Seropositivity for Pathogenic Leptospira in Dogs, Cats, and Horses at a Teaching Veterinary Hospital in Southern Chile
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Serological Samples
2.3. Microscopic Agglutination Test (MAT)
2.4. Interpretation of MAT Results
- (a)
- Dogs: For non-vaccinated animals, titers ≥ 1:100 were classified as positive [39]. For vaccinated dogs, the interpretation depended on the time elapsed since immunization. In dogs vaccinated 1 to 3 months before blood sample collection, titers ≥ 1:400 were considered positive for pathogenic Leptospira spp., while in those vaccinated between 3 to 12 months before sampling, titers ≥ 1:200 were classified as seropositive [25]. The vaccine commonly used in Chile for canine leptospirosis includes the Canicola and Icterohaemorrhagiae serovars. Vaccination data for patients were extracted from their clinical records.
- (b)
- Cats: Titers ≥ 1:100 were classified as positive serological reactions to pathogenic Leptospira spp. [27].
- (c)
2.5. Data Analysis
3. Results
3.1. Seropositivity for Pathogenic Leptospira
3.2. Serogroups Most Frequently Diagnosed in Seropositive Animals
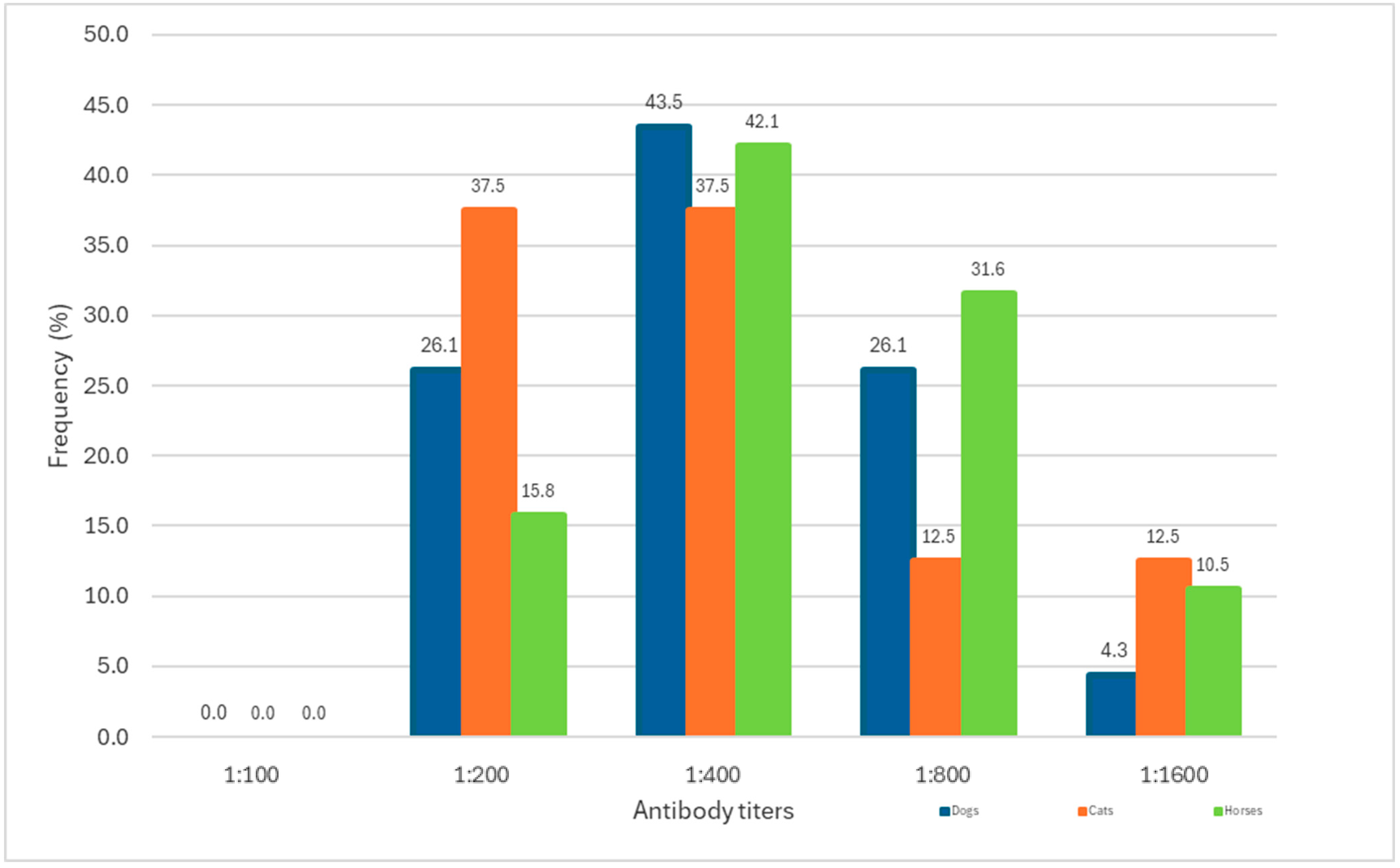
3.3. Antibody Titers Detected in Seropositive Animals
3.4. Characterization of Seropositive Animals by Sex and Age
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MAT | microscopic agglutination test |
| 95% CI | 95% Confidence Interval |
References
- Romero-Vivaz, C.M.; Falconar, A.K. Leptospira spp. y leptospirosis humana. Salud Uninorte 2016, 32, 123–143. [Google Scholar] [CrossRef]
- Garba, B.; Bahaman, A.; Bejo, S.; Zakaria, Z.; Mutalib, A.; Bande, A. Major epidemiological factors associated with leptospirosis in Malaysia. Acta Trop. 2018, 178, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Bouscaren, N.; de Coignac, C.B.; Lastère, S.; Musso, S.; Teissier, Y.; Formont, Y. Leptospirosis in French Polynesia: 11 years of surveillance data, 2007–2017. New Microbes New Infect. 2019, 29, 100518. [Google Scholar] [CrossRef]
- Urbanskas, E.; Karveliene, B.; Radzijevskaja, J. Leptospirosis: Classification, epidemiology, and methods of detection. A review. Biologija 2022, 68, 129–136. [Google Scholar] [CrossRef]
- Coburn, J.; Picardeau, M.; Woods, C.; Veldman, T.; Haake, D. Pathogenesis insights from an ancient and ubiquitous spirochete. PLoS Pathog. 2021, 17, e1009836. [Google Scholar] [CrossRef]
- Schneider, M.; Jancloes, M.; Buss, D.; Aldighieri, S.; Bertherat, E.; Najera, P.; Galan, D.; Durski, K.; Espinal, M. Leptospirosis: A silent epidemic disease. Int. J. Environ. Res. Public Health 2013, 10, 7229–7234. [Google Scholar] [CrossRef]
- Levett, P. Leptospirosis. Clin. Microbiol. Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef]
