Schistosomiasis and Soil Transmitted Helminthiasis Among School Age Children: Impact of 3–5 Annual Rounds of Mass Drug Administration in Ekiti State, Southwest Nigeria
Abstract
1. Introduction
2. Problem Statement
Ethical Clearance
3. Methodology
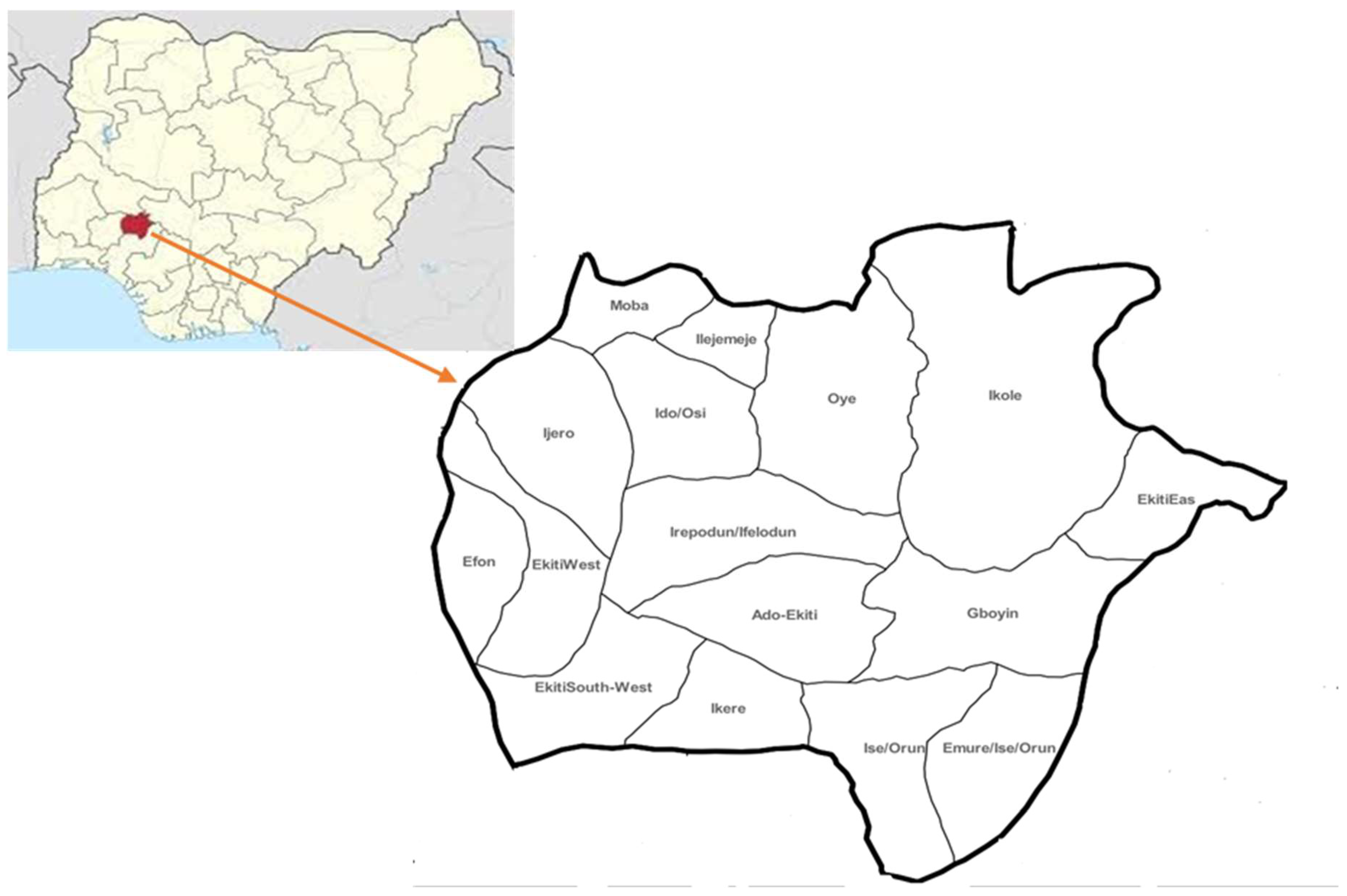
3.1. Study Site and Population
3.2. Study Design: Site Selection and Sample Size
3.3. Stool and Urine Sample Collection at School
3.4. Urine Filtration Technique
3.5. Kato–Katz Technique for Stool Examination
3.6. Data Processing and Analysis
4. Results
4.1. Overall Prevalence of Schistosomiasis and STH
4.2. Prevalence of Schistosomiasis and STH by Sex
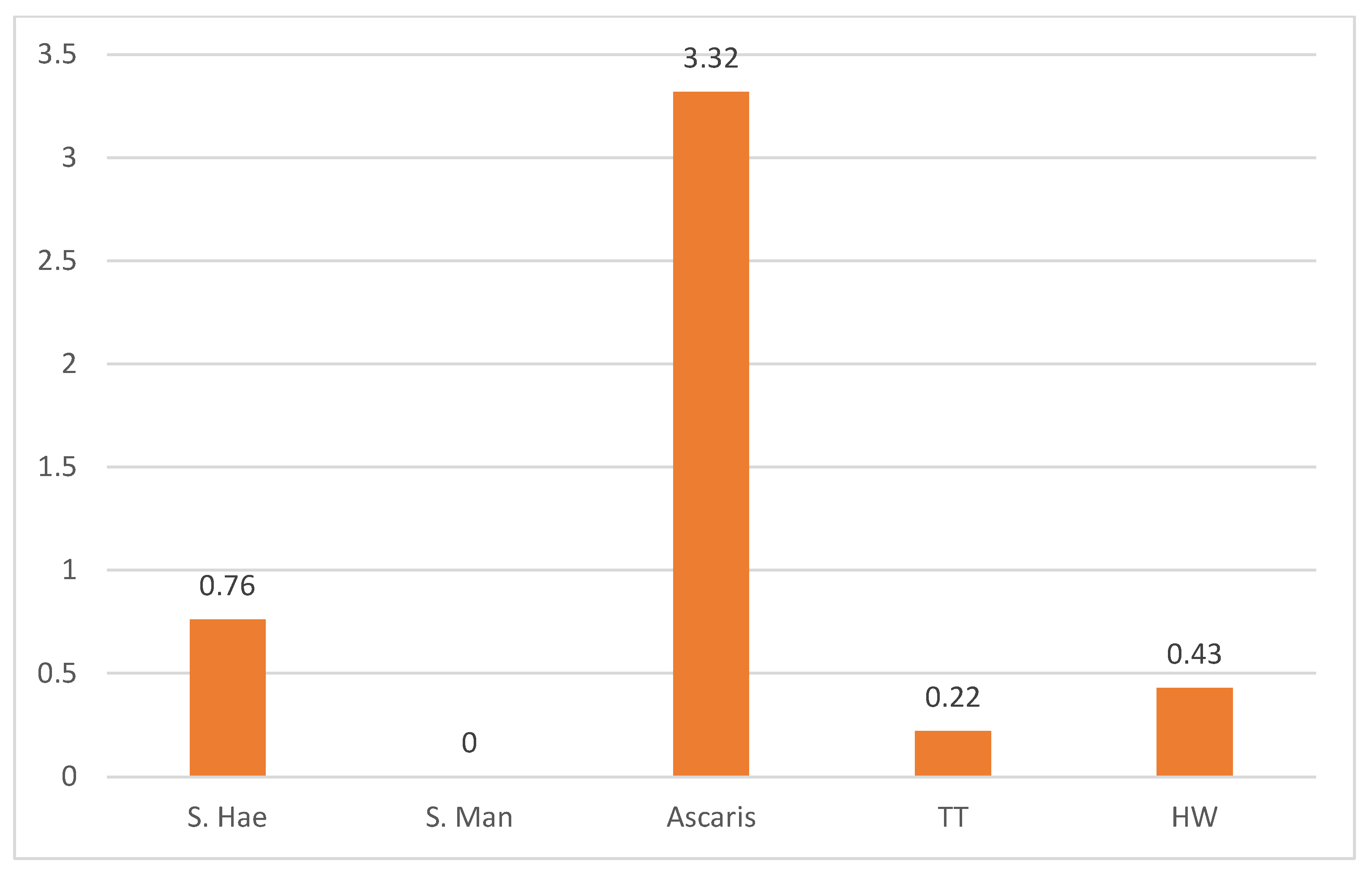
4.3. Distribution of Parasites by Species Across the LGA
4.4. Infection Load Estimation
