Prevalence of Antimicrobial and Colistin Resistance in Enterobacterales in Healthy Pigs in Ghana Before and After Farmer Education
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. General Setting
2.3. Site-Specific Setting
2.4. Sampling
2.5. Study Population
2.6. Specimen Processing and Laboratory Procedures
2.6.1. Bacterial Isolation
2.6.2. Antimicrobial Susceptibility Testing
2.6.3. Molecular Detection of mcr-1 Gene
2.7. Data Variables and Sources of Data
2.8. Data Analysis
3. Results
3.1. Characteristics of the Pigs, Farms, and Antimicrobials Used in the Greater Accra Region of Ghana in 2022 and 2024
3.2. Enterobacterales of Healthy Pigs in 2022 and 2024
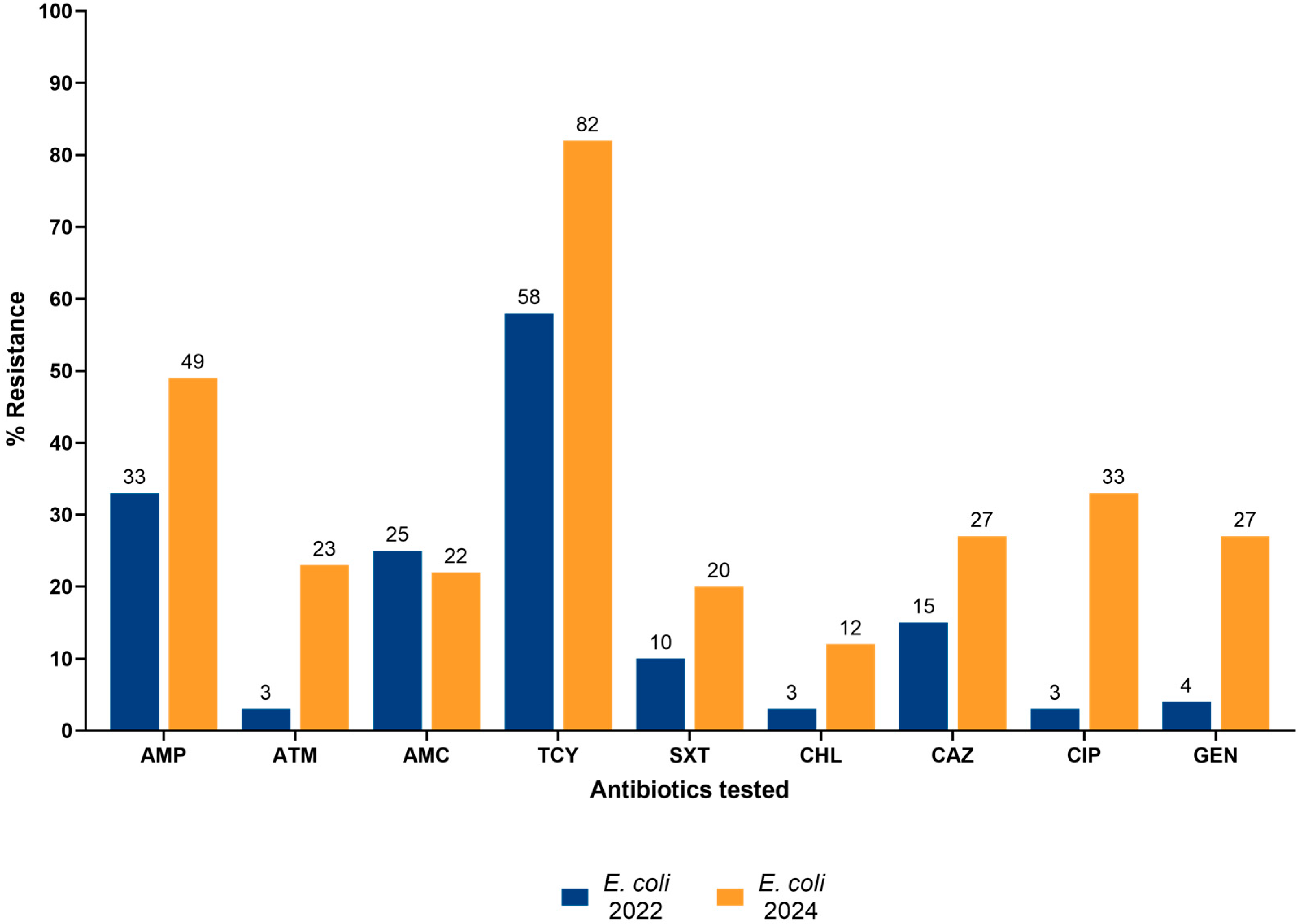
3.3. AMR and Colistin Resistance of Enterobacterales
3.4. Characteristics Associated with MDR and Colistin Resistance in 2024
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Founou, L.L.; Founou, R.C.; Ntshobeni, N.; Govinden, U.; Bester, L.A.; Chenia, H.Y.; Djoko, C.F.; Essack, S.Y. Emergence and Spread of Extended Spectrum β-Lactamase Producing Enterobacteriaceae (ESBL-PE) in Pigs and Exposed Workers: A Multicentre Comparative Study between Cameroon and South Africa. Pathogens 2019, 8, 10. [Google Scholar] [CrossRef]
- Ikwap, K.; Gertzell, E.; Hansson, I.; Dahlin, L.; Selling, K.; Magnusson, U.; Dione, M.; Jacobson, M. The Presence of Antibiotic-Resistant Staphylococcus spp. and Escherichia coli in Smallholder Pig Farms in Uganda. BMC Vet. Res. 2021, 17, 31. [Google Scholar] [CrossRef]
- Kimera, Z.I.; Mgaya, F.X.; Misinzo, G.; Mshana, S.E.; Moremi, N.; Matee, M.I. Multidrug-Resistant, Including Extended-Spectrum Beta Lactamase-Producing and Quinolone-Resistant, Escherichia coli Isolated from Poultry and Domestic Pigs in Dar Es Salaam, Tanzania. Antibiotics 2021, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Effelsberg, N.; Kobusch, I.; Linnemann, S.; Hofmann, F.; Schollenbruch, H.; Mellmann, A.; Boelhauve, M.; Köck, R.; Cuny, C. Prevalence and Zoonotic Transmission of Colistin-Resistant and Carbapenemase-Producing Enterobacterales on German Pig Farms. One Health 2021, 13, 100354. [Google Scholar] [CrossRef]
