The Etiology and Antimicrobial Susceptibility of Community-Onset Urinary Tract Infections in a Low-Resource/High-Resistance Area of Latin America
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Bacterial Isolation, Identification and Antimicrobial Susceptibility Testing
2.3. Molecular Characterization of Acquired Resistance Genes
2.4. Statistical Analysis
3. Results
3.1. Antibiotic Prescribing Patterns in Uncomplicated and Complicated Community-Onset Urinary Tract Infections
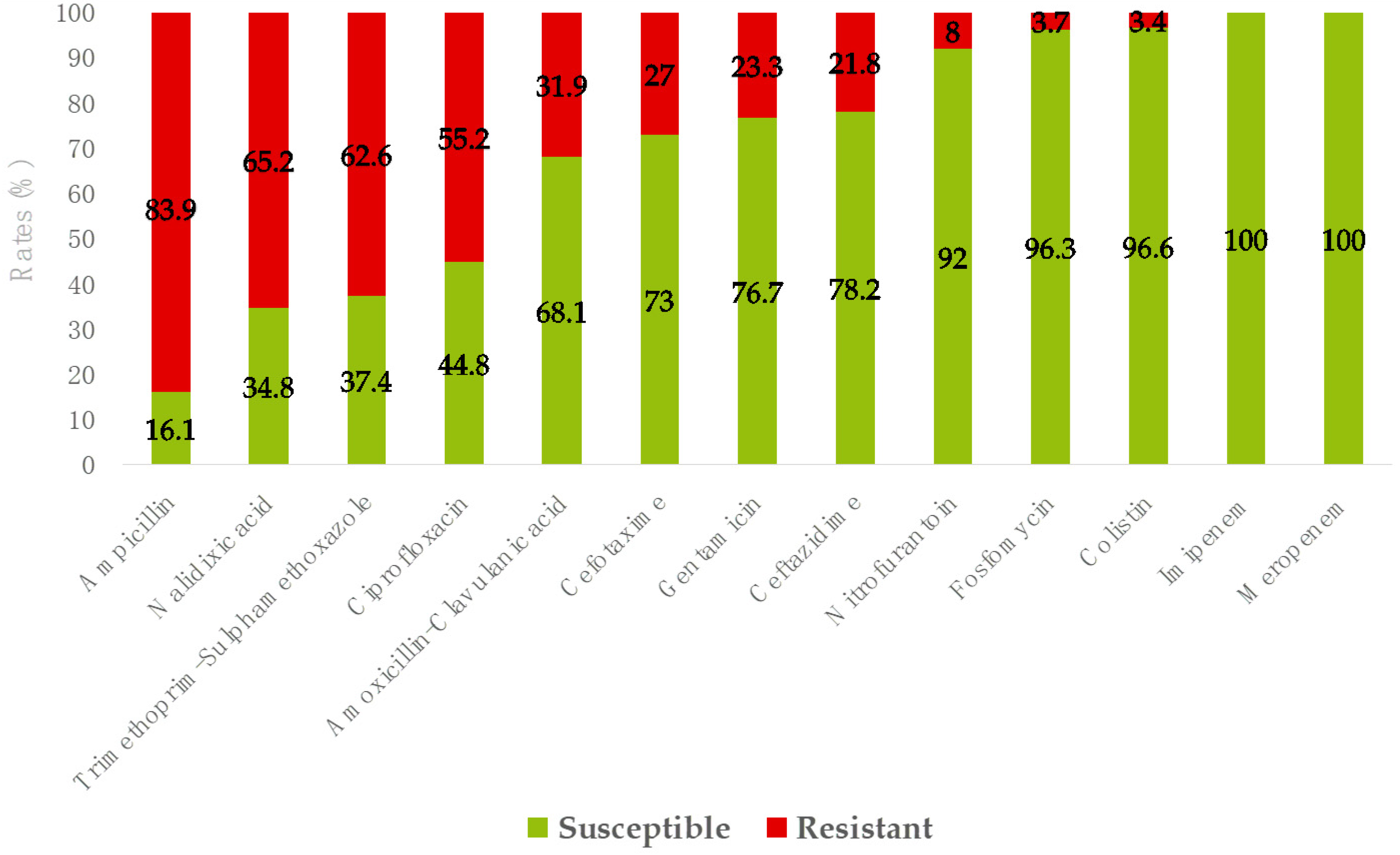
3.2. Etiology and Antimicrobial Resistance of Community-Onset Urinary Tract Infections
3.3. Molecular Characterization of Acquired Resistance Genes Among E. coli
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| MDR | Multi-drug resistant |
| UTI | Urinary tract infection |
| CO | Community onset |
| uUTI | Uncomplicated urinary tract infection |
| cUTI | Complicated urinary tract infection |
| ESBL | Extended-spectrum β-lactamase |
References
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary Tract Infections: Epidemiology, Mechanisms of Infection and Treatment Options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Levison, M.E.; Kaye, D. Treatment of Complicated Urinary Tract Infections With an Emphasis on Drug-Resistant Gram-Negative Uropathogens. Curr. Infect. Dis. Rep. 2013, 15, 109–115. [Google Scholar] [CrossRef] [PubMed]
- European Association of Urology (EAU). EAU Guidelines on Urological Infections; European Association of Urology (EAU): Arnhem, The Netherlands, 2022. [Google Scholar]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef] [PubMed]
- Al Lawati, H.; Blair, B.M.; Larnard, J. Urinary Tract Infections: Core Curriculum 2024. Am. J. Kidney Dis. 2024, 83, 90–100. [Google Scholar] [CrossRef]
- Kolman, K.B. Cystitis and Pyelonephritis. Prim. Care Clin. Off. Pract. 2019, 46, 191–202. [Google Scholar] [CrossRef]
- Medina, M.; Castillo-Pino, E. An Introduction to the Epidemiology and Burden of Urinary Tract Infections. Ther. Adv. Urol. 2019, 11, 175628721983217. [Google Scholar] [CrossRef]
- ASUSS. Normas de Diagnóstico y Tratamiento de Nefrología; Autoridad de Supervisión de la Seguridad Social de Corto Plazo: La Paz, Bolivia, 2019. [Google Scholar]
- Prieto Valtueña, J.M.; Yuste Ara, J.R. La Clínica y El Laboratorio: Interpretación de Análisis y Pruebas Funcionales. Exploración de Los Síndromes. Cuadro Biológico de Las Enfermedades, 23rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9788491133018. [Google Scholar]