- Wang, W.; Gao, Y.; Ji, J.; Huang, Z.; Xiong, B.; Xiang, S. Trends and advances in Leptospira, a bibliometric analysis. Front. Microbiol. 2025, 15, 1514738. [Google Scholar] [CrossRef]
- Mathieu, P. Virulence of the zoonotic agent of leptospirosis: Still terra incognita? Nat. Rev. Microbiol. 2017, 15, 297–307. [Google Scholar] [CrossRef]
- Haake, D.A.; Levett, P. Leptospirosis in humans. In Leptospira and Leptospirosis; Springer: Berlin, Germany, 2014. [Google Scholar]
- Azócar-Aedo, L.; Smits, H.; Monti, G. Leptospirosis in dogs and cats: Epidemiology, clinical disease, zoonotic implications, and prevention. Arch. Med. Vet. 2014, 46, 337–348. [Google Scholar] [CrossRef]
- Goarant, C. Leptospirosis: Risk factors and management challenges in developing countries. Res. Rep. Trop. Med. 2016, 7, 49–62. [Google Scholar] [CrossRef]
- Barragan, V.; Olivas, S.; Keim, P.; Pearson, T. Critical knowledge gaps in our understanding of environmental cycling and transmission of Leptospira spp. Appl. Environ. Microbiol. 2017, 83, e01190-17. [Google Scholar] [CrossRef]
- Sykes, J.E.; Francey, T.; Schuller, S.; Stoddard, R.; Cowgill, L.; Moore, G. 2023 Updated ACVIM consensus statement on leptospirosis in dogs. J. Vet. Intern. Med. 2023, 37, 1966–1982. [Google Scholar] [CrossRef]
- Rodríguez, A.; Ferro, B.; Varona, M.; Santafé, M. Exposure to Leptospira in stray dogs in the city of Cali. Biomédica 2004, 24, 291–295. [Google Scholar] [CrossRef]
- Lima, M.; Mittestainer, J.; de Rocha, J.; De Carvalho, R.; Verotti, B.; Pellicciari, P.; Victoria, C.; Langoni, H. Principal zoonoses in small animals: A brief review. Vet. Zootec. 2017, 24, 84–106. [Google Scholar] [CrossRef]
- Bolin, C. Diagnosis of leptospirosis: A reemerging disease of companion animals. Semin. Vet. Med. Surg. Small Anim. 1996, 11, 166–171. [Google Scholar] [CrossRef]
- Murillo, M.; Goris, M.; Ahmed, A.; Cuenca, R.; Pastor, J. Leptospirosis in cats. Current literature review to guide diagnosis and management. J. Feline Med. Surg. 2020, 22, 216–228. [Google Scholar] [CrossRef]
- Ricardo, T.; Azócar-Aedo, L.; Signorini, M.; Previtali, A. Leptospiral infection in domestic cats: Systematic review with meta-analysis. Prev. Vet. Med. 2023, 212, 105851. [Google Scholar] [CrossRef] [PubMed]
- Ellis, W. Animal leptospirosis. Curr. Top. Microbiol. Immunol. 2015, 387, 99–137. [Google Scholar] [CrossRef] [PubMed]
- Malalana, F.; Blundell, R.; Pinchbeck, G.; Mcgowan, C. The role of Leptospira spp. in horses affected with recurrent uveitis in the UK. Equine Vet. J. 2017, 49, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Divers, T.; Chang, Y.; Irby, N.; Smith, J.; Carter, C. Leptospirosis: An important infectious disease in North American horses. Equine Vet. J. 2019, 51, 287–292. [Google Scholar] [CrossRef]