- Samir, A.; Abdel-Moein, K.A.; Zaher, H.M. Predominance of Enterotoxigenic Escherichia coli among ESBL/Plasmid-Mediated AmpC-Producing Strains Isolated from Diarrheic Foals: A Public Health Concern. Acta Vet. Scand. 2024, 66, 54. [Google Scholar] [CrossRef] [PubMed]
- Michael, C.A.; Dominey-Howes, D.; Labbate, M. The Antimicrobial Resistance Crisis: Causes, Consequences, and Management. Front. Public Health 2014, 2, 145. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Madec, J.-Y.; Haenni, M.; Nordmann, P.; Poirel, L. Extended-Spectrum β-Lactamase/AmpC- and Carbapenemase-Producing Enterobacteriaceae in Animals: A Threat for Humans? Clin. Microbiol. Infect. 2017, 23, 826–833. [Google Scholar] [CrossRef]
- Poirel, L.; Madec, J.-Y.; Lupo, A.; Schink, A.-K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Ling, Z.; Yin, W.; Shen, Z.; Wang, Y.; Shen, J.; Walsh, T.R. Epidemiology of Mobile Colistin Resistance Genes Mcr-1 to Mcr-9. J. Antimicrob. Chemother. 2020, 75, 3087–3095. [Google Scholar] [CrossRef]
- Jaja, I.F.; Oguttu, J.; Jaja, C.-J.I.; Green, E. Prevalence and Distribution of Antimicrobial Resistance Determinants of Escherichia coli Isolates Obtained from Meat in South Africa. PLoS ONE 2020, 15, e0216914. [Google Scholar] [CrossRef]
- Ngbede, E.O.; Poudel, A.; Kalalah, A.; Yang, Y.; Adekanmbi, F.; Adikwu, A.A.; Adamu, A.M.; Mamfe, L.M.; Daniel, S.T.; Useh, N.M.; et al. Identification of Mobile Colistin Resistance Genes (Mcr-1.1, Mcr-5 and Mcr-8.1) in Enterobacteriaceae and Alcaligenes Faecalis of Human and Animal Origin, Nigeria. Int. J. Antimicrob. Agents 2020, 56, 106108. [Google Scholar] [CrossRef]
- Ohene Larbi, R.; Adeapena, W.; Ayim-Akonor, M.; Ansa, E.D.O.; Tweya, H.; Terry, R.F.; Labi, A.-K.; Harries, A.D. Antimicrobial, Multi-Drug and Colistin Resistance in Enterobacteriaceae in Healthy Pigs in the Greater Accra Region of Ghana, 2022: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 10449. [Google Scholar] [CrossRef]
- Communicating Research Findings. Available online: https://tdr.who.int/activities/sort-it-operational-research-and-training/communicating-research-findings (accessed on 12 July 2024).
