Prognostic Value of Ratios of Inflammatory Markers in the Prognosis of Crimean–Congo Hemorrhagic Fever
Abstract
1. Introduction
2. Material and Method
2.1. Study Population
2.2. Laboratory Analysis
2.3. Statistical Analyses
2.4. Ethical Approval
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buyuktuna, S.A.; Bolat, S.; Doğan, K.; Çakır, Y.; Dogan, H.O. Assessment of Serum Beta 2-Microglobulin Levels in Crimean-Congo Hemorrhagic Fever Patients: Implications for Immune Activation and Disease Pathogenesis. Cumhur. Sci. J. 2024, 45, 338–342. [Google Scholar]
- Frank, M.G.; Weaver, G.; Raabe, V.; Research, E.C.S.S.P. Crimean-Congo hemorrhagic fever virus for clinicians—Epidemiology, clinical manifestations, and prevention. Emerg. Infect. Dis. 2024, 30, 854. [Google Scholar] [CrossRef]
- Büyüktuna, S.A.; Yerlitaş, S.İ.; Zararsız, G.E.; Doğan, K.; Kablan, D.; Bağcı, G.; Özer, S.; Baysal, C.; Çakır, Y.; Cephe, A.; et al. Exploring free amino acid profiles in Crimean-Congo hemorrhagic fever patients: Implications for disease progression. J. Med. Virol. 2024, 96, e29637. [Google Scholar] [PubMed]
- Logiudice, J.; Alberti, M.; Ciccarone, A.; Rossi, B.; Tiecco, G.; De Francesco, M.A.; Quiros-Roldan, E. Introduction of Vector-Borne Infections in Europe: Emerging and Re-Emerging Viral Pathogens with Potential Impact on One Health. Pathogens 2025, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Büyüktuna, S.A.; Doğan, H.O. Diagnosis, Prognosis and Clinical Trial in Crimean-Congo Hemorrhagic Fever. In Human Viruses: Diseases, Treatments and Vaccines: The New Insights; Springer: Cham, Switzerland, 2021; pp. 207–219. [Google Scholar]
- Sajid, M.; Hamid, M.; Ayyub, U.; Azam, M. Crimean-Congo Hemorrhagic Fever Virus: Advanced Insights into Virology, Pathogenesis, Pathology, Diagnosis, and Treatment. Hosts Viruses 2024, 11, 78–85. [Google Scholar]
- Bakir, M.; Öksüz, C.; Karakeçili, F.; Baykam, N.; Barut, Ş.; Büyüktuna, S.A.; Özkurt, Z.; Öz, M.; Barkay, O.; Akdoğan, Ö.; et al. Which scoring system is effective in predicting mortality in patients with Crimean Congo hemorrhagic fever? A validation study. Pathog. Glob. Health 2022, 116, 193–200. [Google Scholar] [CrossRef]
