Lymphatic Filariasis Increases Tissue Compressibility and Extracellular Fluid in Lower Limbs of Asymptomatic Young People in Central Myanmar
Abstract
:1. Introduction
2. Materials and Methods
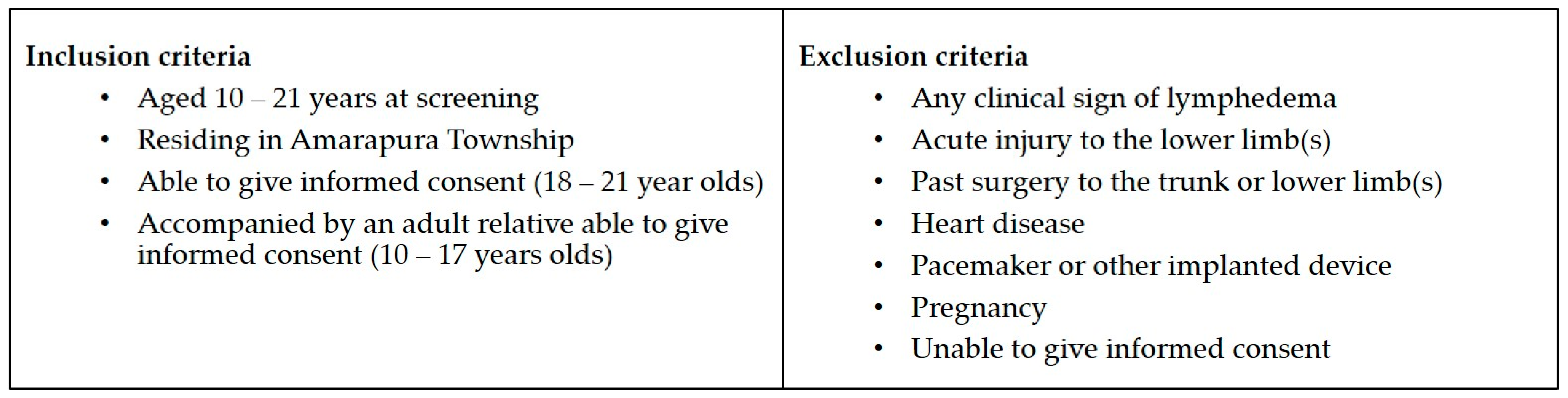
2.1. Study Site Selection, Participant Recruitment, and Screening
2.2. Screening and Baseline Data Collection
2.2.1. Device Measures
2.2.2. Blood Collection and Processing/Storage
2.3. Data Analysis
3. Results
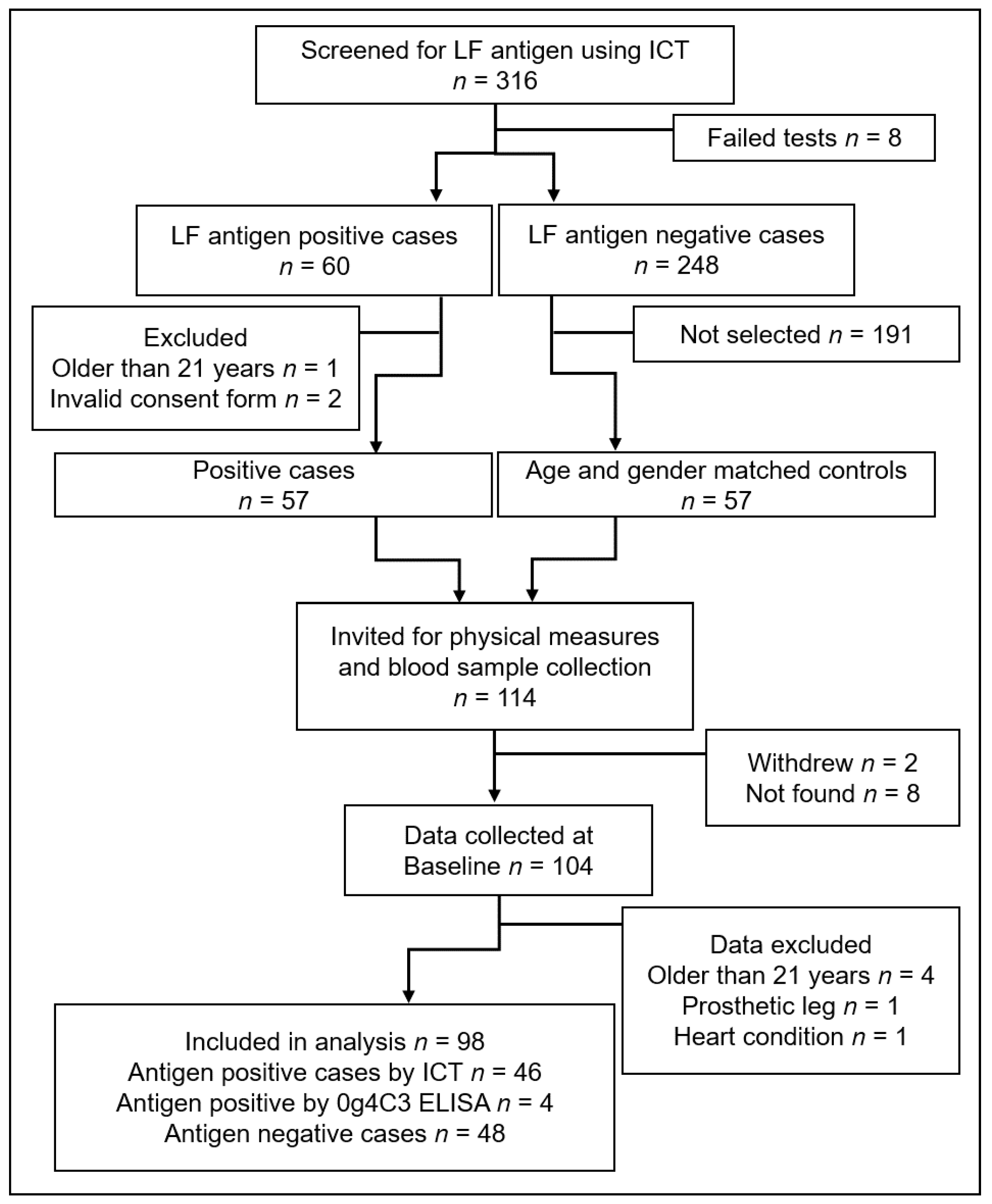
3.1. Participants
3.1.1. Participant Characteristics
3.2. Moderating Factors Associated with Device Measures
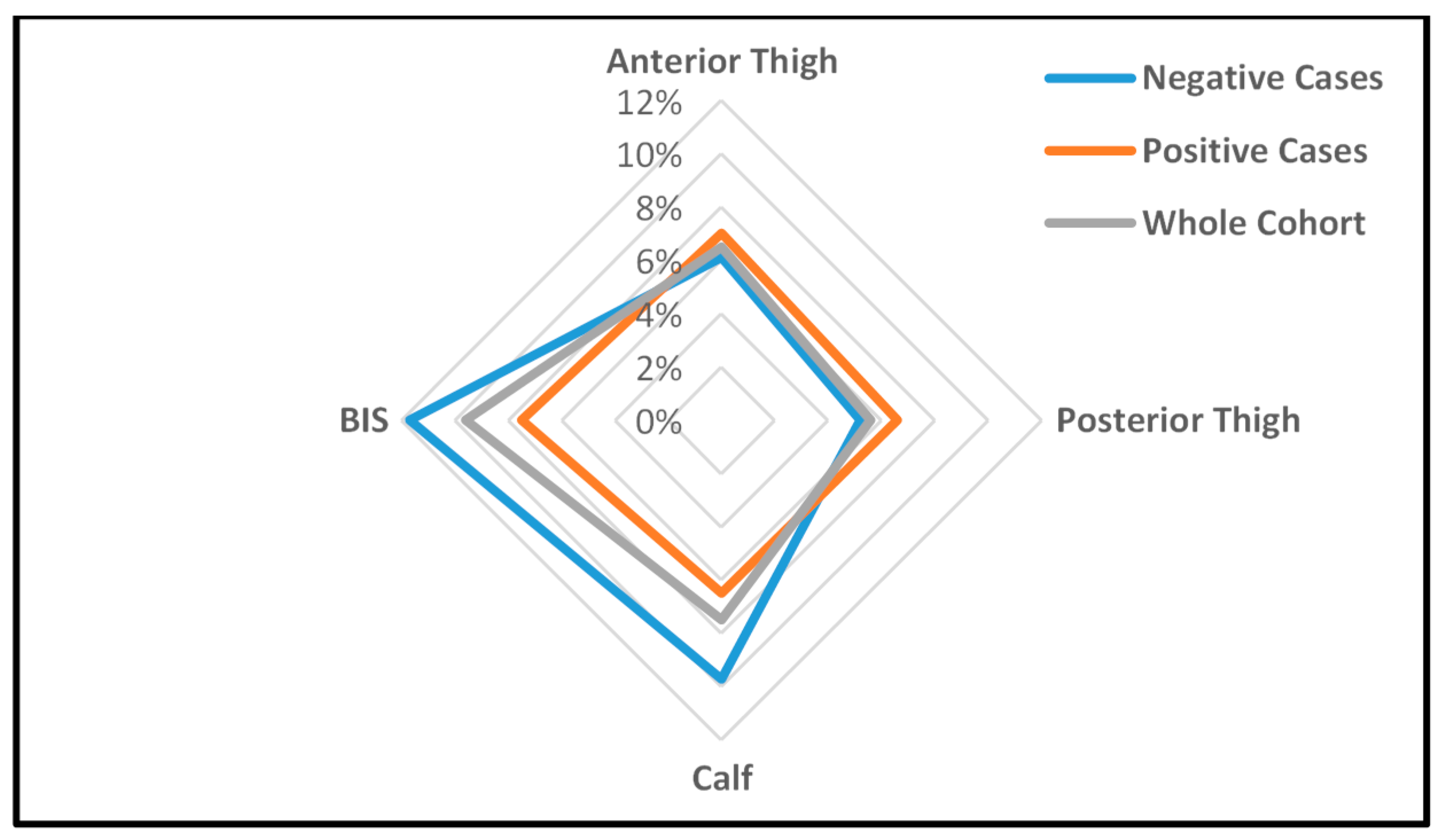
3.2.1. Effect of Infection on Device Measures
3.2.2. Effect of Infection Status, Age, Gender, Body Composition, and Hydration on Device Measures
3.3. Patterns of Tissue Compressibility and Free Fluid in Dominant and Non-Dominant Legs
4. Discussion
Supplementary Materials
Acknowledgments
- Louise Kelly-Hope, Centre for Neglected Tropical Diseases, Liverpool School of Tropical Medicine for early advice on the study design and country selection.
- Myanmar Ministry of Health and Sports, for permission to conduct the study, translation of participant information documents and data collection support.
- Vector Borne Disease Control, Mandalay, for access to sentinel site records and providing research assistants.
- Public Health Laboratory and Staff, Mandalay, for blood separation and short-term storage of plasma.
- Department of Medical Research and Staff, Yangon, for long term storage of plasma and processing of Og4C3 ELISAQ assays.
- Impedimed Australia, for loan of an SFB7 back-up unit and donation of electrodes.
- Delfin Finland, for loan of a SkinFibroMeter.
- JCU Physiotherapy, for use of a Tonometer and Indurometer.
- Cellabs Australia, for Og4C3 reagents.
- Pentagon Freight, for provision of international freight services.
- Singapore International Airlines, for discounted airfares.
- Kyaw San Tun, Mandalay, for interpretation and transport services.
