Beyond the Usual Suspects: Weeksella virosa as a Potential Human and Animal Pathogen
Abstract
1. Introduction
2. Weeksella virosa—A Brief Taxonomic History and Emerging Genetic Perspectives
2.1. A Brief Taxonomic History of W. virosa
2.2. Genomic Perspectives
3. Weeksella virosa: A Rare Pathogen in Humans
4. Challenges in the Microbiological Diagnosis of Weeksella virosa
4.1. Phenotypical Characteristics
4.2. Biochemical Characteristics
4.3. Laboratory Diagnosis of W. virosa
5. Antibiotic Resistance and Treatment Challenges
| Reference/Antibiotic | Mardy et al., 1988 [4] | Reina et al., 1990 [5] | Fass et al., 1996 [63] | Tamayo et al., 2003 [7] | Manogaran et al., 2004 [10] | Slenker et al., 2012 [8] | Toescu et al., 2017 [32] | Unalan et al., 2019 [14] | Vaquera-Aparicio et al., 2020 [33] | Campbell et al., 2020 [34] | de la Fuente García Peña et al., 2024 [18] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of isolates (n) | n = 6 | n = 3 | n = 8 | n = 1 | n = 1 | n = 1 | n = 1 | n = 1 | n = 1 | n = 1 | n = 1 |
| AST technique | DD | MIC | MIC | DD | n/a | MIC | n/a | MIC | MIC | MIC | DD and MIC |
| Penicillin | S | ||||||||||
| Ampicillin | S | S ^ | R | S | S | ||||||
| Ampicillin–Sulbactam | S | R | |||||||||
| Amoxicillin | S | ||||||||||
| Amoxicillin–Clavulanic Acid | S | ||||||||||
| Carbenicillin | S | ||||||||||
| Ticarcillin | S | ||||||||||
| Azlocillin | S | ||||||||||
| Mezlocillin | S | ||||||||||
| Piperacillin | S | S | S | S | S | ||||||
| Piperacillin–Tazobactam | S | S | S | R | |||||||
| Cefazolin | S | # | |||||||||
| Cefuroxime | S | # | S | ||||||||
| Cefoxitin | S | # | R | ||||||||
| Cefotaxime | S | R | # | R | |||||||
| Ceftriaxone | S | S | S | S | # | R | R | ||||
| Ceftazidime | S | S | S | S | S | # | R | I | R | ||
| Cefoperazone | S | # | |||||||||
| Cefepime | S | # | R | R | R | ||||||
| Aztreonam | S | S | S | ||||||||
| Imipenem | S | S | R | S ^^ | S ^^ | S | R | ||||
| Meropenem | S | S | S | S | R | ||||||
| Ertapenem | R | ||||||||||
| Doripenem | R | ||||||||||
| Streptomycin | S | ||||||||||
| Neomycin | R | ||||||||||
| Kanamycin | R | R | |||||||||
| Gentamicin | R | R | R | S | R | S | R | ||||
| Tobramycin | R | R * | R | R | |||||||
| Amikacin | R | R | R | I | S | R | |||||
| Nalidixic Acid | R | R | |||||||||
| Norfloxacin | S | ||||||||||
| Ciprofloxacin | S | S | R | R | R | R | I | S | S | ||
| Ofloxacin | S | ||||||||||
| Levofloxacin | R | ||||||||||
| Tetracycline | S | R | S | S | |||||||
| Tigecycline | I | ||||||||||
| Trimethoprim | R | ||||||||||
| Trimethoprim–Sulfamethoxazole | R | I ** | S | R | |||||||
| Erythromycin | S | ||||||||||
| Polymyxin B | |||||||||||
| Colistin | S | ||||||||||
| Cetrimide | |||||||||||
| Nitrofurantoin | R | ||||||||||
| Chloramphenicol | S | S | S | ||||||||
| Novobiocin | S |
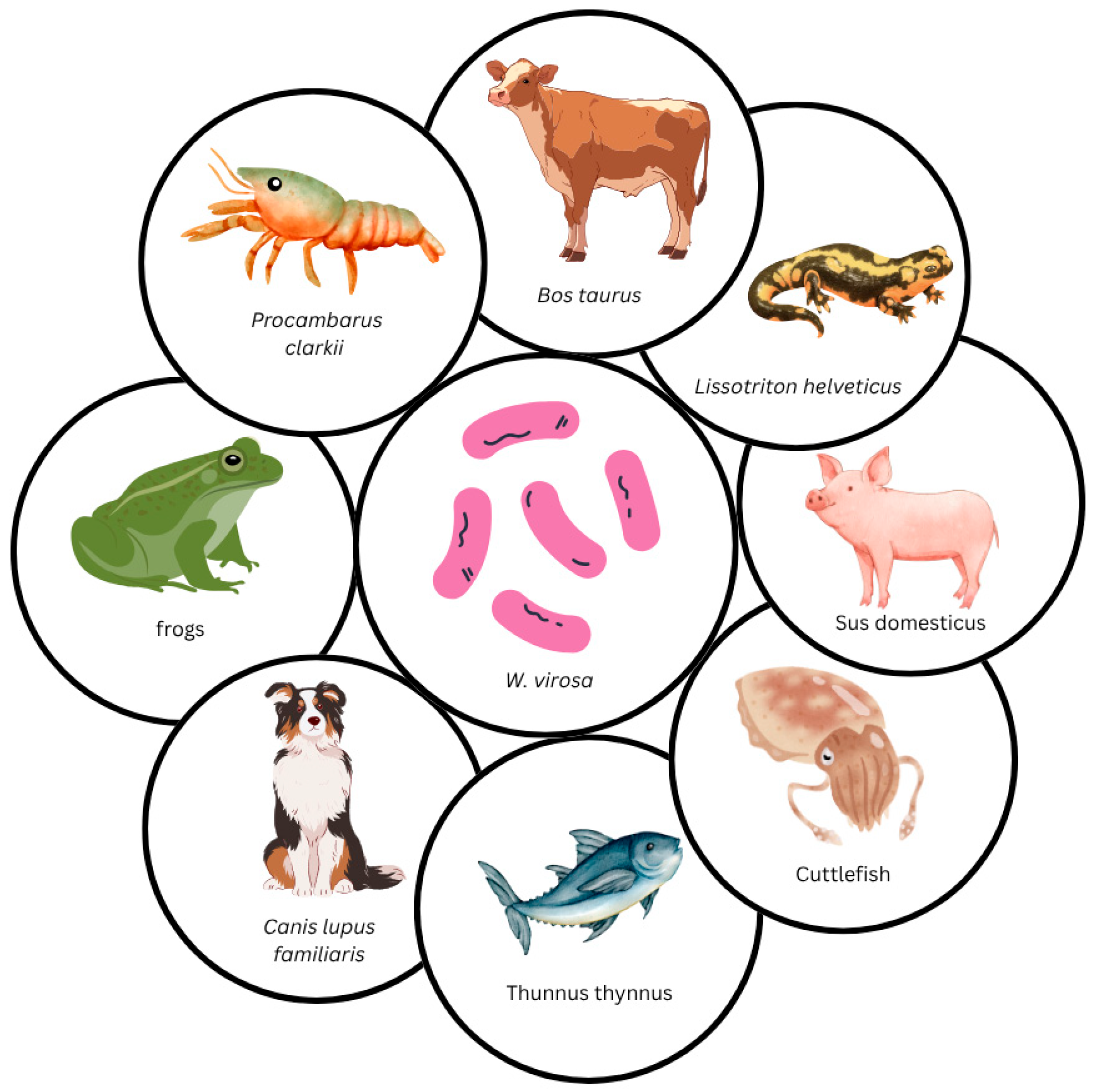
6. Weeksella virosa Infections and Isolation in Animals