- Bakır, M.; Gözel, M.G.; Köksal, I.; Aşık, Z.; Günal, Ö.; Yılmaz, H.; But, A.; Yılmaz, G.; Engin, A. Validation of a severity grading score (SGS) system for predicting the course of disease and mortality in patients with Crimean-Congo hemorrhagic fever (CCHF). Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 325–330. [Google Scholar]
- Ergonul, O.; Tuncbilek, S.; Baykam, N.; Celikbas, A.; Dokuzoguz, B. Evaluation of serum levels of interleukin (IL)–6, IL-10, and tumor necrosis factor–α in patients with Crimean-Congo hemorrhagic fever. J. Infect. Dis. 2006, 193, 941–944. [Google Scholar]
- Eren, S.H.; Zengin, S.; Büyüktuna, S.A.; Gözel, M.G. Clinical severity in forecasting platelet to lymphocyte ratio in Crimean–Congo hemorrhagic fever patients. J. Med. Microbiol. 2016, 65, 1100–1104. [Google Scholar]
- Kriplani, A.; Pandit, S.; Chawla, A.; de la Rosette, J.J.; Laguna, P.; Jayadeva Reddy, S.; Somani, B.K. Neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR) and lymphocyte–monocyte ratio (LMR) in predicting systemic inflammatory response syndrome (SIRS) and sepsis after percutaneous nephrolithotomy (PNL). Urolithiasis 2022, 50, 341–348. [Google Scholar]
- Wang, G.; Mivefroshan, A.; Yaghoobpoor, S.; Khanzadeh, S.; Siri, G.; Rahmani, F.; Aleseidi, S. Prognostic value of platelet to lymphocyte ratio in sepsis: A systematic review and meta-analysis. BioMed Res. Int. 2022, 2022, 9056363. [Google Scholar]
- Yang, A.P.; Liu, J.P.; Tao, W.Q.; Li, H.M. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int. Immunopharmacol. 2020, 84, 106504. [Google Scholar] [PubMed]
- Hawman, D.W.; Feldmann, H. Crimean–Congo haemorrhagic fever virus. Nat. Rev. Microbiol. 2023, 21, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Elaldi, N.; Kaya, S. Crimean-Congo hemorrhagic fever. J. Microbiol. Infect. Dis. 2014, 4, 1–9. [Google Scholar]
- Bastug, A.; Kayaaslan, B.; Kazancioglu, S.; Aslaner, H.; But, A.; Akinci, E.; Yetkin, M.A.; Eren, S.; Bodur, H. Crimean-Congo hemorrhagic fever: Prognostic factors and the association of leukocyte counts with mortality. Jpn. J. Infect. Dis. 2016, 69, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Hatipoglu, C.A.; Bulut, C.; Yetkin, M.A.; Ertem, G.T.; Erdinc, F.S.; Kilic, E.K.; Sari, T.; Kinikli, S.; Oral, B.; Demiroz, A.P. Evaluation of clinical and laboratory predictors of fatality in patients with Crimean-Congo haemorrhagic fever in a tertiary care hospital in Turkey. Scand. J. Infect. Dis. 2010, 42, 516–521. [Google Scholar]