Author Contributions
Conflicts of Interest
References
- World Health Organization. Wha50.29 elimination of lymphatic filariasis as a public health problem. In World Health Assembly Resolutions and Decisions, 3rd ed.; Ninth Plenary Meeting, 13 May 1997—Committee A, Third Report; Hbk, R., Ed.; World Health Organization: Geneva, Switzerland, 1997; Volume III. [Google Scholar]
- Guyton, A.C.; Hall, J.E. Textbook of Medical Physiology, 11th ed.; Elselvier Inc.: Philadelphia, PA, USA, 2006. [Google Scholar]
- International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2016 consensus document of the international society of lymphology. Lymphology 2016, 49, 170–184. [Google Scholar]
- Stout Gergich, N.L.; Pfalzer, L.A.; McGarvey, C.; Springer, B.; Gerber, L.H.; Soballe, P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer 2008, 112, 2809–2819. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, G.; Addiss, D.; Dreyer, P.; Noroes, J. Basic Lymphoedema Management, Treatment and Prevention Problems Associated with Lymphatic Filariasis; Hollis Publishing Company: Hollis, NH, USA, 2002. [Google Scholar]
- Shenoy, R.K. Clinical and pathological aspects of filarial lymphedema and its management. Korean J. Parasitol. 2008, 46, 119–125. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Progress Report 2000–2009 and Strategic Plan 2010–2020 of the Global Programme to Eliminate Lymphatic Filariasis: Halfway towards Eliminating Lymphatic Filariasis; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Addiss, D.G. Mass treatment of filariasis in New Guinea. N. Engl. J. Med. 2003, 348, 1179–1181. [Google Scholar] [PubMed]
- Douglass, J.; Graves, P.; Gordon, S. Self-care for management of secondary lymphedema: A systematic review. PLoS Negl. Trop. Dis. 2016, 10, e0004740. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Melrose, W.; Warner, J.; Buttner, P.; Ward, L. Lymphatic filariasis: A method to identify subclinical lower limb change in PNG adolescents. PLoS Negl. Trop. Dis. 2011, 5, e1242. [Google Scholar] [CrossRef] [PubMed]
- Douglass, J.; Graves, P.; Gordon, S. Intrarater reliability of tonometry and bioimpedance spectroscopy to measure tissue compressibility and extracellular fluid in the legs of healthy young people in Australia and Myanmar. Lymphat. Res. Biol. 2017, 15, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Lawenda, B.D.; Mondry, T.E.; Johnstone, P.A.S. Lymphedema: A primer on the identification and management of a chronic condition in oncologic treatment. CA Cancer J. Clin. 2009, 59, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Douglass, J.; Graves, P.; Gordon, S. Moderating factors in tissue tonometry and bio-impedance spectroscopy measures in the lower extremity of healthy young people in Australia and Myanmar. Lym. Res. Biol. 2017. in submit. [Google Scholar]
- Biomath. Available online: http://biomath.info/power/ttestnoninf.htm (accessed on 14 August 2017).
- Pallotta, O.; McEwen, M.; Tilley, S.; Wonders, T.; Waters, M.; Piller, N. A new way to assess superficial changes to lymphoedema. J. Lymphoedema 2011, 6, 34–40. [Google Scholar]