7. Limitations and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holmes, B.; Steigerwalt, A.G.; Weaver, R.E.; Brenner, D.J. Weeksella virosa gen. no v., sp. nov. (Formerly group IIf), found in human clinical specimens. Syst. Appl. Microbiol. 1986, 8, 185–190. [Google Scholar] [CrossRef]
- García-López, M.; Meier-Kolthoff, J.P.; Tindall, B.J.; Gronow, S.; Woyke, T.; Kyrpides, N.C.; Hahnke, R.L.; Göker, M. Analysis of 1000 Type-Strain Genomes Improves Taxonomic Classification of Bacteroidetes. Front. Microbiol. 2019, 10, 2083. [Google Scholar] [CrossRef]
- Leung, W.K.; Jin, L.J.; Yam, W.C.; Samaranayake, L.P. Oral colonization of aerobic and. Oral. Microbiol. Immunol. 2001, 16, 1–9. [Google Scholar] [CrossRef]
- Mardy, C.; Holmest, B. Incidence of vaginal Weeksella virosa (formerly group IIf). J. Clin. Pathol. 1988, 41, 211–214. [Google Scholar] [CrossRef]
- Reina, J.; Gil, J.; Salva, F.; Gomez, J.; Alomar, P. Microbiological Characteristics of Weeksella virosa (Formerly CDC Group IIf) Isolated from the Human Genitourinary Tract. J. Clin. Microbiol. 1990, 28, 2357–2359. [Google Scholar] [CrossRef]
- Owen, R.J.; Holmes, B. Identification and classification of Flavobacterium species from clinical sources. In The Flavobacterium-Cytophaga Group, Proceedings of the International Symposium on Yellow-Pigmented Gram-Negative Bacteria of the Flavobacterium-Cytophaga Group, Braunschweig, Germany, 8–11 July 1980; Reichenbach, H., Weeks, O.B., Eds.; Verlag Chemie: Weinheim, Germany, 1981; pp. 39–50. [Google Scholar]
- Tamayo, E.J.N.; Melgarejo, S.C.; Martínez, N.R. Sepsis due to Weeksella virosa in wounds made by animal bite. A case report. Medisur 2003, 1, 136–139. [Google Scholar]
- Slenker, A.K.; Hess, B.D.; Jungkind, D.L.; DeSimone, J.A. Fatal case of Weeksella virosa sepsis. J. Clin. Microbiol. 2012, 50, 4166–4167. [Google Scholar] [CrossRef]
- Boixeda, D.; Luis, D.A.; Meseguer, M.A.; Aller, R.; Martin de Argila, C.; Lopez Sanroman, A. A case of spontaneous peritonitis caused by Weeksella virosa. Eur. J. Gastroenterol. Hepatol. 1998, 10, 897–898. [Google Scholar] [CrossRef]
- Manogaran, M.; Marnejon, T.; Sarac, E. Pneumonia and sepsis due to Weeksella virosa in an immunocompromised patient. Infect. Dis. Clin. Pract. 2004, 12, 286–287. [Google Scholar] [CrossRef]
- Faber, M.D.; del Busto, R.; Cruz, C.; Mezger, E. Response of Weeksella virosa peritonitis to imipenem/cilastin. Adv. Perit. Dial. 1991, 7, 133–134. [Google Scholar]
- Lang, E.; Teshima, H.; Lucas, S.; Lapidus, A.; Hammon, N.; Deshpande, S.; Nolan, M.; Cheng, J.-F.; Pitluck, S.; Liolios, K.; et al. Complete genome sequence of Weeksella virosa type strain (9751 T). Stand. Genomic. Sci. 2011, 4, 81–90. [Google Scholar] [CrossRef][Green Version]
- Tan, J.B.X.; Tng, A.R.K.; Htay, H. A Case of Relapsing Peritoneal Dialysis-Associated Peritonitis by Dokdonella koreensis. Case Rep. Infect. Dis. 2018, 2018, 1–4. [Google Scholar] [CrossRef]