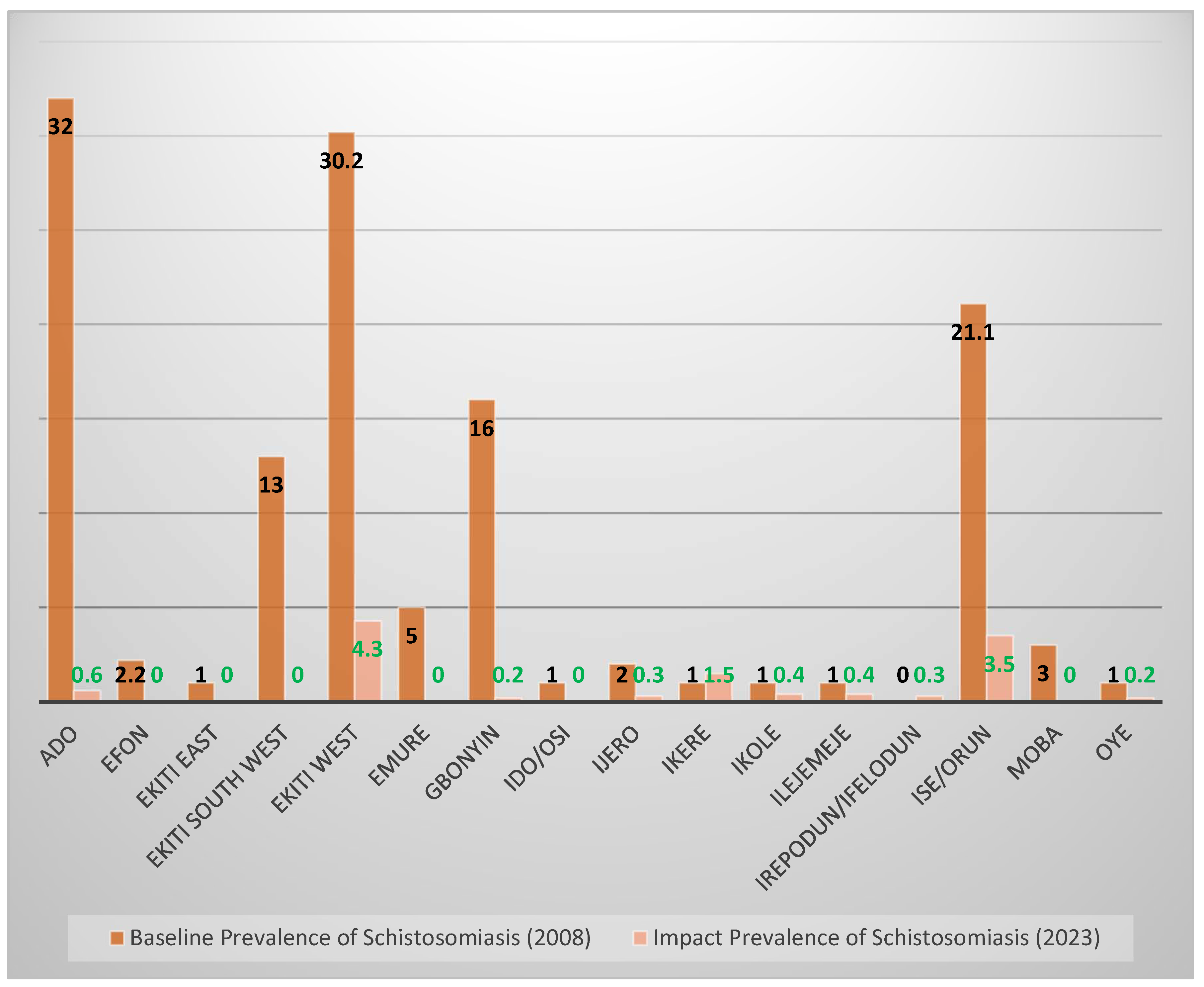
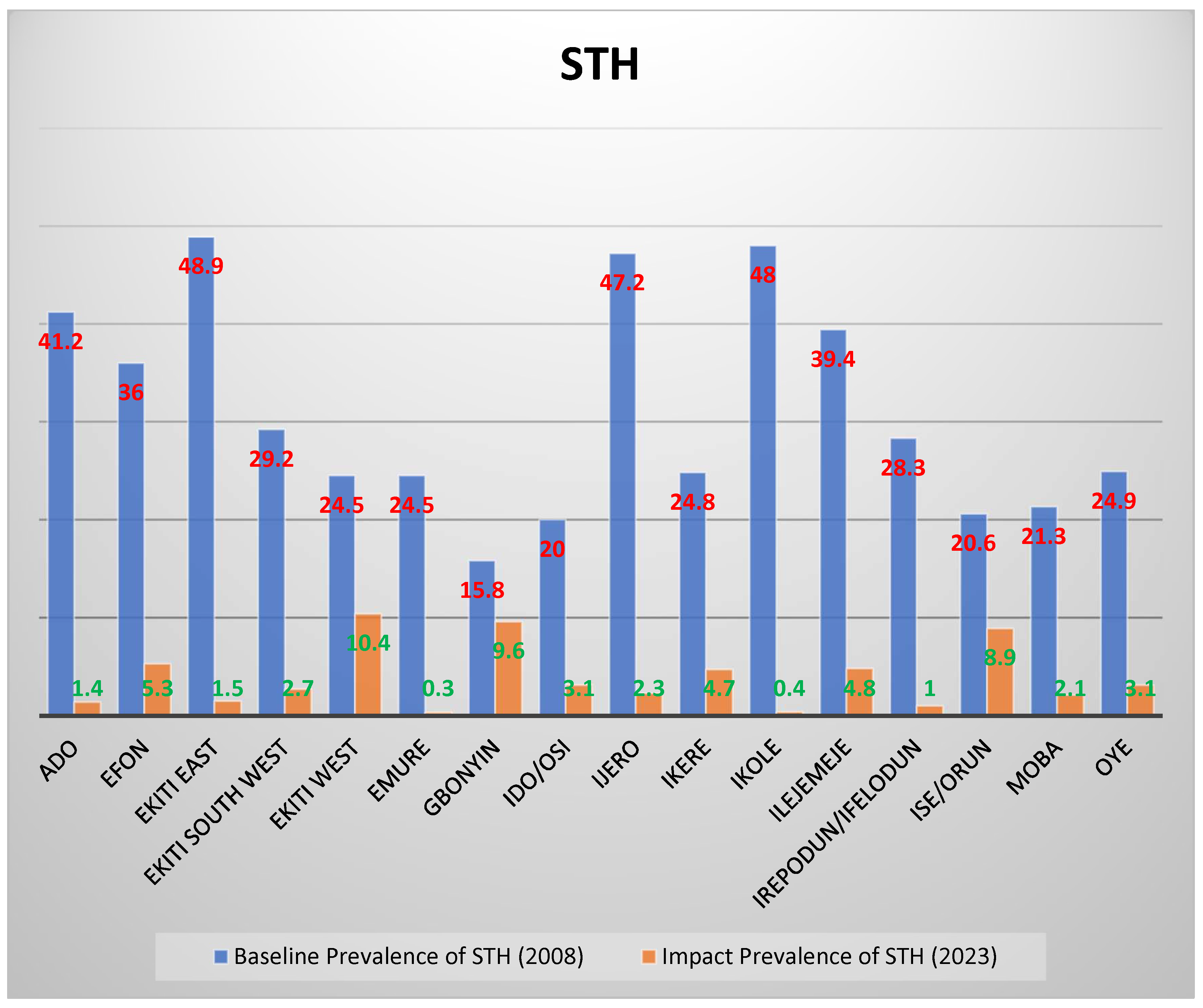
4.5. Prevalence After 2–3 Rounds of Treatment Compared with Baseline Prevalence
5. Discussion
6. Limitations of the Study
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steinmann, P.; Keiser, J.; Bos, R.; Tanner, M.; Utzinger, J. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 2006, 6, 411–425. [Google Scholar] [PubMed]
- Hotez, P.J.; Yamey, G. The evolving scope of PLoS Neglected Tropical Diseases. PLoS Negl. Trop. Dis. 2009, 3, e379. [Google Scholar] [CrossRef]
- WHO. Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis: Report of a WHO Expert Committee; WHO Technical Report Series No. 912; World Health Organization: Geneva, Switzerland, 2002; pp. 1–57.
