Serologic Evidence of Circulation of Six Arboviruses (Dengue Virus, Chikungunya Virus, Zika Virus, Rift Valley Virus, Yellow Fever Virus, Crimean-Congo Hemorrhagic Fever Virus) in Four Regions of Burkina Faso, West Africa
Abstract
1. Introduction
2. Materials and Methods
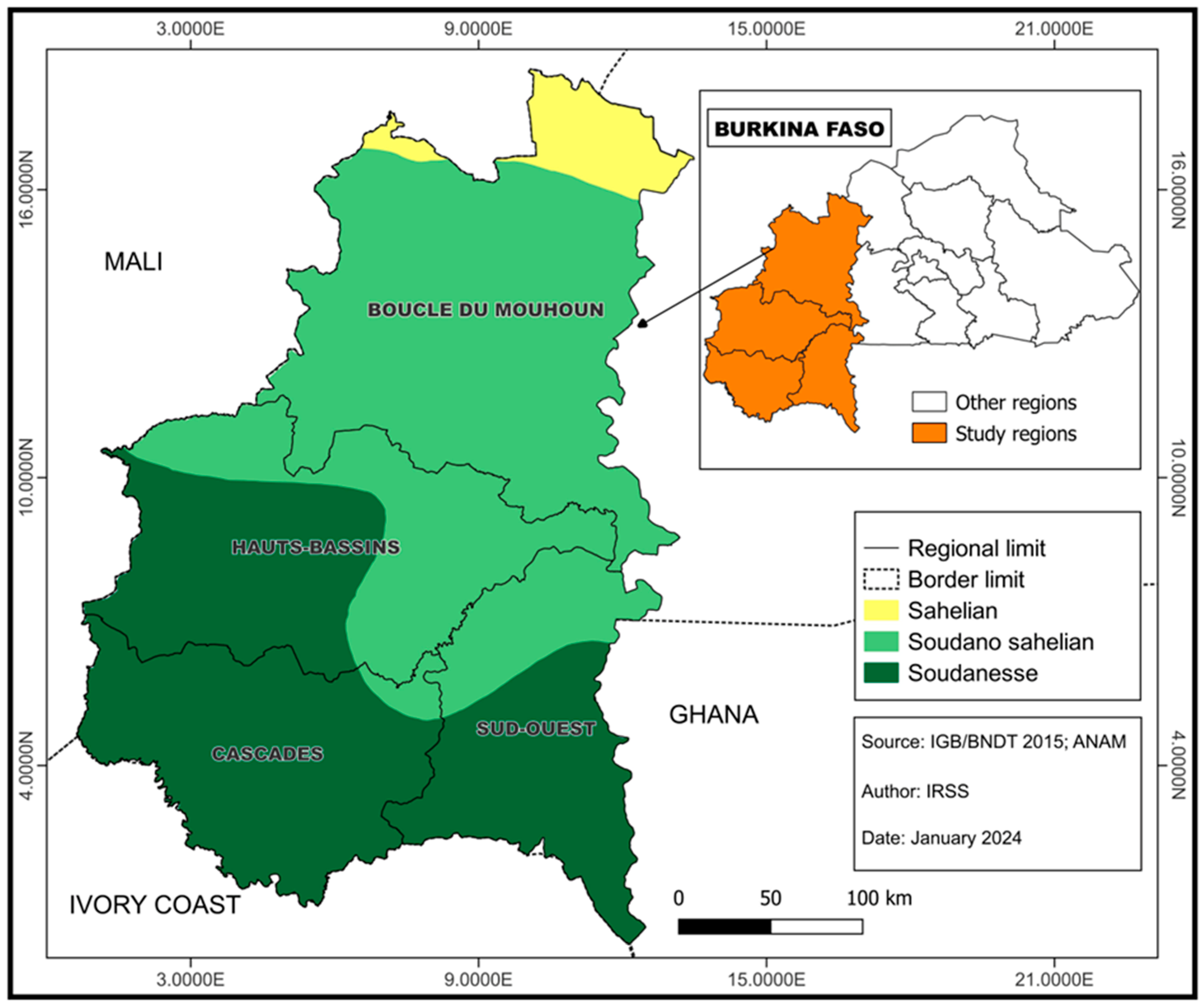
2.1. Study Design
2.2. Sample Size Calculation
2.3. Sample Selection and Data Collection
2.4. Serological Detection
2.5. Statistical Analysis
3. Results
3.1. Study Population
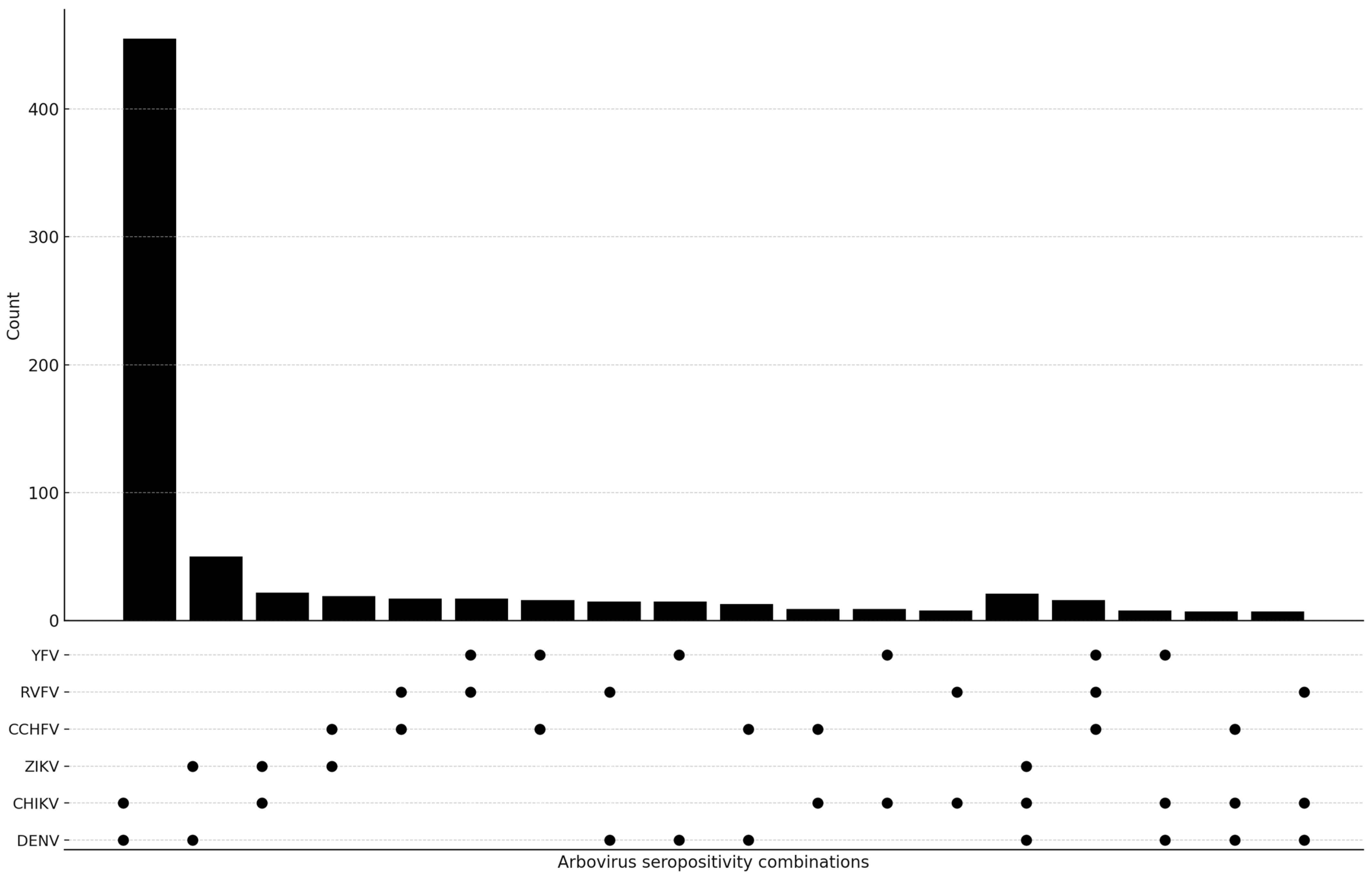
3.2. Seroprevalence of Antibodies Against Arboviruses
3.3. Associations Between Participant Characteristics and Seropositivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutiérrez-Vera, E.; Patiño, L.; Castillo-Segovia, M.; Mora-Valencia, V.; Montesdeoca-Agurto, J.; Regato-Arrata, M. Seroprevalence of arboviruses in Ecuador: Implications for improved surveillance. Biomédica 2021, 41, 247–259. [Google Scholar] [CrossRef]
- Ribeiro, G.S.; Hamer, G.L.; Diallo, M.; Kitron, U.; Ko, A.I.; Weaver, S.C. Influence of herd immunity in the cyclical nature of arboviruses. Curr. Opin. Virol. 2020, 40, 1–10. [Google Scholar] [CrossRef]