- Unalan, T.; Karagoz, A.; Bayhan, C.; Ozsurekci, Y.; Hazirolan, G. An unusual case of peritoneal dialysis-associated bacterial peritonitis caused by Weeksella virosa. Acta Microbiol. Immunol. Hung. 2021, 68, 62–64. [Google Scholar] [CrossRef]
- Hashimoto, M.; Asai, S.; Umezawa, K.; Tanitsu, R.; Miyazawa, M.; Kobayashi, M.; Kawakami, Y.; Sekine, Y.; Suzuki, Y.; Miyachi, H.; et al. Methods of Cleaning Taps to Prevent Hospital-Associated Infections: An Environmental Survey-Based Study. Infect. Dis. Rep. 2023, 15, 142–149. [Google Scholar] [CrossRef]
- Zanetti, F.; De Luca, G.; Leoni, E.; Sacchetti, R. NF-GNB in drinking water dispensed from point-of-use devices. Ann. Agric. Environ. Med. 2014, 21, 29–34. [Google Scholar]
- Gunasekara, T.; Kudavidanage, B.; Peelawattage, M.; Meedin, F.; Guruge, L.; Nanayakkara, G.; Nanayakkara, M.; Fernando, S. Bacterial Contamination of Anaesthetists Hands, Personal Mobile Phones and Wrist Watches used during Theatre Sessions. Sri Lankan J. Anaesthesiol. 2009, 17, 11. [Google Scholar] [CrossRef]
- de la Fuente García Peña, L.A.; Mendoza García, A.U.; Villegas-Dominguez, J.E.; Márquez Celedonio, F.G.; Arana Vidal, H.; Azuara Díaz, K. Ventilator-associated pneumonia by Weeksella virosa: Case report. BMC Infect Dis. 2024, 24, 6. [Google Scholar] [CrossRef]
- Pickett, M.J.; Pedersen, M.M. Nonfermentative Bacilli Associated with Man: II. Detection and Identification. Am. J. Clin. Pathol. 1970, 54, 164–177. [Google Scholar] [CrossRef]
- Olsen, H.; Ravn, T. Flavobacterium meningosepticum isolated from the genitals. Acta Pathol. Microbiol. Scand. Sect. B Microbiol. Immunol. 1971, 79, 102–106. [Google Scholar] [CrossRef]
- Owen, R.J.; Snell, J.J.S. Comparison of group IIf with Flavobacterium and Moraxella. Antonie Van Leeuwenhoek 1973, 39, 473–480. [Google Scholar] [CrossRef]
- Garzon, M.; Mainali, S.; Chacon, M.F.; Azizzadeh-Roodpish, S. A computational approach to biological pathogenicity. Mol. Genet. Genom. 2022, 297, 1741–1754. [Google Scholar] [CrossRef]
- Holmes, B. The Genera Flavobacterium, Sphingobacterium, and Weeksella; Springer: New York, NY, USA, 1992. [Google Scholar]
- National Center for Biotechnology Information. NIH. 2025. NCBI Genome Database. Available online: https://www.ncbi.nlm.nih.gov/datasets/genome/?taxon=1014 (accessed on 20 May 2025).
- Oren, A.; Garrity, G.M. List of novel names and novel combinations previously effectively, but not validly, published. Int. J. Syst. Evol. Microbiol. 2017, 67, 2075–2078. [Google Scholar] [CrossRef]
- Sankar, S.; Lo, C.; Fall, B.; Sambe-Ba, B.; Mediannikov, O.; Diallo, I.; Labas, N.; Faye, N.; Wade, B.; Raoult, D.; et al. Noncontiguous finished genome sequence and description of Weeksella massiliensis sp. nov. New Microbes New Infect. 2015, 8, 89–98. [Google Scholar] [CrossRef]
- Diop, K.; Bretelle, F.; Michelle, C.; Richez, M.; Rathored, J.; Raoult, D.; Fournier, P.-E.; Fenollar, F. Taxonogenomics and description of Vaginella massiliensis gen. nov., sp. nov., strain Marseille P2517T, a new bacterial genus isolated from the human vagina. New Microbes New Infect. 2017, 15, 94–103. [Google Scholar] [CrossRef]