- Weinstein, M.P.; Limbago, B.; Patel, J.B.; Mathers, A.J.; Burnham, C.-A.; Mazzulli, T.; Campeau, S.; Munro, S.D.; Conville, P.S.; de Danies, M.O.S.; et al. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Lewis II, J.S.; Weinstein, M.P.; Bobenchik, A.M.; Campeau, S.; Cullen, S.K.; Dingle, T.; Galas, M.F.; Humphries, R.M.; Kirn, T.J.; Limbago, B.; et al. Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Jarlier, V.; Nicolas, M.-H.; Fournier, G.; Philippon, A. Extended Broad-Spectrum β-Lactamases Conferring Transferable Resistance to Newer β-Lactam Agents in Enterobacteriaceae: Hospital Prevalence and Susceptibility Patterns. Clin. Infect. Dis. 1988, 10, 867–878. [Google Scholar] [CrossRef]
- Giani, T.; Antonelli, A.; Caltagirone, M.; Mauri, C.; Nicchi, J.; Arena, F.; Nucleo, E.; Bracco, S.; Pantosti, A.; Luzzaro, F.; et al. Evolving Beta-Lactamase Epidemiology in Enterobacteriaceae from Italian Nationwide Surveillance, October 2013: KPC-Carbapenemase Spreading among Outpatients. Eurosurveillance 2017, 22, 30583. [Google Scholar] [CrossRef]
- Foglietta, G.; De Carolis, E.; Mattana, G.; Onori, M.; Agosta, M.; Niccolai, C.; Di Pilato, V.; Rossolini, G.M.; Sanguinetti, M.; Perno, C.F.; et al. “CORE” a New Assay for Rapid Identification of Klebsiella Pneumoniae COlistin REsistant Strains by MALDI-TOF MS in Positive-Ion Mode. Front. Microbiol. 2023, 14, 1045289. [Google Scholar] [CrossRef]
- Coppi, M.; Cannatelli, A.; Antonelli, A.; Baccani, I.; Di Pilato, V.; Sennati, S.; Giani, T.; Rossolini, G.M. A Simple Phenotypic Method for Screening of MCR-1-Mediated Colistin Resistance. Clin. Microbiol. Infect. 2018, 24, 201.e1–201.e3. [Google Scholar] [CrossRef] [PubMed]
- Bartoloni, A.; Sennati, S.; Di Maggio, T.; Mantella, A.; Riccobono, E.; Strohmeyer, M.; Revollo, C.; Villagran, A.L.; Pallecchi, L.; Rossolini, G.M. Antimicrobial Susceptibility and Emerging Resistance Determinants (blaCTX-M, RmtB, FosA3) in clinical isolates from Urinary Tract Infections in the Bolivian Chaco. Int. J. Infect. Dis. 2016, 43, 1–6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boncompagni, S.R.; Micieli, M.; Di Maggio, T.; Mantella, A.; Villagrán, A.L.; Briggesth Miranda, T.; Revollo, C.; Poma, V.; Gamboa, H.; Spinicci, M.; et al. Relevant Increase of CTX-M-Producing Escherichia coli Carriage in School-Aged Children from Rural Areas of the Bolivian Chaco in a Three-Year Period. Int. J. Infect. Dis. 2022, 121, 126–129. [Google Scholar] [CrossRef]