- Yilmaz, G.; Koksal, I.; Topbas, M.; Yilmaz, H.; Aksoy, F. The effectiveness of routine laboratory findings in determining disease severity in patients with Crimean-Congo hemorrhagic fever: Severity prediction criteria. J. Clin. Virol. 2010, 47, 361–365. [Google Scholar]
- Arslan, M.; Yilmaz, G.; Mentese, A.; Yilmaz, H.; Karahan, S.C.; Koksal, I. Importance of endothelial dysfunction biomarkers in patients with Crimean-Congo hemorrhagic fever. J. Med. Virol. 2017, 89, 2084–2091. [Google Scholar]
- Soylu, Ü.; Demirtaş, E.; Büyüktuna, S.A.; Korkmaz, İ.; Tekin, Y.K.; Yurtbay, S. Evaluation of the demographic and laboratory data of patients diagnosed with Crimean-Congo hemorrhagic fever in the emergency department and their relationship with morbidity and mortality. Evaluation 2021, 20, 12–18. [Google Scholar] [CrossRef]
- Kazancioğlu, S.; Akinci, E.; Baştuğ, A.; Kayaaslan, B.; But, A.; Aslaner, H.; Eren, S.S.; Yetkin, M.A.; Bodur, H. Does the course of laboratory parameters help us to predict the outcome of CCHF? Turk. J. Med. Sci. 2016, 46, 328–334. [Google Scholar]
- Ergonul, O.; Celikbas, A.; Baykam, N.; Eren, S.; Dokuzoguz, B. Analysis of risk-factors among patients with Crimean-Congo haemorrhagic fever virus infection: Severity criteria revisited. Clin. Microbiol. Infect. 2006, 12, 551–554. [Google Scholar]
- Onguru, P.; Dagdas, S.; Bodur, H.; Yilmaz, M.; Akinci, E.; Eren, S.; Ozet, G. Coagulopathy parameters in patients with Crimean-Congo hemorrhagic fever and its relation with mortality. J. Clin. Lab. Anal. 2010, 24, 163–166. [Google Scholar] [PubMed]
- Çevik, M.A.; Erbay, A.; Bodur, H.; Gülderen, E.; Baştuğ, A.; Kubar, A.; Akıncı, E. Clinical and laboratory features of Crimean-Congo hemorrhagic fever: Predictors of fatality. Int. J. Infect. Dis. 2008, 12, 374–379. [Google Scholar]
- Wuillemin, W.; Korte, W.; Waser, G.; Lämmle, B. Usefulness of the D-dimer/fibrinogen ratio to predict deep venous thrombosis. J. Thromb. Haemost. 2005, 3, 385–387. [Google Scholar]
- Ozturk, B.; Tutuncu, E.; Kuscu, F.; Gurbuz, Y.; Sencan, I.; Tuzun, H. Evaluation of factors predictive of the prognosis in Crimean-Congo hemorrhagic fever: New suggestions. Int. J. Infect. Dis. 2012, 16, e89–e93. [Google Scholar] [PubMed]