- Ghana Statistical Services. Available online: https://www.statsghana.gov.gh/ (accessed on 18 June 2024).
- FAO. Monitoring and Surveillance of Antimicrobial Resistance in Bacteria from Healthy Food Animals Intended for Consumption; FAO: Bangkok, Thailand, 2019. [Google Scholar]
- Sato, W.; Sukmawinata, E.; Uemura, R.; Kanda, T.; Kusano, K.; Kambayashi, Y.; Sato, T.; Ishikawa, Y.; Toya, R.; Sueyoshi, M. Antimicrobial Resistance Profiles and Phylogenetic Groups of Escherichia coli Isolated from Healthy Thoroughbred Racehorses in Japan. J. Equine Sci. 2020, 31, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Cheesbrough, M. Monica Medical Manual for Tropical Countries; Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Performance Standards for Antimicrobial Susceptibility Testing, 34th Edition. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 10 July 2024).
- OIE. OIE Standards, Guidelines and Resolution on Antimicrobial Resistance and the Use of Antimicrobial Agents, 2nd ed.; OIE: Paris, France, 2015; ISBN 978-92-95115-06-4. [Google Scholar]
- Collignon, P.C.; Conly, J.M.; Andremont, A.; McEwen, S.A.; Aidara-Kane, A.; for the World Health Organization Advisory Group, Bogotá Meeting on Integrated Surveillance of Antimicrobial Resistance (WHO-AGISAR); Agerso, Y.; Andremont, A.; Collignon, P.; Conly, J.; et al. World Health Organization Ranking of Antimicrobials According to Their Importance in Human Medicine: A Critical Step for Developing Risk Management Strategies to Control Antimicrobial Resistance From Food Animal Production. Clin. Infect. Dis. 2016, 63, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Abdul Momin, M.H.F.; Bean, D.C.; Hendriksen, R.S.; Haenni, M.; Phee, L.M.; Wareham, D.W. CHROMagar COL-APSE: A Selective Bacterial Culture Medium for the Isolation and Differentiation of Colistin-Resistant Gram-Negative Pathogens. J. Med. Microbiol. 2017, 66, 1554–1561. [Google Scholar] [CrossRef]
- Aanensen, D.M.; Huntley, D.M.; Menegazzo, M.; Powell, C.I.; Spratt, B.G. EpiCollect+: Linking Smartphones to Web Applications for Complex Data Collection Projects. F1000Research 2014, 3, 199. [Google Scholar] [CrossRef]
- John, O.S. Surveillance of Antimicrobial Resistance in Pigs Using Escherichia coli Faecal Isolates. CIB Technol. 2014, 3, 78–83. [Google Scholar]
- Lalruatdiki, A.; Dutta, T.K.; Roychoudhury, P.; Subudhi, P.K. Extended-Spectrum β-Lactamases Producing Multidrug Resistance Escherichia coli, Salmonella and Klebsiella Pneumoniae in Pig Population of Assam and Meghalaya, India. Vet. World 2018, 11, 868. [Google Scholar] [CrossRef]
- Olu-Taiwo, M.A.; Egyir, B.; Owusu-Nyantakyi, C.; Forson, A.O.; Opintan, J.A. Molecular Characterization of Multidrug-Resistant Escherichia coli in the Greater Accra Region, Ghana: A ‘One Health’ Approach. One Health Outlook 2025, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Dogbatse, R.; Folitse, R.; Nyarku, R.E.; Burimuah, V.; Amemor, E.; Emikpe, B.O. Antibiotic Use and Knowledge of Antibiotic Resistance among Pig Farmers in Ejisu and Juaben Municipalities of Ashanti Region, Ghana. PAMJ—One Health 2023, 12. [Google Scholar] [CrossRef]