- Pallecchi, L.; Malossi, M.; Mantella, A.; Gotuzzo, E.; Trigoso, C.; Bartoloni, A.; Paradisi, F.; Kronvall, G.; Rossolini, G.M. Detection of CTX-M-Type β-Lactamase Genes in Fecal Escherichia Coli Isolates from Healthy Children in Bolivia and Peru. Antimicrob. Agents Chemother. 2004, 48, 4556–4561. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.E.; Weidner, W.; Pilatz, A.; Naber, K.G. Urinary Tract Infections and Bacterial Prostatitis in Men. Curr. Opin. Infect. Dis. 2014, 27, 97–101. [Google Scholar] [CrossRef]
- von Wintersdorff, C.J.H.; Penders, J.; van Niekerk, J.M.; Mills, N.D.; Majumder, S.; van Alphen, L.B.; Savelkoul, P.H.M.; Wolffs, P.F.G. Dissemination of Antimicrobial Resistance in Microbial Ecosystems through Horizontal Gene Transfer. Front. Microbiol. 2016, 7, 173. [Google Scholar] [CrossRef]
- Giani, T.; Sennati, S.; Antonelli, A.; Di Pilato, V.; di Maggio, T.; Mantella, A.; Niccolai, C.; Spinicci, M.; Monasterio, J.; Castellanos, P.; et al. High Prevalence of Carriage of mcr-1-Positive Enteric Bacteria among Healthy Children from Rural Communities in the Chaco Region, Bolivia, September to October 2016. Eurosurveillance 2018, 23, 1800115. [Google Scholar] [CrossRef]
- SENASAG (Servicio Nacional de Sanidad Agropecuaria e Inocuidad Alimentaria). Resolución Administrativa No. 158; Base de datos FAOLEX: Potosí, Bolivia, 2019. [Google Scholar]
- Woodford, N.; Fagan, E.J.; Ellington, M.J. Multiplex PCR for Rapid Detection of Genes Encoding CTX-M Extended-Spectrum β-Lactamases. J. Antimicrob. Chemother. 2006, 57, 154–155. [Google Scholar] [CrossRef]
- Riccobono, E.; Di Pilato, V.; Villagran, A.L.; Bartoloni, A.; Rossolini, G.M.; Pallecchi, L. Complete Sequence of PV404, a Novel IncI1 Plasmid Harbouring BlaCTX-M-14 in an Original Genetic Context. Int. J. Antimicrob. Agents 2014, 44, 374–376. [Google Scholar] [CrossRef]
- Cannatelli, A.; Giani, T.; Antonelli, A.; Principe, L.; Luzzaro, F.; Rossolini, G.M. First Detection of the Mcr-1 Colistin Resistance Gene in Escherichia Coli in Italy. Antimicrob. Agents Chemother. 2016, 60, 3257–3258. [Google Scholar] [CrossRef]
- Di Pilato, V.; Arena, F.; Tascini, C.; Cannatelli, A.; Henrici De Angelis, L.; Fortunato, S.; Giani, T.; Menichetti, F.; Rossolini, G.M. Mcr-1.2, a New Mcr Variant Carried on a Transferable Plasmid from a Colistin-Resistant KPC Carbapenemase-Producing Klebsiella Pneumoniae Strain of Sequence Type 512. Antimicrob. Agents Chemother. 2016, 60, 5612–5615. [Google Scholar] [CrossRef] [PubMed]