- Masson, J.; Douglass, J.; Roineau, M.; Aye, K.; Htwe, K.; Warner, J.; Graves, P. Relative performance and predictive values of plasma and dried blood spots with filter paper sampling techniques and dilutions of the lymphatic filariasis Og4c3 antigen ELISA for samples from Myanmar. Trop. Med. Infect. Dis. 2017, 2, 7. [Google Scholar] [CrossRef]
- Onis, M.D.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.C.; Janda, M.; Cornish, B.; Battistutta, D.; Newman, B. Lymphedema after breast cancer: Incidence, risk factors, and effect on upper body function. J. Clin. Oncol. 2008, 26, 3536–3542. [Google Scholar] [CrossRef] [PubMed]
- Nutman, T.B. Insights into the pathogenesis of disease in human lymphatic filariasis. Lymphat. Res. Biol. 2013, 11, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.A. Lymphedema and subclinical lymphostasis (microlymphedema) facilitate cutaneous infection, inflammatory dermatoses, and neoplasia: A locus minoris resistentiae. Clin. Dermatol. 2014, 32, 599–615. [Google Scholar] [CrossRef] [PubMed]
| LF Antigen-Positive Cases | LF Antigen-Negative Controls | Mean Diff (95% CI) | p= | |
|---|---|---|---|---|
| n = 50 | n = 48 | |||
| Age in years—mean (SD) | 15.20 (3.38) | 15.48 (3.46) | 0.28 (−1.09, 1.07) | 0.691 a |
| Gender | ||||
| Female n (%) | 27 (54%) | 28 (58%) | 0.410 b | |
| Male n (%) | 23 (46%) | 20 (42%) | 0.410 b | |
| Height in cm—mean (SD) | 151.80 (12.56) | 152.20 (11.52) | 0.399 (−4.44, 5.24) | 0.870 a |
| Weight in kg—mean (SD) | 42.27 (12.81) | 42.30 (10.12) | 0.028 (−4.617, 4.670) | 0.990 a |
| BMI in kg/m2—mean (SD) | 18.05 (3.46) | 18.03 (2.65) | −0.012 (−1.239, 1.216) | 0.985 a |
| Body composition n = (%) | 0.976 c | |||
| Median weight | 41 (82%) | 40 (83%) | ||
| Underweight > −2SD | 7 (14%) | 6 (13%) | ||
| Overweight > +1SD | 2 (4%) | 2 (4%) | ||
| Dominant leg right/left | 47/3 | 47/1 | 0.324 b | |
| Occupation n = (%) | 0.395 c | |||
| Student | 14 (28%) | 13 (27%) | ||
| Working/other | 32/4 (72%) | 34/1 (73%) | ||
| Drank liquid n = 97 | 0.590 c | |||
| <60 min | 13 (26%) | 12 (26%) | ||
| >60 min | 37 (74%) | 35 (74%) (1 NA) | ||
| Consumed 2013 MDA n (%) | 17 (34%) | 22 (46%) | 0.383 c |
| Measurement Point Indurometer | Positive n = 50 | Negative n = 48 | Mean Difference (%) | Direction in Positive Cases | p= |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | ||||
| Dominant anterior thigh | 4.80 (0.76) | 4.72 (0.69) | 0.05 (1.1%) | Softer | 0.731 |
| Non-dominant anterior thigh | 5.10 (0.88) | 5.00 (0.69) | 0.10 (1.9%) | Softer | 0.546 |
| Dominant posterior thigh | 4.13 (0.93) | 4.06 (0.87) | 0.07 (1.7%) | Softer | 0.701 |
| Non-dominant posterior thigh | 3.88 (0.83) | 3.86 (0.95) | 0.02 (0.4%) | Softer | 0.933 |
| Dominant calf | 2.91 (0.57) | 2.70 (0.68) | 0.21 (7.8%) | Softer | 0.096 |
| Non-dominant calf | 2.73 (0.65) | 2.46 (0.65) | 0.27 (11.1%) *,# | Softer | 0.021 |
| BIS | |||||
| Dominant leg n = 47/45 | 2.44 (0.46) | 2.56 (0.45) | 0.12 (4.9%) # | More fluid | 0.220 |
| Non-dominant leg n = 46/44 | 2.62 (0.56) | 2.86 (0.59) | 0.24 (9.2%) # | More fluid | 0.053 |
| Indurometer | BIS | ||||||
|---|---|---|---|---|---|---|---|
| Higher Values = Increased Tissue Compressibility | Lower Values = Increased ECF | ||||||