- Buckel, A.; Afakye, K.; Koka, E.; Price, C.; Kabali, E.; Caudell, M.A. Understanding the Factors Influencing Biosecurity Adoption on Smallholder Poultry Farms in Ghana: A Qualitative Analysis Using the COM-B Model and Theoretical Domains Framework. Front. Vet. Sci. 2024, 11, 1324233. [Google Scholar] [CrossRef]
- Phares, C.A.; Danquah, A.; Atiah, K.; Agyei, F.K.; Michael, O.-T. Antibiotics Utilization and Farmers’ Knowledge of Its Effects on Soil Ecosystem in the Coastal Drylands of Ghana. PLoS ONE 2020, 15, e0228777. [Google Scholar] [CrossRef] [PubMed]
- Sekyere, J.O. Antibiotic Types and Handling Practices in Disease Management among Pig Farms in Ashanti Region, Ghana. J. Vet. Med. 2014, 2014, 531952. [Google Scholar] [CrossRef] [PubMed]
- Boateng, M.; Amoah, K.; Okai, D.; Acheampong, M.; Atuahene, P. Assessment of the Status of Pig Production in the Greater Accra Region of Ghana. Ghana. J. Anim. Sci. 2021, 12, 49–58. [Google Scholar]
- Toya, R.; Sasaki, Y.; Uemura, R.; Sueyoshi, M. Indications and Patterns of Antimicrobial Use in Pig Farms in the Southern Kyushu, Japan: Large Amounts of Tetracyclines Used to Treat Respiratory Disease in Post-Weaning and Fattening Pigs. J. Vet. Med. Sci. 2021, 83, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.d.S.; Duarte, M.E.; Saraiva, A.; de Oliveira, L.L.; Teixeira, L.M.; Rocha, G.C. Effect of Antibiotics and Low-Crude Protein Diets on Growth Performance, Health, Immune Response, and Fecal Microbiota of Growing Pigs. J. Anim. Sci. 2023, 101, skad357. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Okoh, A.I.; Lues, R. Prevalence of Multidrug-Resistant Bacteria (Enteropathogens) Recovered from a Blend of Pig Manure and Pinewood Saw Dust during Anaerobic Co-Digestion in a Steel Biodigester. Int. J. Environ. Res. Public Health 2023, 20, 984. [Google Scholar] [CrossRef]
- Humphrey, M.; Larrouy-Maumus, G.J.; Furniss, R.C.D.; Mavridou, D.A.I.; Sabnis, A.; Edwards, A.M. Colistin Resistance in Escherichia coli Confers Protection of the Cytoplasmic but Not Outer Membrane from the Polymyxin Antibiotic. Microbiology 2021, 167, 001104. [Google Scholar] [CrossRef]
- Huang, X.; Yu, L.; Chen, X.; Zhi, C.; Yao, X.; Liu, Y.; Wu, S.; Guo, Z.; Yi, L.; Zeng, Z.; et al. High Prevalence of Colistin Resistance and Mcr-1 Gene in Escherichia coli Isolated from Food Animals in China. Front. Microbiol. 2017, 8, 562. [Google Scholar] [CrossRef]