- Kaya, S.; Elaldi, N.; Kubar, A.; Gursoy, N.; Yilmaz, M.; Karakus, G.; Gunes, T.; Polat, Z.; Gozel, M.G.; Engin, A.; et al. Sequential determination of serum viral titers, virus-specific IgG antibodies, and TNF-α, IL-6, IL-10, and IFN-γ levels in patients with Crimean-Congo hemorrhagic fever. BMC Infect. Dis. 2014, 14, 1–8. [Google Scholar]
- Ergönül, Ö. Crimean-Congo haemorrhagic fever. Lancet Infect. Dis. 2006, 6, 203–214. [Google Scholar]
- Papa, A.; Bino, S.; Velo, E.; Harxhi, A.; Kota, M.; Antoniadis, A. Cytokine levels in Crimean-Congo hemorrhagic fever. J. Clin. Virol. 2006, 36, 272–276. [Google Scholar] [PubMed]
- Sari, İ.; Bakır, S.; Engin, A.; Aydın, H.; Poyraz, Ö. Some acute phase reactants and cholesterol levels in serum of patient with Crimean-Congo haemorrhagic fever. Bosn. J. Basic Med. Sci. 2013, 13, 21. [Google Scholar]
- Bozkurt, I.; Esen, S. Association between severity grading score and acute phase reactants in patients with Crimean Congo hemorrhagic fever. Pathog. Glob. Health 2021, 115, 496–498. [Google Scholar]
- Wu, X.; Liu, Y.; Gao, L.; Yan, Z.; Zhao, Q.; Chen, F.; Xie, Q.; Zhang, X. Development and Application of a Reverse-Transcription Recombinase-Aided Amplification Assay for Porcine Epidemic Diarrhea Virus. Viruses 2022, 14, 591. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, L.T.; Kelly-Cirino, C. Diagnostic tests for Crimean-Congo haemorrhagic fever: A widespread tickborne disease. BMJ Glob. Health 2019, 4, e001114. [Google Scholar] [PubMed]
- Semper, A.E.; Olver, J.; Warner, J.; Cehovin, A.; Fay, P.C.; Hart, P.J.; Golding, J.P.; Benassi, V.; Preziosi, M.-P.; Al-Asadi, K.H.R.; et al. Research and product development for Crimean-Congo haemorrhagic fever: Priorities for 2024-30. Lancet Infect. Dis. 2025, 25, e223–e234. [Google Scholar] [CrossRef] [PubMed]
| Variables | Patients (n = 60) | Controls (n = 30) | p-Value |
|---|---|---|---|
| Age (Years) ± SD | 49.1 ± 15.4 | 47.9 ± 9.3 | 0.648 |
| Gender | 0.872 | ||
| Male, n (%) | 41 (68.3) | 21 (70) | |
| Female, n (%) | 19 (31.7) | 9 (30) | |
| Comorbidities | |||
| Hypertension | 12 (20.0) | ||
| Diabetes mellitus | 6 (10.0) | ||
| Coronary artery disease | 4 (6.7) | ||
| Chronic obstructive lung disease | 7 (11.7) | ||
| Malignancy | 2 (3.3) | ||
| Laboratory findings | |||