- Uribe, M.; Azócar-Aedo, L.; Gallardo, M. Serosurveillance of pathogenic Leptospira in ruminants from a veterinary teaching hospital. Austral J. Vet. Sci. 2025, 57, e5709. [Google Scholar] [CrossRef]
- Tuemmers, C.; Tüders, C.; Rojas, C.; Serri, M.; Espinoza, R.; Castillo, C. Prevalence of leptospirosis in vague dogs captured in Temuco city, 2011. Rev. Chil. Infect. 2013, 30, 252–257. [Google Scholar] [CrossRef]
- Azócar-Aedo, L.; Monti, G. Seroprevalence of pathogenic Leptospira spp. in domestic dogs from southern Chile and risk factors associated with different environments. Prev. Vet. Med. 2023, 206, 105707. [Google Scholar] [CrossRef]
- Dorsch, R.; Ojeda, J.; Salgado, M.; Monti, G.; Collado, B.; Tomckowiack, C.; Tejeda, C.; Müller, A.; Eberhard, T.; Klaasen, H.; et al. Cats shedding pathogenic Leptospira spp.—An underestimated zoonotic risk? PLoS ONE 2020, 22, e0239991. [Google Scholar] [CrossRef] [PubMed]
- Azócar-Aedo, L.; Monti, G.; Jara, R. Leptospira spp. in domestic cats from different environments: Prevalence of antibodies and risk factors associated with the seropositivity. Animals 2014, 4, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Tuemmers, C.; Quezada, G.; Morales, R.; Serri, M. Seroprevalence of Leptospira spp. in draft horses from indigenous communities in the Araucanía Region, Chile. Rev. Chil. Infectol. 2021, 38, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Moreno, T.; Ramírez, R.; Azócar-Aedo, L. Seroprevalence of pathogenic Leptospira, infecting serogroups and antibody titers detected in horses from Los Lagos region, southern Chile. Chil. J. Agric. Anim. Sci. 2024, 40, 341–352. [Google Scholar] [CrossRef]
- Zamora, J.; Riedemann, S.; Tadich, N. A serological survey of leptospirosis in sheep in Chile. Rev. Latinoam. Microbiol. 1999, 41, 73–76. [Google Scholar]
- Zamora, J.; Kruze, J.; Riedemann, S. Leptospirosis de los animales domésticos en el Sur de Chile. Estudio Serológico. Zoon Public. Health 1975, 22, 544–555. [Google Scholar] [CrossRef]
- Díaz, E.; Arroyo, G.; Sáenz, C.; Mena, L.; Barragán, V. Leptospirosis in horses: Sentinels for a neglected zoonosis? A systematic review. Vet. World 2023, 16, 2110–2119. [Google Scholar] [CrossRef]
- Ricardo, T.; Azócar-Aedo, L.; Monti, G.; Previtali, A. Seroprevalence of pathogenic Leptospira serogroups in asymptomatic domestic dogs and cats: Systematic review and meta-analysis. Front. Vet. Sci. 2024, 11, 1301959. [Google Scholar] [CrossRef]
- OMSA (Organización Mundial de Sanidad Animal). Código Sanitario Para Los Animales Terrestres. 2023. Available online: https://www.woah.org/fileadmin/Home/esp/Health_standards/tahc/current/chapitre_surveillance_general.pdf (accessed on 21 December 2024).