- Humphry, R.W.; Evans, J.; Webster, C.; Tongue, S.C.; Innocent, G.T.; Gunn, G.J. An Empirical Comparison of Isolate-Based and Sample-Based Definitions of Antimicrobial Resistance and Their Effect on Estimates of Prevalence. Prev. Vet. Med. 2018, 150, 143–150. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Bastidas-Caldes, C.; Guerrero-Freire, S.; Ortuño-Gutiérrez, N.; Sunyoto, T.; Gomes-Dias, C.A.; Ramírez, M.S.; Calero-Cáceres, W.; Harries, A.D.; Rey, J.; De Waard, J.H.; et al. Colistin Resistance in Escherichia coli and Klebsiella Pneumoniae in Humans and Backyard Animals in Ecuador. Rev. Panam. Salud Pública 2023, 47, 1. [Google Scholar] [CrossRef] [PubMed]
- Lentz, S.A.; de Lima-Morales, D.; Cuppertino, V.M.; Nunes, L.d.S.; da Motta, A.S.; Zavascki, A.P.; Barth, A.L.; Martins, A.F. Letter to the Editor: Escherichia coli Harbouring Mcr-1 Gene Isolated from Poultry Not Exposed to Polymyxins in Brazil. Euro Surveill. 2016, 21, 30267. [Google Scholar] [CrossRef] [PubMed]
- Pillonetto, M.; Mazzetti, A.; Becker, G.N.; Siebra, C.A.; Arend, L.N.; Barth, A.L. Low Level of Polymyxin Resistance among Nonclonal Mcr-1–Positive Escherichia coli from Human Sources in Brazil. Diagn. Microbiol. Infect. Dis. 2019, 93, 140–142. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Zhang, R.; Chen, Y.; Shen, Y.; Hu, F.; Liu, D.; Lu, J.; Guo, Y.; Xia, X. Changes in Colistin Resistance and Mcr-1 Abundance in Escherichia coli of Animal and Human Origins Following the Ban of Colistin-Positive Additives in China: An Epidemiological Comparative Study. Lancet Infect. Dis. 2020, 20, 1161–1171. [Google Scholar] [CrossRef]
- GBD 2021 Antimicrobial Resistance Collaborators. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | 2022 | 2024 | p Value # | |||
|---|---|---|---|---|---|---|
| n | (%) * | n | (%) * | |||
| Pigs | Total | 140 | 140 | |||
| Sex | ||||||
| Male | 75 | (53.6) | 71 | (50.7) | 0.635 | |
| Female | 65 | (46.4) | 69 | (49.3) | ||
| Age in months | Median (IQR) | 4 | (3–5) | 4 | (3–5) | 0.739 |
| Farms | Total | 14 | 14 | |||
| Annual production turnover | ||||||
| 1–100 | 6 | (42.9) | 5 | (35.7) | 0.410 | |
| 101–300 | 5 | (35.7) | 8 | (57.2) | ||
| >300 | 3 | (21.4) | 1 | (7.1) | ||
| Source of farm feed | ||||||
| Commercial feed | 0 | 0 | 1 | (7.1) | 0.501 | |
| Self-made feed | 12 | (85.7) | 10 | (71.5) | ||
| Both | 2 | (14.3) | 3 | (21.4) | ||
| Antimicrobial use in last 12 months | ||||||
| Yes | 14 | (100) | 13 | (92.9) | 0.500 | |
| No | 0 | 0 | 1 | (7.1) | ||
| Variable | 2022 | 2024 | p-Value | ||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Total pig rectal swabs | 140 | (100) | 140 | (100) | |
| Enterobacterales | |||||
| Present | 137 | (97.9) | 138 | (98.6) | 0.999 |
| Absent | 3 | (2.1) | 2 | (1.4) | |
| Type of Enterobacterales | |||||
| Escherichia coli only | 117 | (85.4) | 130 | (94.2) | 0.017 |
| Enterobacter spp. only | 0 | (0) | 0 | (0) | - |
| Escherichia coli and Enterobacter spp. | 20 | (14.6) | 8 | (5.8) | 0.018 |
| Variable | 2022 | 2024 | ||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Total | 137 | (100) | 138 | (100) |