| HGB (g/dL) | 13.8 ± 1.6 | 14.7 ± 1.6 | 0.009 |
| PLT (109/L) | 82 (53–112) | 247 (192–272) | <0.001 |
| WBC (109/L) | 2.59 (1.6–4.28) | 7.0 (5.9–9.0) | <0.001 |
| INR | 1.0 (0.9–1.2) | 1.0 (1.0–1.10) | 0.382 |
| aPTT (s) | 32 (29–35) | 29 (26–31) | 0.002 |
| D-dimer (mg/L FEU) | 2.2 (0.9–7.03) | 0.2 (0.2–0.2) | <0.001 |
| Fibrinogen (mg/dL) | 268 ± 50 | 270 ± 56 | 0.846 |
| AST (U/L) | 88 (46–191) | 17 (15–20) | <0.001 |
| ALT (U/L) | 53 (26–113) | 18 (13–25) | <0.001 |
| GGT (U/L) | 45 (23–95) | 19 (13–29) | <0.001 |
| ALP (U/L) | 75 (60–94) | 67 (60–79) | 0.139 |
| Creatinine (mg/dL) | 0.8 (0.7–1.0) | 0.8 (0.7–0.9) | 0.196 |
| CRP (mg/L) | 12.0 (4.0–35) | 0.95 (0.41–2.16) | <0.001 |
| IL-6 (ng/L) | 22 (13–76) | 1.5 (1.5–1.99) | <0.001 |
| Treatment, n (%) | |||
| Ribavirin | 6 (10.0) | ||
| Steroid | 22 (36.7) | ||
| Thrombocytes | 28 (46.7) | ||
| Fresh frozen plasma | 41 (68.3) | ||
| Antibiotics | 20 (33.3) | ||
| ICU need, n (%) | 7 (11.7) | ||
| Mortality, n (%) | 6 (10.0) |
| Variables | Patients (n = 60) | Control (n = 30) | p-Value |
|---|---|---|---|
| WBC/PLT | 0.03 (0.02–0.05) | 0.03 (0.02–0.04) | 0.909 |
| WBC/INR | 2.33 (1.65–3.74) | 7.48 (5.9–8.9) | <0.001 |
| WBC/aPTT | 0.08 (0.05–0.12) | 0.26 (0.22–0.33) | <0.001 |
| WBC/D-dimer | 0.96 (0.36–2.53) | 35 (29.5–45) | <0.001 |
| WBC/Fibrinogen | 0.01 (0.01–0.02) | 0.03 (0.02–0.04) | <0.001 |
| WBC/CRP | 0.19 (0.08–0.55) | 6.81 (3.35–17.5) | <0.001 |
| WBC/IL-6 | 0.11 (0.04–0.18) | 3.97 (3.38–5.67) | <0.001 |
| Laboratory Finding | Non-Fatal Cases (n = 54) | Fatal Cases (n = 6) | p-Value |
|---|---|---|---|
| HGB (g/dL) | 13.94 ± 1.51 | 12.22 ± 1.79 | 0.012 |
| PLT (109/L) | 85 (57–115) | 38 (31–51) | 0.005 |
| WBC (109/L) | 2.4 (1.6–3.5) | 6.8 (4.9–9.8) | 0.010 |
| INR | 1.0 (0.9–1.1) | 1.5 (1.5–1.6) | <0.001 |
| aPTT (s) | 32 (28–35) | 45 (42–64) | <0.001 |
| D-dimer (mg/L FEU) | 1.85 (0.81–6.0) | 38.0 (15.0–45.0) | <0.001 |
| Fibrinogen (mg/dL) | 273 ± 48 | 226 ± 61 | 0.029 |
| CRP (mg/L) | 10.0 (4.0–26.0) | 101 (35–166) | 0.001 |
| IL-6 (ng/L) | 18.5 (12.0–53.0) | 314 (79–585) | <0.001 |
| WBC/PLT | 0.03 (0.02–0.04) | 0.15 (0.1–0.32) | <0.001 |
| WBC/INR | 2.33 (1.6–3.38) | 5.22 (3.27–6.53) | 0.043 |
| WBC/aPTT | 0.08 (0.05–0.11) | 0.16 (0.11–0.22) | 0.074 |
| WBC/D-dimer | 1.13 (0.5–3.0) | 0.17 (0.11–0.4) | 0.002 |