- Rabinowitz, P.; Scotch, M.; Conti, L. Human and animal sentinels for shared health risks. Vet. Ital. 2009, 45, 23–24. [Google Scholar]
- Hernández, R.; Fernández, C.; Baptista, L. Metodología de la Investigación, 6th ed.; Mc Graw Hill Educación: Mexico DF, Mexico, 2011. [Google Scholar]
- Faine, S.; Adler, B.; Bolin, C.; Perolat, P. Leptospira and Leptospirosis, 2nd ed.; MedSci: Melbourne, Australia, 1999. [Google Scholar]
- WHO-ILS. Human Leptospirosis: Guidance for Diagnosis, Surveillance and Control; NLM classification: WC 420; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Miotto, B.A.; Tozzi, B.; De Souza, M.; Alves, A.; Zanolli, L.; Heinemann, M.; Moreno, M.; Lilenbaum, W.; Hagiwara, M. Diagnosis of acute canine leptospirosis using multiple laboratory tests and characterization of isolated strains. BMC Vet. Res. 2018, 14, 222. [Google Scholar] [CrossRef]
- Tapia, A. Frecuencia de Presentación de Sueros Reaccionantes a Leptospira interrogans y Leptospira borgpetersenii en una Población de Equinos de tiro Urbano de la Región Metropolitana de Chile. Bachelor’s Thesis, Universidad de Chile, Santiago, Chile, 2014. [Google Scholar]
- Bolwell, C.; Adams, B.; Collins-Emerson, J.; Scarfe, K.; Nisa, S.; Gordon, E.; Rogers, C.; Benschop, J. Longitudinal testing of Leptospira antibodies in horses located near a leptospirosis outbreak in alpacas. Vet. Sci. 2022, 9, 426. [Google Scholar] [CrossRef]
- Romanowski, T.; Días, R.; Heinemann, M.; Carvalho, S.; Silva, T.; Martins, A.; Caetano, G.; Ferreira, J.; Santos, J.; Borsanelli, A. Seroprevalence of equine leptospirosis in the state of Goiás, Brazil. Vet. Sci. 2023, 10, 590. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Riedemann, S. Frequency of canine leptospirosis in dogs attending veterinary practices determined through Microscopic Agglutination Test and comparison with isolation and immunofluorescence techniques. Arch. Med. Vet. 2007, 39, 269–274. [Google Scholar] [CrossRef]
- Beaglehole, R.; Bonita, R.; Kjellstrõm, T. Basic Epidemiology; PAHO and WHO: Washington, DC, USA, 2003. [Google Scholar]
- Santiago, M.; Hervada, H.; Naveira, G.; Silva, C.; Fariñas, H.; Vásquez, E.; Bacallao, J.; Mujica, O. El programa EPIDAT: Usos y perspectivas. Rev. Panam. Salud Publica 2010, 27, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Noordhuizen, J.; Frankena, K.; Van der Hoofd, C.; Graat, E. Application of Quantitative Methods in Veterinary Epidemiology, 1st ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 1997. [Google Scholar]
- Vogt, A.; Rodan, I.; Brown, M.; Brown, S.; Buffington, C.; Forman, M.; Neilson, J.; Sparkes, A. AAFP-AAHA: Feline life stage guidelines. J. Feline Med. Surg. 2010, 12, 43–54. [Google Scholar] [CrossRef]
- Harvey, N. How Old Is My Dog? Identification of rational age groupings in pet dogs based upon normative age-linked processes. Front. Vet. Sci. 2010, 8, 643085. [Google Scholar] [CrossRef]