- Kabatereine, N.B.; Vennervald, B.J.; Ouma, J.H.; Kemijumbi, J.; Butterworth, A.E.; Dunne, D.W.; Fulford AJ, C. Adult resistance to schistosomiasis mansoni: Age-dependence to reinfection remains constant in communities with diverse exposure patterns. Parasitology 1999, 118, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Lackey, E.K.; Horrall, S. Schistosomiasis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025; Available online: https://www.ncbi.nlm.nih.gov/books/NBK554434/ (accessed on 18 January 2025).
- Abossie, A.; Seid, M. Assessment of the prevalence of intestinal parasitosis and associated risk factors among primary school children in Chencha town, Southern Ethiopia. BMC Public Health 2014, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Knopp, S.; Steinmann, P.; Keiser, J.; Utzinger, J. Nematode infections: Soil-transmitted helminths and trichinella. Infect. Dis. Clin. North Am. 2012, 26, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Bethony, J.; Brooker, S.; Albonico, M.; Geiger, S.M.; Loukas, A.; Diemert, D.; Hotez, P.J. Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet 2006, 367, 1521–1532. [Google Scholar] [PubMed]
- de Silva, N.R.; Brooker, S.; Hotez, P.J.; Montresor, A.; Engels, D.; Savioli, L. Soil-transmitted helminth infections: Updating the global picture. Trends Parasitol. 2003, 19, 547–551. [Google Scholar] [CrossRef] [PubMed]
- WHO. Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers; World Health Organization Press: Geneva, Switzerland, 2006.
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals—A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2020.
- WHO-AFRO. Guide for Mapping Neglected Tropical Diseases Targeted by Preventive Chemotherapy in the African Region; World Health Organization-Regional Office for Africa: Brazzaville, Congo, 2014.
- Lauren Thomas Systematic Sampling|A Step-by-Step Guide with Examples 2020. Revised 2023. Available online: https://www.scribbr.com/author/laurenthomas (accessed on 21 January 2025).
- Lengeler, C.; Mshinda, H.; Morona, D.; deSavigny, D. Urinary schistosomiasis: Testing with urine filtration and reagent sticks for haematuria provides a comparable prevalence estimate. Acta Trop. 1993, 53, 39–50. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Basic laboratory methods in medical parasitology; World Health Organization: Geneva, Switzerland, 1991.
