mechanisms. Mediators Inflamm. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Hesse, M.; Modolell, M.; La Flamme, A.C.; Schito, M.; Fuentes, J.M.; Cheever, A.W.; Pearce, E.J.; Wynn, T.A. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: Granulomatous pathology is shaped by the pattern of L-arginine metabolism. J. Immunol. 2001, 167, 6533–6544. [Google Scholar] [CrossRef] [PubMed]
- Loukas, A.; Prociv, P. Immune responses in hookworm infections. Clin. Microbiol. Rev. 2001, 14, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.J. Interactions between helminth parasites and allergy. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Shimogawara, R.; Hata, N.; Schuster, A.; Lesshafft, H.; De Oliveira, G.; Ignatius, R.; Akao, N.; Ohta, N.; Feldmeier, H. Hookworm-related cutaneous larva migrans in patients living in an endemic community in Brazil: Immunological patterns before and after ivermectin treatment. Eur. J. Microbiol. Immunol. 2013, 4, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Demmouche, A. Iron deficiency anemia in children and alteration of the immune system. J. Nutr. Food Sci. 2014, 4, 333. [Google Scholar]
- Özcan, A.; Çakmak, M.; Toraman, A.R.; Çolak, A.; Yazgan, H.; Demirdöven, M.; Yokuş, O.; Gürel, A. Evaluation of leucocyte and its subgroups in irondeficiency anemia. Int. J. Med. Med. Sci. 2011, 3, 135–138. [Google Scholar]
- Shichino, S.; Abe, J.; Ueha, S.; Otsuji, M.; Tsukui, T.; Kosugi-Kanaya, M.; Shand, F.H.; Hashimoto, S.; Suzuki, H.I.; Morikawa, T.; et al. Reduced supply of monocyte-derived macrophages leads to a transition from nodular to diffuse lesions and tissue cell activation in silica-induced pulmonary fibrosis in mice. Am. J. Pathol. 2015, 185, 2923–2938. [Google Scholar] [CrossRef] [PubMed]
- Das, I.; Saha, K.; Mukhopadhyay, D.; Roy, S.; Raychaudhuri, G.; Chatterjee, M.; Mitra, P.K. Impact of iron deficiency anemia on cell-mediated and humoral immunity in children: A case control study. J. Nat. Sci. Biol. Med. 2014, 5, 158–163. [Google Scholar] [PubMed]
- Rafieemehr, H.; Rafiee, M.; Mahmoodi, M. Association between percentage of TCD4 and TCD8 lymphocytes with iron status in female adolescents. Iran. J. Blood Cancer 2017, 9, 59–63. [Google Scholar]
- Niedermeier, M.; Reich, B.; Gomez, M.R.; Denzel, A.; Schmidbauer, K.; Göbel, N.; Talke, Y.; Schweda, F.; Mack, M. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc. Nat. Assoc. Sci. USA 2009, 106, 17892–17897. [Google Scholar] [CrossRef] [PubMed]
- Puoti, M.; Bonacini, M.; Spinetti, A.; Putzolu, V.; Govindarajan, S.; Zaltron, S.; Favret, M.; Callea, F.; Gargiulo, F.; Donato, F.; et al. Liver fibrosis progression is related to CD4 cell depletion in patients coinfected with hepatitis C virus and human immunodeficiency virus. J. Infect. Dis. 2001, 183, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Mastroianni, C.M.; Lichtner, M.; Mascia, C.; Zuccalà, P.; Vullo, V. Molecular mechanisms of liver fibrosis in HIV/HCV coinfection. Int. J. Mol. Sci. 2014, 15, 9184–9208. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Famakinde, D.O.; Adenusi, A.A. Involvement of Hookworm Co-Infection in the Pathogenesis and Progression of Podoconiosis: Possible Immunological Mechanism. Trop. Med. Infect. Dis. 2018, 3, 37. https://doi.org/10.3390/tropicalmed3020037
Famakinde DO, Adenusi AA. Involvement of Hookworm Co-Infection in the Pathogenesis and Progression of Podoconiosis: Possible Immunological Mechanism. Tropical Medicine and Infectious Disease. 2018; 3(2):37. https://doi.org/10.3390/tropicalmed3020037
Chicago/Turabian StyleFamakinde, Damilare O., and Adedotun A. Adenusi. 2018. "Involvement of Hookworm Co-Infection in the Pathogenesis and Progression of Podoconiosis: Possible Immunological Mechanism" Tropical Medicine and Infectious Disease 3, no. 2: 37. https://doi.org/10.3390/tropicalmed3020037
APA StyleFamakinde, D. O., & Adenusi, A. A. (2018). Involvement of Hookworm Co-Infection in the Pathogenesis and Progression of Podoconiosis: Possible Immunological Mechanism. Tropical Medicine and Infectious Disease, 3(2), 37. https://doi.org/10.3390/tropicalmed3020037