| WBC/Fibrinogen | 0.01 (0.01–0.01) | 0.03 (0.02–0.06) | 0.002 |
| WBC/CRP | 0.21 (0.08–0.63) | 0.1 (0.03–0.14) | 0.078 |
| WBC/IL-6 | 0.12 (0.04–0.21) | 0.02 (0.02–0.04) | 0.006 |
| Variables | ICU Requirement | No Need for ICU | p-Value |
|---|---|---|---|
| WBC/PLT | 0.16 (0.1–0.32) | 0.03 (0.02–0.04) | <0.001 |
| WBC/INR | 6.18 (3.27–6.53) | 2.33 (1.6–3.2) | 0.013 |
| WBC/aPTT | 0.19 (0.11–0.22) | 0.08 (0.05–0.11) | 0.026 |
| WBC/D-dimer | 0.15 (0.11–0.4) | 1.18 (0.51–3) | 0.001 |
| WBC/Fibrinogen | 0.03 (0.02–0.06) | 0.01 (0.01–0.01) | <0.001 |
| WBC/CRP | 0.09 (0.03–0.14) | 0.21 (0.08–0.63) | 0.061 |
| WBC/IL-6 | 0.02 (0.02–0.05) | 0.12 (0.04–0.21) | 0.006 |
| Variables | Cut-Off Value | AUC | Sensitivity (95% CI) | Specificity (95% CI) | LR (+) (95% CI) | LR (−) (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|
| WBC/PLT | ≤0.045 | 0.512 | 71.7 (58.6–82.5) | 0 (0–11.6) | 0.72 (0.61–0.84) | NA | 0.835 |
| WBC/INR | ≤3.5 | 0.923 | 73.3 (60.3–83.9) | 100 (88.4–100) | NA | 0.27 (0.18–0.41) | <0.001 |
| WBC/APTT | ≤0.12 | 0.917 | 78.3 (65.8–87.9) | 96.7 (82.8–99.9) | 23.5 (3.41–162) | 0.22 (0.14–0.36) | <0.001 |
| WBC/D-dimer | ≤11.1 | 0.990 | 98.3 (91.1–100) | 100 (88.4–100) | NA | 0.017 (0.002–0.12) | <0.001 |
| WBC/Fibrinogen | ≤0.017 | 0.886 | 81.7 (69.6–90.5) | 90.0 (73.5–97.9) | 8.17 (2.77–24.1) | 0.20 (0.12–0.35) | <0.001 |
| WBC/CRP | ≤2.1 | 0.980 | 95.0 (86.1–99.0) | 96.7 (82.8–99.9) | 28.5 (4.15–196) | 0.052 (0.017–0.16) | <0.001 |
| WBC/IL-6 | ≤0.53 | 0.998 | 96.7 (88.5–99.6) | 100 (88.4–100) | NA | 0.033 (0.009–0.13) | <0.001 |
| Variables | Cut-Off Value | AUC | Sensitivity (95% CI) | Specificity (95% CI) | LR (+) (95% CI) | LR (−) (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|
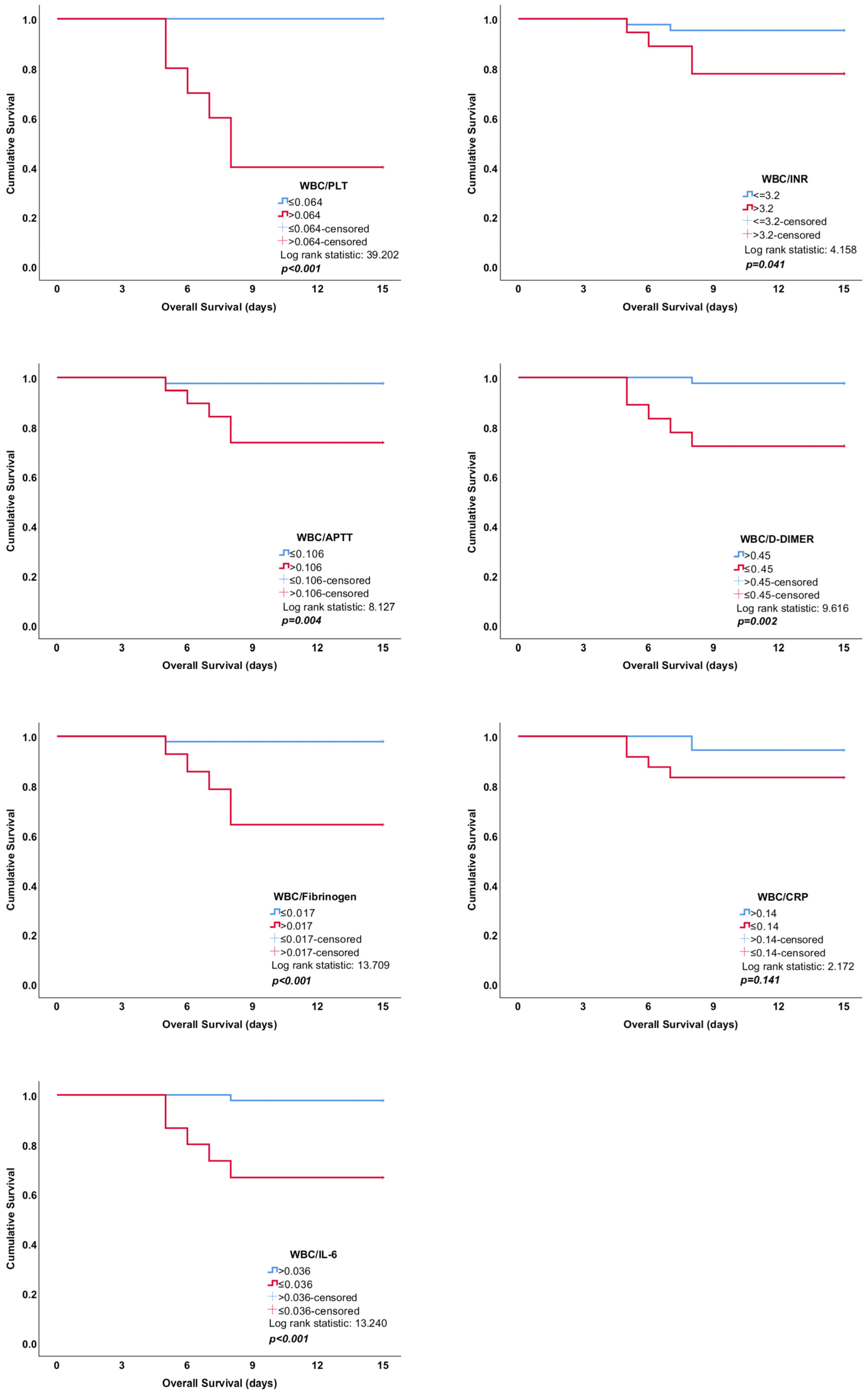
| WBC/PLT | >0.064 | 0.969 | 100 (54.1–100) | 92.6 (82.1–97.9) | 13.5 (5.26–34.7) | 0 | <0.001 |
| WBC/INR | >3.2 | 0.753 | 83.3 (35.9–99.6) | 74.1 (60.3–85.0) | 3.21 (1.81–5.72) | 0.23 (0.037–1.36) | 0.061 |
| WBC/APTT | >0.106 | 0.722 | 83.3 (35.9–99.6) | 74.1 (60.3–85.0) | 3.21 (1.81–5.72) | 0.23 (0.037–1.36) | 0.096 |
| WBC/D-dimer | ≤0.45 | 0.867 | 100 (54.1–100) | 75.9 (62.4–86.5) | 4.15 (2.59–6.67) | 0 | <0.001 |
| WBC/Fibrinogen | >0.017 | 0.880 | 83.3 (35.9–99.6) | 88.9 (77.4–95.8) | 7.5 (3.25–17.3) | 0.19 (0.031–1.12) | <0.001 |