- Ricardo, T.; Bazán, L.; Beltramini, L.; Prieto, Y.; Montiel, A.; Margenet, L.; Schmeling, F.; Chiani, J.; Signorini, M.; Previtali, M. Seroprevalence of Leptospira antibodies in dogs and cats from Santa Fe, a city in East-Central Argentina endemic for leptospirosis. Prev. Vet. Med. 2014, 229, 106239. [Google Scholar] [CrossRef]
- Cruz, J.; Sánchez, A.; Vera, L. Characterization and prevalence of oral diseases in creole horses, Department of Caldas, Colombia. Rev. Med. Vet. 2012, 23, 39–50. [Google Scholar]
- Hazra, A.; Gogtay, N. Biostatistics series module 4: Comparing groups- categorical variables. Indian. J. Dermatol. 2016, 61, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P. Companion animals as sentinels for public health. Vet. Clin. Small Anim. 2009, 39, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Lelu, M.; Muñoz-Zanzi, C.; Higgins, B.; Galloway, R. Seroepidemiology of leptospirosis in dogs from rural and slum communities of Los Ríos Region, Chile. BMC Vet. Res. 2015, 11, 31. [Google Scholar] [CrossRef]
- Guzmán, D.; Diaz, E.; Sáenz, C.; Álvarez, H.; Cueva, R.; Zapata-Ríos, G.; Prado-Vivar, B.; Falconí, M.; Pearson, T.; Barragan, V. Domestic dogs in indigenous Amazonian communities: Key players in Leptospira cycling and transmission? PLOS Neg. Trop. Dis. 2024, 18, e0011671. [Google Scholar] [CrossRef]
- McCreight, K.; Barbosa, L.; Odoi, A.; Reed, P.; Rajeev, S. Leptospira seroprevalence in dogs, cats, and horses in Tennessee, USA. J. Vet. Diagn. Investig. 2024, 37, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Sohn-Hausner, N.; Kmetiuk, L.; da Silva, E.; Langoni, H.; Biondo, A. One Health approach to leptospirosis: Dogs as environmental sentinels for identification and monitoring of human risk areas in Southern Brazil. Trop. Med. Infect. Dis. 2023, 8, 435. [Google Scholar] [CrossRef]
- Toro, A. Determinación a Través del Test de ELISA de Anticuerpos Contra Leptospira en Felinos Pacientes del Hospital Clínico Veterinario de la Universidad de Concepción en Chillán. Bachelor’s Thesis, Universidad de Concepción, Chillán, Chile, 2002. [Google Scholar]
- Azócar-Aedo, L. Global prevalence and epidemiology of leptospirosis in domestic cats, a systematic review and meta-analysis. Vet. Mex. OA 2022, 9, e1129. [Google Scholar] [CrossRef]
- Miotto, B.; Camelo, Q.; Miranda, A.; Mendes de Oliveira, A.; Barreto, M.; Hagiwara, M.; Bergmann, S. Current knowledge on leptospirosis in cats: A systematic review with metanalysis on direct detection, serological response, and clinical data. Res. Vet. Sci. 2024, 167, 105292. [Google Scholar] [CrossRef]
- Bay-Schmith, N. Prevalencia de Leptospirosis Equina en Caballos Jugadores de Polo de la Octava Región de Chile. Bachelor’s Thesis, Universidad de Concepción, Chillán, Chile, 2004. [Google Scholar]
- Troncoso, I.; Toro, I.; Guzmán, A.; Fuentealba, J.; Fischer, K. Serological evaluation of Leptospira interrogans in horses from an equestrian center in Linares province, Chile. CES Med. Vet. Zootec. 2013, 8, 101–107. [Google Scholar]
- Tadich, T.; Tapia, C.; González, D. Seroprevalence of Leptospira spp. in working horses located in the central region of Chile. J. Equine Vet. Sci. 2016, 38, 14–18. [Google Scholar] [CrossRef]