- Xavier, B.B.; Lammens, C.; Ruhal, R.; Kumar-Singh, S.; Butaye, P.; Goossens, H.; Malhotra-Kumar, S. Identification of a Novel Plasmid-Mediated Colistin-Resistance Gene, Mcr-2, in Escherichia Coli, Belgium, June 2016. Eurosurveillance 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Roer, L.; Hansen, F.; Stegger, M.; Sönksen, U.W.; Hasman, H.; Hammerum, A.M. Novel Mcr-3 Variant, Encoding Mobile Colistin Resistance, in an ST131 Escherichia Coli Isolate from Bloodstream Infection, Denmark, 2014. Eurosurveillance 2017, 22. [Google Scholar] [CrossRef]
- Carattoli, A.; Villa, L.; Feudi, C.; Curcio, L.; Orsini, S.; Luppi, A.; Pezzotti, G.; Magistrali, C.F. Novel Plasmid-Mediated Colistin Resistance Mcr-4 Gene in Salmonella and Escherichia Coli, Italy 2013, Spain and Belgium, 2015 to 2016. Eurosurveillance 2017, 22. [Google Scholar] [CrossRef]
- Borowiak, M.; Hammerl, J.A.; Deneke, C.; Fischer, J.; Szabo, I.; Malorny, B. Characterization of Mcr-5 -Harboring Salmonella Enterica Subsp. Enterica Serovar Typhimurium Isolates from Animal and Food Origin in Germany. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]

| Positive Urine Culture | |||
|---|---|---|---|
| Total (n = 361) | Uncomplicated (n = 210) | Complicated (n = 151) | |
| Age: median (IQR) | 38.5 (30) | 42 (25) | 55 (35) |
| Age groups, years | |||
| 18–64 | 278 (77) | 175 (83.3) * | 103 (68.2) |
| ≥65 | 83 (23) | 35 (16.7) | 48 (31.8) * |
| Gender | |||
| Females | 308 (65.3) | 210 (100) | 98 (64.9) |
| Males | 53 (14.7) | NA | 53 (35.1) |
| Underlying diseases | |||
| Renal failure | 7 (1.9) | NA | 7 (4.6) |
| Liver cirrhosis | 1 (0.3) | NA | 1 (0.7) |
| Diabetes mellitus | 60 (16.6) | NA | 60 (39.7) |
| Predisposing factors | |||
| Previous UTI | 67 (18.6) | 35 (16.7) | 32 (21.2) |
| Recurrent UTI | 61 (16.9) | 32 (15.2) | 29 (19.2) |
| Catheter | 11 (3) | NA | 11 (7.3) |
| Pregnancy | 38 (10.5) | NA | 38 (25.2) |
| Prostatitis | 5 (1.4) | NA | 5 (3.3) |
| uUTIs (n = 213) | cUTIs (n = 159) | Total UTIs (n = 372) | p Value 1 | ||||
|---|---|---|---|---|---|---|---|
| Species | No. | % | No. | % | No. | % | |
| Escherichia coli | 184 | 86.4 | 138 | 86.8 | 322 | 86.6 | 1 |
| Klebsiella pneumoniae | 10 | 4.7 | 10 | 6.3 | 20 | 5.4 | 0.5 |
| Proteus mirabilis | 4 | 1.9 | 2 | 1.3 | 6 | 1.6 | 1 |
| Morganella morganii | 2 | 0.9 | 2 | 1.3 | 4 | 1.1 | 1 |
| Citrobacter koseri | 2 | 0.9 | - | - | 2 | 0.5 | - |
| Staphylococcus saprophyticus | 2 | 0.9 | - | - | 2 | 0.5 | - |
| Acinetobacter junii | 1 | 0.5 | - | - | 1 | 0.3 | - |
| Acinetobacter pitti | 1 | 0.5 | - | - | 1 | 0.3 | - |
| Pseudomonas mendocina | 1 | 0.5 | - | - | 1 | 0.3 | - |