- Montresor, A.; Deol, A.; à Porta, N.; Lethanh, N.; Jankovic, D. Markov Model Predicts Changes in STH Prevalence during Control Activities Even with a Reduced Amount of Baseline Information. PLoS Neglected Trop. Dis. 2016, 10, e0004371. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Makau-Barasa, L.; Assefa, L.; Aderogba, M.; Bell, D.; Solomon, J.; Urude, R.O.; Nebe, O.J.; A-Enegela, J.; Damen, J.G.; Popoola, S.; et al. Performance evaluation of the AiDx multi-diagnostic automated microscope for the detection of schistosomiasis in Abuja, Nigeria. Sci. Rep. 2023, 13, 14833. [Google Scholar] [CrossRef] [PubMed]
- Onasanya, A.; Bengtson, M.; Agbana, T.; Oladunni, O.; van Engelen, J.; Oladepo, O.; Diehl, J.C. Towards Inclusive Diagnostics for Neglected Tropical Diseases: User Experience of a New Digital Diagnostic Device in Low-Income Settings. Trop. Med. Infect. Dis. 2023, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guideline on Control and Elimination of Human Schistosomiasis 2022; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-004160-8. electronic version.
- World Health Organization. Report of a Meeting to Review the Results of Studies on the Treatment of Schistosomiasis in Preschool-Age Children; WHO: Geneva, Switzerland, 2010.
- Harris-Roxas, B.; Viliani, F.; Bond, A.; Cave, B.; Divall, M.; Furu, P. Health impact assessment: The state of the art. Impact Assess. Proj. Apprais. 2012, 30, 43–52. [Google Scholar] [CrossRef]
- Evan, S.W. Water-based interventions for schistosomiasis control. Path Glob. Health 2014, 108, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Grimes, J.E.; Croll, D.; Harrison, W.E.; Utzinger, J.; Freeman, M.C.; Templeton, M.R. The relationship between water, sanitation and schistosomiasis: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2014, 8, e3296. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.J.; Biritwum, N.K.; Woods, G.; Velleman, Y.; Fleming, F.; Stothard, J.R. Tailoring water, sanitation, and hygiene (WASH) targets for soil-transmitted helminthiasis and schistosomiasis control. Trends Parasitol. 2018, 34, 53–63. [Google Scholar] [PubMed]
- Hailegebriel, T.; Nibret, E.; Munshea, A. Efficacy of Praziquantel for the Treatment of Human Schistosomiasis in Ethiopia: A Systematic Review and Meta-Analysis. J. Trop. Med. 2021, 2021, 2625255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berhanu, M.S.; Atnafie, S.A.; Ali, T.E.; Chekol, A.A.; Kebede, H.B. Efficacy of Praziquantel Treatment and Schistosoma Mansoni Infection among Primary School Children in Kemisse Town, Northeast Ethiopia. Ethiop. J. Health Sci. 2022, 32, 631–640. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nduka, F.; Nebe, O.J.; Njepuome, N.; Dakul, D.A.; Anagbogu, I.A.; Ngege, E. Epidemiological mapping of schistosomiasis and soil-transmitted helminthiasis for intervention strategies in Nigeria. Nig. J. Parasitol. 2019, 40, 124–131. [Google Scholar] [CrossRef]