- Xu, P.; Cheng, A.; Wang, M.; Zhu, D.; Wang, X. Bioinformatics analysis of OmpA/MotB protein encoded by OmpA/MotB gene(ORF 648bp) of Riemerella anatipestifer. Adv. Mater. Res. 2013, 647, 304–309. [Google Scholar]
- Zhang, C.; Rikihisa, Y. Proposal to transfer “Aegyptianella ranarum”, an intracellular bacterium of frog red blood cells, to the family Flavobacteriaceae as “Candidatus Hemobacterium ranarum” comb. nov. Environ. Microbiol. 2004, 6, 568–573. [Google Scholar] [CrossRef]
- Meharwal, S.; Taneja, N.; Sharma, S.K.; Meera, S. Complicated nosocomial UTI caused by nonfermenters. Indian, J. Urol. 2002, 18, 123–128. [Google Scholar]
- Melo, G.B.; Bispo, P.J.M.; Campos Pignatari, A.C.; Höfling-Lima, A.L. Real-time polymerase chain reaction test to discriminate between contamination and intraocular infection after cataract surgery. J. Cataract. Refract. Surg. 2011, 37, 1244–1250. [Google Scholar] [CrossRef]
- Toescu, S.M.; Lacey, S.; Low, H.L. First report of postoperative intracranial Weeksella virosa infection. Acta Neurochir. 2017, 159, 2235–2238. [Google Scholar] [CrossRef]
- Vaquera-Aparicio, D.N.; Mascareñas-De Los Santos, A.H.; Casillas-Vega, N.; Riojas-Hernández, P.; Llaca-Díaz, J.; Herrera-Benavente, I.; Castillo-Bejarano, J.I. Bacteremia due to Weeksella virosa in a pediatric patient with embryonal rhabdomyosarcoma. Bol. Med. Hosp. Infant. Mex. 2020, 77, 149–152. [Google Scholar] [CrossRef]
- Campbell, I.M.; Congdom, M.; Capucilli, P.S.; Fox, W.W. A not so common infection in an extremely low-birth-weight infant. Clin. Pediatr. 2020, 44, 1–3. [Google Scholar] [CrossRef]
- Dilip, B.; Angelo, M.A.; Wasif, H. Fatal Septic Shock due to Weeksella virosa in 69-Year-Old Male. Spartan Med. Res. J. 2024, 9, 123109. [Google Scholar] [CrossRef]
- The Editorial Board. Weeksella †,‡. In Bergey’s Manual of Systematics of Archaea and Bacteria; Trujillo, M.E., Dedysh, S., DeVos, P., Hedlund, B., Kämpfer, P., Rainey, F.A., Whitman, W.B., Eds.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Walker, J.T.; Jhutty, A.; Parks, S.; Willis, C.; Copley, V.; Turton, J.F.; Hoffman, P.; Bennett, A. Investigation of healthcare-acquired infections associated with Pseudomonas aeruginosa biofilms in taps in neonatal units in Northern Ireland. J. Hosp. Infect. 2014, 86, 16–23. [Google Scholar] [CrossRef]
- Gromkova, R.; Dangor, Y.; Miller, S.D. V-factor (NAD) Independent Haemophilus parainfluenzae Recovered from a Human Isolation of Weeksella virosa (Formerly CDC Group IIf) from a Vaginal Sample. Eur. J. Clin. Microbiol. Infect. Dis. 1989, 8, 569. [Google Scholar] [CrossRef]
- Martínez, M.A.; Ovalle, A. Weeksella Virosa. Rev. Chil. Infectología 2011, 28, 429–430. [Google Scholar] [CrossRef]