| No MDR | 110 | (80.3) | 77 | (55.8) ** |
| MDR | 27 | (19.7) | 61 | (44.2) ** |
| Resistance to 3 antimicrobial classes | 14 | (10.2) | 18 | (13.0) |
| Resistance to 4 antimicrobial classes | 7 | (5.1) | 8 | (5.8) |
| Resistance to 5 antimicrobial classes | 6 | (4.4) | 8 | (5.8) |
| Resistance to 6 antimicrobial classes | 0 | (0) | 10 | (7.2) |
| Resistance to 7 antimicrobial classes | 0 | (0) | 12 | (8.7) |
| Resistance to 8 antimicrobial classes | 0 | (0) | 3 | (2.2) |
| Resistance to 9 antimicrobial classes | 0 | (0) | 2 | (1.4) |
| Variable | n | (%) |
|---|---|---|
| Total samples | 140 | |
| Phenotypic Colistin resistance | ||
| E. coli | 61 | (43.6) |
| Enterobacter spp. | 5 | (3.6) |
| Molecular Colistin resistance * | ||
| E. coli | 31 | (50.8) ** |
| Enterobacter spp. | 2 | (40.0) ** |
| Variable | Total | MDR Present * | PR | 95% CI | p Value | |
|---|---|---|---|---|---|---|
| n | (%) | |||||
| Pig Characteristics | ||||||
| Total | 138 | 61 | (44.2) | |||
| Sex | ||||||
| Male | 70 | 26 | (37.1) | 1 | ||
| Female | 68 | 35 | (51.5) | 1.4 | (1.0–2.0) | 0.094 |
| Age in months | ||||||
| 2–4 months | 99 | 44 | (44.4) | 1.2 | (0.7–2.2) | 0.446 |
| 5–7 months | 25 | 9 | (36.0) | 1 | ||
| 8–12 months | 14 | 8 | (57.1) | 1.7 | (0.8–3.3) | 0.158 |
| Farm Characteristics | ||||||
| Annual production turnover | ||||||
| 30–100 | 50 | 33 | (66.0) | 2.3 | (1.6–3.5) | <0.001 |
| 101–300 | 78 | 22 | (28.2) | 1 | ||
| >300 | 10 | 6 | (60.0) | 2.1 | (1.2–4.0) | 0.017 |
| Source of farm feed | ||||||
| Commercial | 10 | 6 | (60.0) | 1.9 | (1.1–3.4) | 0.032 |
| Self-made | 98 | 31 | (31.6) | 1 | ||
| Both | 30 | 24 | (80.0) | 2.5 | (1.8–3.6) | <0.001 |
| Change of farm feed from 2022 | ||||||
| Yes | 50 | 30 | (60.0) | 1.7 | (1.2–2.5) | 0.004 |
| No | 88 | 31 | (35.2) | 1 | ||
| Antimicrobial usage | ||||||
| For treatment only | 59 | 23 | (39.0) | 1 | ||
| For prophylaxis only | 49 | 21 | (42.9) | 1.1 | (0.7–1.7) | 0.683 |
| For both prophylaxis and treatment | 20 | 11 | (55.0) | 1.4 | (0.9–2.4) | 0.185 |
| No antimicrobials for prophylaxis or treatment | 10 | 6 | (60.0) | 1.5 | (0.9–2.8) | 0.158 |
| Frequency of prophylaxis * | ||||||
| Monthly | 40 | 23 | (57.5) | 1.2 | (0.7–2.2) | 0.385 |
| Every two months | 20 | 9 | (45.0) | 1 | ||
| Vet consulted for treating sick pigs * | ||||||
| Yes | 39 | 15 | (38.5) | 1 | ||
| No | 40 | 19 | (47.5) | 1.2 | (0.7–2.1) | 0.421 |
| Variable | Total | Colistin Resistance | PR | 95% CI | p Value | |
|---|---|---|---|---|---|---|
| n | (%) | |||||
| Pig Characteristics | ||||||
| Total | 140 | 66 | (47.1) | |||
| Sex | ||||||
| Male | 71 | 39 | (54.9) | 1.4 | (1.0–2.0) | 0.065 |
| Female | 69 | 27 | (39.1) | 1 | ||
| Age in months | ||||||
| 2–4 months | 100 | 48 | (48.0) | 1.6 | (0.8–2.9) | 0.122 |
| 5–7 months | 26 | 8 | (30.8) | 1 | ||
| 8–12 months | 14 | 10 | (71.4) | 2.3 | (1.2–4.5) | 0.018 |