| WBC/CRP | ≤0.14 | 0.722 | 83.3 (35.9–99.6) | 63.0 (48.7–75.7) | 2.25 (1.37–3.71) | 0.26 (0.044–1.60) | 0.028 |
| WBC/IL-6 | ≤0.036 | 0.830 | 83.3 (35.9–99.6) | 81.5 (68.6–90.7) | 4.5 (2.32–8.74) | 0.2 (0.034–1.23) | <0.001 |
| Variables | Cut-Off Value | AUC | Sensitivity (95% CI) | Specificity (95% CI) | LR (+) (95% CI) | LR (−) (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|
| WBC/PLT | >0.064 | 0.984 | 100 (59.0–100) | 94.3 (84.3–98.8) | 17.7 (5.89–53.0) | 0 | <0.001 |
| WBC/INR | >3.2 | 0.784 | 85.7 (42.1–99.6) | 75.5 (61.7–86.2) | 3.49 (1.99–6.12) | 0.19 (0.031–1.17) | 0.017 |
| WBC/APTT | >0.106 | 0.757 | 85.7 (42.1–99.6) | 75.5 (61.7–86.2) | 3.49 (1.99–6.12) | 0.19 (0.031–1.17) | 0.030 |
| WBC/D-dimer | ≤0.45 | 0.879 | 100 (59.0–100) | 77.4 (63.8–87.7) | 4.42 (2.69–7.26) | 0 | <0.001 |
| WBC/Fibrinogen | >0.017 | 0.903 | 85.7 (42.1–99.6) | 90.6 (79.3–96.9) | 9.09 (3.74 22.1) | 0.16 (0.026–0.97) | <0.001 |
| WBC/CRP | ≤0.14 | 0.720 | 85.7 (42.1–99.6) | 64.2 (49.8–76.9) | 2.39 (1.49–3.83) | 0.22 (0.036–1.38) | 0.014 |
| WBC/IL-6 | ≤0.048 | 0.814 | 85.7 (42.1–99.6) | 71.7 (57.7–83.2) | 3.03 (1.79–5.12) | 0.2 (0.032–1.23) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasbek, M.; Kıymaz, Y.Ç.; Büyüktuna, S.A.; Yavuz, H. Prognostic Value of Ratios of Inflammatory Markers in the Prognosis of Crimean–Congo Hemorrhagic Fever. Trop. Med. Infect. Dis. 2025, 10, 99. https://doi.org/10.3390/tropicalmed10040099
Hasbek M, Kıymaz YÇ, Büyüktuna SA, Yavuz H. Prognostic Value of Ratios of Inflammatory Markers in the Prognosis of Crimean–Congo Hemorrhagic Fever. Tropical Medicine and Infectious Disease. 2025; 10(4):99. https://doi.org/10.3390/tropicalmed10040099
Chicago/Turabian StyleHasbek, Mürşit, Yasemin Çakır Kıymaz, Seyit Ali Büyüktuna, and Hayrettin Yavuz. 2025. "Prognostic Value of Ratios of Inflammatory Markers in the Prognosis of Crimean–Congo Hemorrhagic Fever" Tropical Medicine and Infectious Disease 10, no. 4: 99. https://doi.org/10.3390/tropicalmed10040099
APA StyleHasbek, M., Kıymaz, Y. Ç., Büyüktuna, S. A., & Yavuz, H. (2025). Prognostic Value of Ratios of Inflammatory Markers in the Prognosis of Crimean–Congo Hemorrhagic Fever. Tropical Medicine and Infectious Disease, 10(4), 99. https://doi.org/10.3390/tropicalmed10040099