- Bombicino, K.A.; Almuzara, M.N.; Famiglietti, A.M.R.; Vay, C. Evaluation of pyrrolidonyl arylamidase for the identification of nonfermenting Gram-negative rods. Diagn. Microbiol. Infect. Dis. 2007, 57, 101–103. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, L.; Sun, C. Algoriella xinjiangensis gen. nov., sp. nov., a new psychrotolerant bacterium of the family Flavobacteriaceae. Antonie Van Leeuwenhoek 2015, 108, 1107–1116. [Google Scholar] [CrossRef]
- Longshaw, M.; Bateman, K.S.; Stebbing, P.; Stentiford, G.D.; Hockley, F.A. Disease risks associated with the importation and release of non-native crayfish species into mainland Britain. Aquat. Biol. 2012, 16, 1–15. [Google Scholar] [CrossRef]
- Almuzara, M.; Barberis, C.; Traglia, G.; Famiglietti, A.; Ramirez, M.S.; Vay, C. Evaluation of matrix-assisted laser desorption ionization-time-of-flight mass spectrometry for species identification of Nonfermenting Gram-Negative Bacilli. J. Microbiol. Methods 2015, 112, 24–27. [Google Scholar] [CrossRef]
- Saffert, R.T.; Cunningham, S.A.; Ihde, S.M.; Monson Jobe, K.E.; Mandrekar, J.; Patel, R. Comparison of Bruker Biotyper matrix-assisted laser desorption ionization-time of flight mass spectrometer to BD phoenix automated microbiology system for identification of gram-negative bacilli. J. Clin. Microbiol. 2011, 49, 887–892. [Google Scholar] [CrossRef]
- Rocca, M.F.; Danze, D.; D’Angiolo, G.; Etcheverry, P.; Martínez, C.; Prieto, M. MALDI-TOF MS experience with the identification of complex microorganisms using two devices in a National Reference Laboratory. Microbiol. Spectr. 2025, 13, e0167724. [Google Scholar] [CrossRef]
- Chung, Y.; Han, M.; Kim, J.S. Comparative evaluation of bruker biotyper and asta microidsys for species identification in a clinical microbiology laboratory. Diagnostics 2021, 11, 1683. [Google Scholar] [CrossRef]
- Lallemand, E.; Coiffier, G.; Arvieux, C.; Brillet, E.; Guggenbuhl, P.; Jolivet-Gougeon, A. MALDI-TOF MS performance compared to direct examination, culture, and 16S rDNA PCR for the rapid diagnosis of bone and joint infections. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 857–866. [Google Scholar] [CrossRef]
- Koyama, S.; Watanabe, N.; Naruse, H.; Mitsutake, K.; Ebihara, Y. Utility of direct microorganism species identification and antimicrobial susceptibility tests in urine samples. J. Infect. Chemother. 2024, 31, 102590. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Sharma, S.; Krishnaswamy, V.; Chaturvedi, R.; Sharma, A. Epidemiology of rare bacterial, parasitic, and fungal pathogens in India. IJID Reg. 2024, 11, 100359. [Google Scholar] [CrossRef]
- Varga, J.J.; Zhao, C.Y.; Davis, J.D.; Hao, Y.; Farrell, J.M.; Gurney, J.R.; Voit, E.; Brown, S.P.; Young, V.B. Antibiotics Drive Expansion of Rare Pathogens in a Chronic Infection Microbiome Model. mSphere 2022, 7, e0031822. [Google Scholar] [CrossRef]
- EUCAST (European Committee on Antimicrobial Susceptibility Testing). Expected Resistant Phenotypes. Version 1.2, 2023. [Internet]. EUCAST. Available online: https://www.eucast.org/expert_rules_and_expected_phenotypes/expected_phenotypes (accessed on 25 May 2025).