- Hall, A.; Holland, C. Geographical variation in Ascaris lumbricoides fecundity and its implications for helminth control. Parasitol. Today 2000, 16, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Bundy, D.A.P.; de Silva, N.; Appleby, L.J.; Brooker, S.J. Intestinal Nematodes: Ascariasis. In Hunter’s Tropical Medicine and Emerging Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2020; pp. 840–844. [Google Scholar] [CrossRef]
| LGA | No. of Respondents | Prevalence of Schistosomiasis | Prevalence of STH | ||||
|---|---|---|---|---|---|---|---|
| No. Positive | Prev. % | 95% CI | No. Positive | Prev. % | 95% CI | ||
| Ado | 658 | 4 | 0.6 | 0.14–1.20 | 9 | 1.4 | 0.48–2.26 |
| Efon | 416 | 0 | 0 | 0.00 | 22 | 5.3 | 3.13–7.44 |
| Ekiti East | 618 | 0 | 0 | 0.00 | 9 | 1.5 | 0.51–2.40 |
| Ekiti South West | 556 | 0 | 0 | 0.00 | 15 | 2.7 | 1.35–4.04 |
| Ekiti West | 469 | 20 | 4.3 | 2.44–6.09 | 49 | 10.4 | 7.68–13.22 |
| Emure | 320 | 0 | 0 | 0.00 | 1 | 0.3 | ‒0.30–0.90 |
| Gbonyin | 447 | 1 | 0.2 | 0.02–0.60 | 43 | 9.6 | 6.89–12.35 |
| Ido/Osi | 509 | 0 | 0 | 0.00 | 16 | 3.1 | 1.63–4.66 |
| Ijero | 303 | 1 | 0.3 | 0.03–0.98 | 7 | 2.3 | 0.62–4.00 |
| Ikere | 550 | 8 | 1.5 | 0.40–2.46 | 26 | 4.7 | 2.95–6.50 |
| Ikole | 527 | 2 | 0.4 | 0.10–0.90 | 2 | 0.4 | 0.15–0.90 |
| Ilejemeje | 546 | 2 | 0.4 | 0.14–0.87 | 26 | 4.8 | 2.98–6.55 |
| Irepodun/Ifelodun | 396 | 1 | 0.3 | 0.02–0.70 | 4 | 1 | 0.03–2.00 |
| Ise/Orun | 517 | 18 | 3.5 | 1.90–5.10 | 46 | 8.9 | 6.44–11.35 |
| Moba | 420 | 0 | 0 | 0.00 | 8 | 1.9 | 0.60–3.21 |
| Oye | 418 | 1 | 0.2 | 0.23–0.71 | 13 | 3.1 | 1.45–4.77 |
| Total | 7670 | 58 | 0.8 | 0.56–0.95 | 296 | 3.9 | 3.43–4.29 |
| Disease | Total Sampled | Male | Female | p-Value | ||
|---|---|---|---|---|---|---|
| Sampled | No. Positive (Prevalence) | Sampled | No. Positive (Prevalence) | |||
| Schistosomiasis | 7670 | 3823 | 29 (0.76%) | 3847 | 29 (0.75%) | p > 0.05 |
| STH | 7670 | 3823 | 151(3.95%) | 3847 | 145 (3.77%) | p > 0.05 |
| LGA | No. Examined | Schistosomiasis | STH | |||||
|---|---|---|---|---|---|---|---|---|
| No. Positive | Light Infection (< 50 Eggs per mL) | Heavy Infection (> 50 Eggs per mL) | No. Positive | Light Infection (1–999 epg) | Moderate Infection (1000–9999 epg) | Heavy Infection (≥10,000 epg) | ||
| Ado | 658 | 4 | 3 (75%) | 1 (25%) | 9 | 9 (100%) | 0 (0%) | 0 (0%) |
| Efon | 416 | 0 | 0 (0%) | 0 (0%) | 22 | 22 (100%) | 0 (0%) | 0 (0%) |
| Ekiti East | 618 | 0 | 0 (0%) | 0 (0%) | 9 | 9 (100%) | 0 (0%) | 0 (0%) |
| Ekiti South West | 556 | 0 | 0 (0%) | 0 (0%) | 15 | 15 (100%) | 0 (0%) | 0 (0%) |