- Orlando, S.; Páez, K.; Sánchez, E.; De la Cruz, C.; Calderón, J.; Arcos, F.; Torres-Lasso, P.; Calvopiña, M.; Garcia-Bereguiain, M. Racehorses from a breeding farm in Tropical Ecuador have a high seroprevalence of anti-Leptospira spp. antibodies: A paradigm for leptospirosis management from a One Health perspective. Front. Trop. Dis. 2023, 4, 1061038. [Google Scholar] [CrossRef]
- Simbizi, B.; Saulez, M.; Potts, A.; Lötter, C.; Gummow, B. A study of leptospirosis in South African horses and associated risk factors. Prev. Vet. Med. 2016, 134, 6–15. [Google Scholar] [CrossRef]
- Baharom, M.; Ahmad, N.; Hod, R.; Ha’afar, M.; Arsad, F.; Tangang, F.; Ismail, R.; Mohamed, N.; Radi, M.; Osma, Y. Environmental and occupational factors associated with leptospirosis: A systematic review. Heliyon 2024, 10, e23473. [Google Scholar] [CrossRef]
- Nurfitri, F.; Sukesi, T.; Mulasari, S. Risk factor analysis of leptospirosis incidence in endemic areas. Adv. Healthc. Res. 2025, 3, 297–313. [Google Scholar] [CrossRef]
- Talukder, H.; Muñoz-Zanzi, C.; Salgado, M.; Berg, S.; Yang, A. Identifying the drivers related to animal reservoirs, environment, and socio-demography of human leptospirosis in different community types of Southern Chile: An application of machine learning algorithm in One Health perspective. Pathogens 2024, 13, 687. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, P.; Scotch, M.; Conti, L. Animals as sentinels: Using comparative medicine to move beyond the laboratory. ILAR J. 2010, 51, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Scotch, M.; Odofin, L.; Rabinowitz, P. Linkages between animal and human health sentinel data. BMC Vet. Res. 2009, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Cilia, G.; Bertelloni, F.; Angelini, M.; Cerri, D.; Fratini, F. Leptospira survey in wild boar (Sus. scrofa) hunted in Tuscany, central Italy. Pathogens 2020, 9, 377. [Google Scholar] [CrossRef]
- Cilia, G.; Bertelloni, F.; Fratini, F. Leptospira infections in domestic and wild animals. Pathogens 2020, 9, 573. [Google Scholar] [CrossRef]
- Balcázar, L.; Azócar-Aedo, L.; Barrera, V.; Meniconi, G.; Muñoz, V.; Valencia-Soto, C. Detection of antibodies for pathogenic Leptospira in wild mammals and birds from southern Chile—First record of seropositivity in a guiña (Leopardus guigna). Animals 2024, 14, 601. [Google Scholar] [CrossRef]
- Luna, A.; Moles, C.; Gavaldón, R.; Nava, V.; Salazar, G. La leptospirosis canina y su problemática en México. Rev. Salud Anim. 2008, 30, 1–11. [Google Scholar]
- Ojeda, J.; Salgado, M.; Encina, C.; Santamaria, C.; Monti, G. Evidence of interspecies transmission of pathogenic Leptospira between livestock and a domestic cat dwelling in a dairy cattle farm. J. Vet. Med. Sci. 2018, 80, 1305–1308. [Google Scholar] [CrossRef]
- Wegmann, E. Leptospirosis en caballos. Arch. Med. Vet. 1983, 15, 59–64. [Google Scholar]
- WHO (World Health Organization). Report of the First Meeting of the Leptospirosis Burden Epidemiology Reference Group. Available online: https://www.paho.org/en/node/44086 (accessed on 28 August 2025).