- Aber, R.C.; Wennersten, C.; Ring, R.C.M. Antimicrobial Susceptibility of Flavobacteria. Antimicrob. Agents Chemother. 1978, 14, 483–487. [Google Scholar] [CrossRef]
- Górniak, D.; Świątecki, A.; Kowalik, J.; Grzesiak, J.; Jastrzębski, J.; Zdanowski, M.K. High antagonistic activity and antibiotic resistance of flavobacteria of polar microbial freshwater mats on King George Island in maritime Antarctica. Sci. Rep. 2025, 15, 13615. [Google Scholar] [CrossRef]
- Gray, A.T. Mandell, Douglass, and Bennet’s Principles and Practice of Infectious Diseases. J. Am. Med. Assoc. 2010, 304, 318. [Google Scholar]
- EUCAST Guidance on When There Are no Breakpoints in Breakpoint Tables? 2024. Available online: https://www.eucast.org/clinical_breakpoints_and_dosing/when_there_are_no_breakpoints (accessed on 23 July 2025).
- Pawar, M.; Mehta, Y.; Purohit, A.; Trehan, N.; Rosenthal, V.D. Resistance in gram-negative bacilli in a cardiac intensive care unit in India: Risk factors and outcome. Ann. Card. Anaesth. 2008, 11, 20–26. [Google Scholar] [CrossRef]
- Koncz, M.; Stirling, T.; Mehdi, H.H.; Méhi, O.; Eszenyi, B.; Asbóth, A.; Apjok, G.; Tóth, Á.; Orosz, L.; Vásárhelyi, B.M.; et al. Genomic surveillance as a scalable framework for precision phage therapy against antibiotic-resistant pathogens. Cell 2024, 187, 5901–5918.e28. [Google Scholar] [CrossRef]
- Anastassopoulou, C.; Ferous, S.; Petsimeri, A.; Gioula, G.; Tsakris, A. Phage-Based Therapy in Combination with Antibiotics: A Promising Alternative against Multidrug-Resistant Gram-Negative Pathogens. Pathogens 2024, 13, 896. [Google Scholar] [CrossRef]
- Aranaga, C.; Pantoja, L.D.; Martínez, E.A.; Falco, A. Phage Therapy in the Era of Multidrug Resistance in Bacteria: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 4577. [Google Scholar] [CrossRef]
- Iordache, D.; Baci, G.M.; Căpriță, O.; Farkas, A.; Lup, A.; Butiuc-Keul, A. Correlation between CRISPR Loci Diversity in Three Enterobacterial Taxa. Int. J. Mol. Sci. 2022, 23, 12766. [Google Scholar] [CrossRef]
- Butiuc-Keul, A.; Farkas, A.; Carpa, R.; Iordache, D. CRISPR-Cas System: The Powerful Modulator of Accessory Genomes in Prokaryotes. Microb. Physiol. 2022, 32, 2–17. [Google Scholar] [CrossRef]
- Fass, R.J.; Barnishan, J.; Solomon, M.C.; Ayers, L.W. In Vitro Activities of Quinolones, -Lactams, Tobramycin, and Trimethoprim-Sulfamethoxazole against Nonfermentative Gram-Negative Bacilli Downloaded from [Internet]. Antimicrob. Agents Chemother. 1996, 40, 1412–1418. [Google Scholar] [CrossRef]
- Brun, R.; Urraro, C.; Medaglia, C.; Russo, V.; Borzacchiello, G.; Roperto, F.; Roperto, S. Lymphoepithelioma-like Carcinoma of the Urinary Bladder in a Cow Associated with Bovine Papillomavirus Type-2. J. Comp. Pathol. 2008, 139, 121–125. [Google Scholar] [CrossRef]
- Hamon, M.; Dequeant, B.; Decambron, A.; Reyes-Gomez, E.; Manassero, M. Leiomyoma in the nasal cavity of a dog. J. Small Anim. Pract. 2019, 60, 319–322. [Google Scholar] [CrossRef]