| Farm Characteristics | ||||||
| Annual production turnover | ||||||
| 30–100 | 50 | 27 | (54.0) | 1.8 | (0.7–4.8) | 0.189 |
| 101–300 | 80 | 36 | (45.0) | 1.5 | (0.6–4.0) | 0.396 |
| >300 | 10 | 3 | (30.0) | 1 | ||
| Source of farm feed | ||||||
| Commercial | 10 | 7 | (70.0) | 1.6 | (1.0–2.5) | 0.151 |
| Self-made | 100 | 45 | (45.0) | 1 | ||
| Both | 30 | 14 | (46.7) | 1.0 | (0.7–1.6) | 0.87 |
| Change of farm feed from 2022 | ||||||
| Yes | 50 | 30 | (60.0) | 1.5 | (1.1–2.1) | 0.025 |
| No | 90 | 36 | (40.0) | 1 | ||
| Antimicrobial usage | ||||||
| For treatment only | 60 | 26 | (43.3) | 1.7 | (0.8–3.9) | 0.155 |
| For prophylaxis only | 50 | 28 | (56.0) | 2.2 | (1.0–5.0) | 0.021 |
| For both prophylaxis and treatment | 20 | 5 | (25.0) | 1 | ||
| No antimicrobials for prophylaxis or treatment | 10 | 7 | (70.0) | 2.8 | (1.2–6.6) | 0.027 |
| Frequency of prophylaxis * | ||||||
| Monthly | 40 | 18 | (45.0) | 1 | ||
| Every two months | 20 | 9 | (45.0) | 1 | (0.6–1.8) | 0.998 |
| Every three months | 10 | 6 | (60.0) | 1.5 | (0.7–2.5) | 0.424 |
| Vet consulted for treating sick pigs * | ||||||
| Yes | 40 | 15 | (37.5) | 1 | ||
| No | 40 | 16 | (40.0) | 1.1 | (0.6–1.9) | 0.823 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amegayibor, E.F.; Ohene Larbi, R.; Ayim-Akonor, M.; Ansa, E.D.O.; Thekkur, P.; Owusu, H.; Terry, R.F.; Harries, A.D.; Sasu, B.K.; Hedidor, G.K.; et al. Prevalence of Antimicrobial and Colistin Resistance in Enterobacterales in Healthy Pigs in Ghana Before and After Farmer Education. Trop. Med. Infect. Dis. 2025, 10, 266. https://doi.org/10.3390/tropicalmed10090266
Amegayibor EF, Ohene Larbi R, Ayim-Akonor M, Ansa EDO, Thekkur P, Owusu H, Terry RF, Harries AD, Sasu BK, Hedidor GK, et al. Prevalence of Antimicrobial and Colistin Resistance in Enterobacterales in Healthy Pigs in Ghana Before and After Farmer Education. Tropical Medicine and Infectious Disease. 2025; 10(9):266. https://doi.org/10.3390/tropicalmed10090266
Chicago/Turabian StyleAmegayibor, Elvis Fiam, Rita Ohene Larbi, Matilda Ayim-Akonor, Ebenezer D. O. Ansa, Pruthu Thekkur, Helena Owusu, Robert Fraser Terry, Anthony D. Harries, Benjamin Kissi Sasu, George Kwesi Hedidor, and et al. 2025. "Prevalence of Antimicrobial and Colistin Resistance in Enterobacterales in Healthy Pigs in Ghana Before and After Farmer Education" Tropical Medicine and Infectious Disease 10, no. 9: 266. https://doi.org/10.3390/tropicalmed10090266
APA StyleAmegayibor, E. F., Ohene Larbi, R., Ayim-Akonor, M., Ansa, E. D. O., Thekkur, P., Owusu, H., Terry, R. F., Harries, A. D., Sasu, B. K., Hedidor, G. K., & Mills, R. O. (2025). Prevalence of Antimicrobial and Colistin Resistance in Enterobacterales in Healthy Pigs in Ghana Before and After Farmer Education. Tropical Medicine and Infectious Disease, 10(9), 266. https://doi.org/10.3390/tropicalmed10090266