- Greene, C.; Sykes, J.; Brown, C.; Hartmann, K. Leptospirosis. In Enfermedades Infecciosas del Perro y el Gato; Greene, C., Ed.; Intermédica: Buenos Aires, Argentina, 2008. [Google Scholar]
- Riedemann, S.; Zamora, J.; Cabezas, X. Detección de aglutininas antileptospira en sueros de roedores silvestres del área rural de Valdivia, Chile. Av. Cienc. Vet. 1994, 9, 1. [Google Scholar] [CrossRef]
- Luna, J.; Salgado, M.; Tejeda, C.; Moroni, M.; Monti, G. Assessment of risk factors in synanthropic and wild rodents infected by pathogenic Leptospira spp. captured in Southern Chile. Animals 2020, 10, 2133. [Google Scholar] [CrossRef]
- Correa, J.; Bucarey, S.; Cattan, P.; Landaeta-Aqueveque, L.; Ramírez-Estrada, J. Renal carriage of Leptospira species in rodents from Mediterranean Chile: The Norway rat (Rattus norvegicus) as a relevant host in agricultural lands. Acta Trop. 2017, 176, 105–108. [Google Scholar] [CrossRef] [PubMed]
- MINSAL (Ministerio de Salud). Decreto 7. Aprueba el Reglamento Sobre Notificación de Enfermedades Transmisibles de Declaración Obligatoria y su Vigilancia; Ministerio de Salud: Santiago, Chile, 2020. [Google Scholar]
- MINSAL (Ministerio de Salud). Informe Epidemiológico Anual Leptospirosis, Chile; Ministerio de Salud: Santiago, Chile, 2021. [Google Scholar]

| Serogroups | Serovars | Strain |
|---|---|---|
| Pomona | Pomona | Pomona |
| Canicola | Canicola | Hond Utrech IV |
| Icterohaemorrhagiae | Icterohaemorrhagiae | Icterohaemorrhagiae |
| Autumnalis | Autumnalis | Akiyami A |
| Australis | Bratislava | Jez Bratislava |
| Sejroe | Hardjo | Ar |
| Tarassovi | Tarassovi | Perepelitsin |
| Grippotyphosa | Grippotyphosa | Sag |
| Antibody Titers | |||||||
|---|---|---|---|---|---|---|---|
| Animal Species | Serogroups | 1:100 | 1:200 | 1:400 | 1:800 | 1:1600 | Total |
| Dogs | Canicola | 1 | 0 | 1 | 0 | 0 | 2 |
| Tarassovi | 0 | 5 | 8 | 6 | 1 | 20 | |
| Canicola-Tarassovi | 0 | 0 | 1 | 0 | 0 | 1 | |
| Cats | Tarassovi | 0 | 3 | 3 | 1 | 1 | 8 |
| Horses | Canicola | 0 | 1 | 2 | 0 | 0 | 3 |
| Tarassovi | 0 | 2 | 4 | 4 | 2 | 12 | |
| Grippotyphosa | 0 | 0 | 1 | 0 | 0 | 1 | |
| Sejroe | 0 | 0 | 1 | 1 | 0 | 2 | |
| Icterohaemorrhagiae | 0 | 0 | 0 | 1 | 0 | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azócar-Aedo, L.; Meniconi, G.; Pino-Olguín, C.; Gallardo, M. Seropositivity for Pathogenic Leptospira in Dogs, Cats, and Horses at a Teaching Veterinary Hospital in Southern Chile. Trop. Med. Infect. Dis. 2025, 10, 253. https://doi.org/10.3390/tropicalmed10090253
Azócar-Aedo L, Meniconi G, Pino-Olguín C, Gallardo M. Seropositivity for Pathogenic Leptospira in Dogs, Cats, and Horses at a Teaching Veterinary Hospital in Southern Chile. Tropical Medicine and Infectious Disease. 2025; 10(9):253. https://doi.org/10.3390/tropicalmed10090253
Chicago/Turabian StyleAzócar-Aedo, Lucía, Gloria Meniconi, Carolina Pino-Olguín, and María Gallardo. 2025. "Seropositivity for Pathogenic Leptospira in Dogs, Cats, and Horses at a Teaching Veterinary Hospital in Southern Chile" Tropical Medicine and Infectious Disease 10, no. 9: 253. https://doi.org/10.3390/tropicalmed10090253
APA StyleAzócar-Aedo, L., Meniconi, G., Pino-Olguín, C., & Gallardo, M. (2025). Seropositivity for Pathogenic Leptospira in Dogs, Cats, and Horses at a Teaching Veterinary Hospital in Southern Chile. Tropical Medicine and Infectious Disease, 10(9), 253. https://doi.org/10.3390/tropicalmed10090253