| Ekiti West | 469 | 20 | 19 (95%) | 1 (5%) | 49 | 47 (96%) | 2 (4%) | 0 (0%) |
| Emure | 320 | 0 | 0 (0%) | 0 (0%) | 1 | 1 (100%) | 0 (0%) | 0 (0%) |
| Gbonyin | 447 | 1 | 1 (100%) | 0 (0%) | 43 | 43 (100%) | 0 (0%) | 0 (0%) |
| Ido/Osi | 509 | 0 | 0 (0%) | 0 (0%) | 16 | 16 (100%) | 0 (0%) | 0 (0%) |
| Ijero | 303 | 1 | 1 (100%) | 0 (0%) | 7 | 7 (100%) | 0 (0%) | 0 (0%) |
| Ikere | 550 | 8 | 7 (87.5%) | 1 (12.5%) | 26 | 25 (96%) | 1 (4%) | 0 (0%) |
| Ikole | 527 | 2 | 2 (100%) | 0 (0%) | 2 | 2 (100%) | 0 (0%) | 0 (0%) |
| Ilejemeje | 546 | 2 | 2 (100%) | 0 (0%) | 26 | 26 (100%) | 0 (0%) | 0 (0%) |
| Irepodun/Ifelodun | 396 | 1 | 0 (0%) | 1 (100%) | 4 | 3 (75%) | 1 (25%) | 0 (0%) |
| Ise/Orun | 517 | 18 | 16 (89%) | 2 (11%) | 46 | 46 (100%) | 0 (0%) | 0 (0%) |
| Moba | 420 | 0 | 0 (0%) | 0 (0%) | 8 | 8 (100%) | 0 (0%) | 0 (0%) |
| Oye | 418 | 1 | 1 (100%) | 0 (0%) | 13 | 13 (100%) | 0 (0%) | 0 (0%) |
| TOTAL | 7670 | 58 | 52 (89.7%) | 6 (10.3%) | 296 | 292 (98.6%) | 4 (1.4%) | 0 (0.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacob, S.M.; Diehl, J.-C.; Vdovine, G.; Agbana, T.; Popoola, S.; Jujjavarapu, S.; Bell, D.; Ajayi, A.O.; Fadare, J.O.; Akinwumi, A.F.; et al. Schistosomiasis and Soil Transmitted Helminthiasis Among School Age Children: Impact of 3–5 Annual Rounds of Mass Drug Administration in Ekiti State, Southwest Nigeria. Trop. Med. Infect. Dis. 2025, 10, 85. https://doi.org/10.3390/tropicalmed10040085
Jacob SM, Diehl J-C, Vdovine G, Agbana T, Popoola S, Jujjavarapu S, Bell D, Ajayi AO, Fadare JO, Akinwumi AF, et al. Schistosomiasis and Soil Transmitted Helminthiasis Among School Age Children: Impact of 3–5 Annual Rounds of Mass Drug Administration in Ekiti State, Southwest Nigeria. Tropical Medicine and Infectious Disease. 2025; 10(4):85. https://doi.org/10.3390/tropicalmed10040085
Chicago/Turabian StyleJacob, Solomon Monday, Jan-Carel Diehl, Gleb Vdovine, Temitope Agbana, Samuel Popoola, Satyajith Jujjavarapu, David Bell, Akande Oladimeji Ajayi, Joseph O. Fadare, Adebowale F. Akinwumi, and et al. 2025. "Schistosomiasis and Soil Transmitted Helminthiasis Among School Age Children: Impact of 3–5 Annual Rounds of Mass Drug Administration in Ekiti State, Southwest Nigeria" Tropical Medicine and Infectious Disease 10, no. 4: 85. https://doi.org/10.3390/tropicalmed10040085
APA StyleJacob, S. M., Diehl, J.-C., Vdovine, G., Agbana, T., Popoola, S., Jujjavarapu, S., Bell, D., Ajayi, A. O., Fadare, J. O., Akinwumi, A. F., Animashaun, S., Olamiju, F., Aderogba, M. O., & Makau-Barasa, L. (2025). Schistosomiasis and Soil Transmitted Helminthiasis Among School Age Children: Impact of 3–5 Annual Rounds of Mass Drug Administration in Ekiti State, Southwest Nigeria. Tropical Medicine and Infectious Disease, 10(4), 85. https://doi.org/10.3390/tropicalmed10040085