| Posterior Thigh B (SE) | Calf B (SE) | Whole Leg B (SE) | |||||
| Factor | Dominant | Non−dominant | Dominant | Non−dominant | Dominant | Non−dominant | |
| Step 1 | R2= | 0.002 | 0.000 | 0.029 | 0.054 | 0.017 | 0.042 |
| Antigen Positive | 0.070 (0.182) | 0.015 (0.180) | 0.212 (0.126) | 0.272 (0.116) * | −0.117 (0.095) | −0.238 (0.122) | |
| Step 2 | R2= | 0.189 | 0.187 | 0.283 | 0.269 | 0.283 | 0.398 |
| Antigen Positive | 0.093 (0.168) | 0.049 (0.166) | 0.234 (0.111) * | 0.286 (0.104) ** | −0.108 (0.083) | −0.210 (0.099) * | |
| Gender = Female | 0.751 (0.178) ** | 0.679 (0.175) ** | 0.639 (0.117) ** | 0.492 (0.110) ** | 0.230 (0.087) ** | 0.485 (0.103) ** | |
| Older age | 0.022 (0.025) | 0.041 (0.025) | 0.010 (0.017) | 0.024 (0.016) | −0.051 (0.012) ** | −0.061 (0.015) ** | |
| Underweight | 0.136 (0.250) | 0.277 (0.247) | −0.052 (0.165) | −0.094 (0.155) | −0.237 (0.120) | −0.302 (0.142) * | |
| Less Recent Hydration | −0.338 (0.177) | −0.223 (0.174) | −0.139 (0.117) | −0.239 (0.110) * | 0.107 (0.085) | 0.124 (0.101) | |
| Indurometer (n = 98) | BIS (n = 90) | |||
|---|---|---|---|---|
| Anterior Thigh | Posterior Thigh | Calf | Whole Leg | |
| Dominant leg mean (SD) | 4.74 (0.72) | 4.10 (0.90) | 2.81 (0.63) | 2.50 (0.46) |
| Non−dominant leg mean (SD) | 5.05 (0.79) | 3.87 (0.89) | 2.60 (0.59) | 2.74 (0.59) |
| Mean difference (SD) | −0.31 (0.31) | 0.23 (0.23) | 0.21 (0.21) | −0.24 (0.32) |
| 95% CI of the difference | −0.41, −0.21 | 0.11, 0.35 | 0.13, 0.28 | −0.31, −0.17 |
| % difference | 6.5% ** | 5.6% ** | 7.5% ** | 9.6% **,# |
| Direction (dominant leg) | Harder | Softer | Softer | More fluid |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douglass, J.; Graves, P.; Lindsay, D.; Becker, L.; Roineau, M.; Masson, J.; Aye, N.N.; Win, S.S.; Wai, T.; Win, Y.Y.; et al. Lymphatic Filariasis Increases Tissue Compressibility and Extracellular Fluid in Lower Limbs of Asymptomatic Young People in Central Myanmar. Trop. Med. Infect. Dis. 2017, 2, 50. https://doi.org/10.3390/tropicalmed2040050
Douglass J, Graves P, Lindsay D, Becker L, Roineau M, Masson J, Aye NN, Win SS, Wai T, Win YY, et al. Lymphatic Filariasis Increases Tissue Compressibility and Extracellular Fluid in Lower Limbs of Asymptomatic Young People in Central Myanmar. Tropical Medicine and Infectious Disease. 2017; 2(4):50. https://doi.org/10.3390/tropicalmed2040050
Chicago/Turabian StyleDouglass, Janet, Patricia Graves, Daniel Lindsay, Luke Becker, Maureen Roineau, Jesse Masson, Ni Ni Aye, San San Win, Tint Wai, Yi Yi Win, and et al. 2017. "Lymphatic Filariasis Increases Tissue Compressibility and Extracellular Fluid in Lower Limbs of Asymptomatic Young People in Central Myanmar" Tropical Medicine and Infectious Disease 2, no. 4: 50. https://doi.org/10.3390/tropicalmed2040050
APA StyleDouglass, J., Graves, P., Lindsay, D., Becker, L., Roineau, M., Masson, J., Aye, N. N., Win, S. S., Wai, T., Win, Y. Y., & Gordon, S. (2017). Lymphatic Filariasis Increases Tissue Compressibility and Extracellular Fluid in Lower Limbs of Asymptomatic Young People in Central Myanmar. Tropical Medicine and Infectious Disease, 2(4), 50. https://doi.org/10.3390/tropicalmed2040050