- Rich, A.F.; Denk, D.; Sangster, C.R.; Stidworthy, M.F. A retrospective study of pathologic findings in cephalopods (extant subclasses: Coleoidea and Nautiloidea) under laboratory and aquarium management. Vet. Pathol. 2023, 60, 578–598. [Google Scholar] [CrossRef]
- Kapetanović, D.; Smrzlić, I.V.; Valić, D.; Teskeredžić, Z.; Teskeredžić, E.; Teskeredžić, E. Culturable microbiota associated with farmed Atlantic bluefin tuna (Thunnus thynnus). Aquat Living Resour. 2017, 30, 30. [Google Scholar] [CrossRef]
- Edery, S.; Elias, R.; Shiva, C.; Weaver, T.; Reading, R. Cutaneous bacteria of confiscated Telmatobius culeus in Lima, Peru. J. Wildl. Dis. 2021, 57, 900–902. [Google Scholar] [CrossRef]
- Vela, A.I.; García, N.; Latre, M.V.; Casamayor, A.; Sánchez-Porro, C.; Briones, V.; Ventosa, A.; Domínguez, L.; Fernández-Garayzábal, J.F. Aerococcus suis sp. nov., isolated from clinical specimens from swine. Int. J. Syst. Evol. Microbiol. 2007, 57, 1291–1294. [Google Scholar] [CrossRef]
- Hassl, A.; Url, A.; Rebel-Bauder, B. Weeksella virosa HOLMES et al., 1987 colonised epidermal cysts in Hyla crepitans WIED-NEUWIED, 1824. Herpetozoa 2002, 14, 127–131. [Google Scholar]
- González-Hernández, M.; Denoël, M.; Duffus, A.J.L.; Garner, T.W.J.; Cunningham, A.A.; Acevedo-Whitehouse, K. Dermocystid infection and associated skin lesions in free-living palmate newts (Lissotriton helveticus) from Southern France. Parasitol. Int. 2010, 59, 344–350. [Google Scholar] [CrossRef]

| No | Reference | Patient Sex and Age (years) | Comorbidities/Risk Factors | Type of Infection | Sample | Identification Method | AST | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Faber et al., 1991 [11] | F, 33 | End-stage renal failure with peritoneal dialysis | Spontaneous bacterial peritonitis | Peritoneal fluid | n/a | n/a | IMP/Cilastin + AMP | Favorable |
| 2 | Boixeda et al., 1998 [9] | M, 55 | HCV cirrhosis | Spontaneous bacterial peritonitis | Peritoneal fluid | VITEK GNI and API 20E | n/a | FOX | Favorable |
| 3 | Meharwal et al., 2002 [30] | n/a | n/a | UTI | Urine | n/a | n/a | n/a | n/a |
| 4 | Tamayo et al., 2003 [7] | F, 31 | Immunocompetent | Reticular lymphangitis | Wound | Biochemical | Yes | TET | Favorable |
| 5 | Manogaran et al., 2004 [10] | F, 53 | Lymphoma, chronic kidney failure with hemodialysis, diabetes mellitus | Pneumonia and sepsis | Sputum and blood | n/a | Yes | FEP + AB | Patient expired |
| 6 | Melo et al., 2011 [31] | n/a, 50 | n/a | Postoperative infectious endophthalmitis | Aqueous and vitreous samples | Biochemical testing (Phoenix system) and real-time PCR | n/a | VAN + CTZ | Favorable |
| 7 | Slenker et al., 2012 [8] | F, 44 | Obesity, menorrhagia | Labial abscess | Wound | n/a | n/a | Surgical | Favorable |
| F, 26 | Endometriosis, pelvic adhesions, small bowel obstruction, abdominal surgery, diabetes mellitus | UTI | Urine | n/a | n/a | SXT | Favorable | ||
| F, 25 | Pregnancy and vaginal delivery complicated by Amnionitis | Amnionitis | Placenta | n/a | n/a | AMP + CN | Favorable | ||
| F, 31 | Ischemic heart disease, acute myocardial infarction, end-stage renal disease with hemodialysis, smoking, asthma, HCV infection, obesity | Sepsis and suspected pneumonia | Blood | BD Phoenix and 16S gene sequencing | Yes | AZT + TOB + D | Patient expired | ||