| Pseudomonas stutzeri | 1 | 0.5 | - | - | 1 | 0.3 | - |
| Enterobacter cloacae | 1 | 0.5 | - | - | 1 | 0.3 | - |
| Escherichia vulneris | 1 | 0.5 | - | - | 1 | 0.3 | - |
| Klebsiella variicola | 1 | 0.5 | - | - | 1 | 0.3 | - |
| Salmonella spp. | 1 | 0.5 | - | - | 1 | 0.3 | - |
| Stenotrophomonas maltophilia | 1 | 0.5 | - | - | 1 | 0.3 | - |
| Proteus vulgaris | - | - | 2 | 1.3 | 2 | 0.9 | - |
| Klebsiella oxytoca | - | - | 1 | 0.6 | 1 | 0.3 | - |
| Citrobacter freundii | - | - | 1 | 0.6 | 1 | 0.3 | - |
| Enterobacter asburiae | - | - | 1 | 0.6 | 1 | 0.3 | - |
| Enterococcus faecalis | - | - | 1 | 0.6 | 1 | 0.3 | - |
| Serratia marcescens | - | - | 1 | 0.6 | 1 | 0.3 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micieli, M.; Boncompagni, S.R.; Di Maggio, T.; Ramos, Y.B.M.; Mantella, A.; Villagrán, A.L.; Yelma, C.A.R.; Fernández, E.E.F.; Spinicci, M.; Strohmeyer, M.; et al. The Etiology and Antimicrobial Susceptibility of Community-Onset Urinary Tract Infections in a Low-Resource/High-Resistance Area of Latin America. Trop. Med. Infect. Dis. 2025, 10, 64. https://doi.org/10.3390/tropicalmed10030064
Micieli M, Boncompagni SR, Di Maggio T, Ramos YBM, Mantella A, Villagrán AL, Yelma CAR, Fernández EEF, Spinicci M, Strohmeyer M, et al. The Etiology and Antimicrobial Susceptibility of Community-Onset Urinary Tract Infections in a Low-Resource/High-Resistance Area of Latin America. Tropical Medicine and Infectious Disease. 2025; 10(3):64. https://doi.org/10.3390/tropicalmed10030064
Chicago/Turabian StyleMicieli, Maria, Selene Rebecca Boncompagni, Tiziana Di Maggio, Yenny Bertha Mamani Ramos, Antonia Mantella, Ana Liz Villagrán, Carmen Angélica Revollo Yelma, Evelin Esther Fortún Fernández, Michele Spinicci, Marianne Strohmeyer, and et al. 2025. "The Etiology and Antimicrobial Susceptibility of Community-Onset Urinary Tract Infections in a Low-Resource/High-Resistance Area of Latin America" Tropical Medicine and Infectious Disease 10, no. 3: 64. https://doi.org/10.3390/tropicalmed10030064
APA StyleMicieli, M., Boncompagni, S. R., Di Maggio, T., Ramos, Y. B. M., Mantella, A., Villagrán, A. L., Yelma, C. A. R., Fernández, E. E. F., Spinicci, M., Strohmeyer, M., Pallecchi, L., Rossolini, G. M., & Bartoloni, A. (2025). The Etiology and Antimicrobial Susceptibility of Community-Onset Urinary Tract Infections in a Low-Resource/High-Resistance Area of Latin America. Tropical Medicine and Infectious Disease, 10(3), 64. https://doi.org/10.3390/tropicalmed10030064