| 8 | Toescu et al., 2017 [32] | F, 49 | Glucocorticoid use, recurrent malignant meningioma treated with repeated surgeries and whole-brain radiotherapy | Sepsis due to post-surgical ventricular empyema | Extradural and ventricular purulent material, CSF, other tissue samples | 16S rRNA PCR | Yes | CTR + AMX | Favorable (patient expired due to neoplastic complications) |
| 9 | Unalan et al., 2019 [14] | F, 4 | Addison’s disease, terminal kidney failure with peritoneal dialysis | Bacterial peritonitis associated with peritoneal dialysis | Peritoneal and dialysis fluid | BD Phoenix and 16S gene sequencing | Yes | FEP, later MEM and catheter removal | Favorable |
| 10 | Vaquera-Aparicio et al., 2020 [33] | M, 4 | Embryonal rhabdomyosarcoma | Bacteremia | Blood | Sensititre™ ARIS™ 2X ID/AST System-Thermo Fisher Scientific | Yes | IMP/cilstatin, MEM * | Favorable |
| 11 | Campbell et al., 2020 [34] | n/a, 26 weeks | Extremely premature with low birth weight | Neonatal Sepsis | Blood | 16S rRNA PCR | Yes | MEM ** | Favorable |
| 12 | de la Fuente García Peña et al., 2024 [18] | F, 64 | Diabetes mellitus, chronic renal disease, transcatheter aortic valve implantation, post-cardiac arrest syndrome, mechanical ventilation, soft tissue infection in pelvic limb | Ventilator-associated pneumonia | Bronchoalveolar lavage | VITEK and PCR sequencing | Yes | CIP | Favorable |
| 13 | Dilip et al., 2024 [35] | M, 69 | Chronic urinary catheterization, hypertension, hyperlipidemia, benign prostatic hyperplasia | Septic shock | Blood and urine | n/a | n/a | FEP | Patient expired |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colosi, I.A.; Toc, D.A.; Neculicioiu, V.S.; Panaitescu, P.-Ș.; Șchiopu, P.; Pană, A.-G.; Opris, R.V.; Baciu, A.M.; Berar, G.; Botan, A.; et al. Beyond the Usual Suspects: Weeksella virosa as a Potential Human and Animal Pathogen. Trop. Med. Infect. Dis. 2025, 10, 210. https://doi.org/10.3390/tropicalmed10080210
Colosi IA, Toc DA, Neculicioiu VS, Panaitescu P-Ș, Șchiopu P, Pană A-G, Opris RV, Baciu AM, Berar G, Botan A, et al. Beyond the Usual Suspects: Weeksella virosa as a Potential Human and Animal Pathogen. Tropical Medicine and Infectious Disease. 2025; 10(8):210. https://doi.org/10.3390/tropicalmed10080210
Chicago/Turabian StyleColosi, Ioana Alina, Dan Alexandru Toc, Vlad Sever Neculicioiu, Paul-Ștefan Panaitescu, Pavel Șchiopu, Adrian-Gabriel Pană, Razvan Vlad Opris, Alina Mihaela Baciu, George Berar, Alexandru Botan, and et al. 2025. "Beyond the Usual Suspects: Weeksella virosa as a Potential Human and Animal Pathogen" Tropical Medicine and Infectious Disease 10, no. 8: 210. https://doi.org/10.3390/tropicalmed10080210
APA StyleColosi, I. A., Toc, D. A., Neculicioiu, V. S., Panaitescu, P.-Ș., Șchiopu, P., Pană, A.-G., Opris, R. V., Baciu, A. M., Berar, G., Botan, A., & Costache, C. (2025). Beyond the Usual Suspects: Weeksella virosa as a Potential Human and Animal Pathogen. Tropical Medicine and Infectious Disease, 10(8), 210. https://doi.org/10.3390/tropicalmed10080210