- Salgado, B.B.; Maués, F.C.d.J.; Pereira, R.L.; Chiang, J.O.; Freitas, M.N.d.O.; Ferreira, M.S.; Martins, L.C.; Vasconcelos, P.F.d.C.; Ganoza, C.; Lalwani, P. Prevalence of arbovirus antibodies in young healthy adult population in Brazil. Parasit. Vectors 2021, 14, 403. [Google Scholar] [CrossRef]
- Ushijima, Y.; Abe, H.; Ondo, G.N.; Bikangui, R.; Loembé, M.M.; Zadeh, V.R.; Essimengane, J.G.E.; Mbouna, A.V.N.; Bache, E.B.; Agnandji, S.T.; et al. Surveillance of the major pathogenic arboviruses of public health concern in Gabon, Central Africa: Increased risk of West Nile virus and dengue virus infections. BMC Infect. Dis. 2021, 21, 265. [Google Scholar] [CrossRef] [PubMed]
- Fritzell, C.; Rousset, D.; Adde, A.; Kazanji, M.; Van Kerkhove, M.D.; Flamand, C. Current challenges and implications for dengue, chikungunya and Zika seroprevalence studies worldwide: A scoping review. PLoS Negl. Trop. Dis. 2018, 12, e0006533. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Pettersson, J.; Higgs, S.; Charrel, R.; de Lamballerie, X. Emerging arboviruses: Why today? One Health 2017, 4, 1–13. [Google Scholar] [CrossRef]
- Marbán-Castro, E.; Arrieta, G.J.; Martínez, M.J.; González, R.; Bardají, A.; Menéndez, C.; Mattar, S. High Seroprevalence of Antibodies against Arboviruses among Pregnant Women in Rural Caribbean Colombia in the Context of the Zika Virus Epidemic. Antibodies 2020, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.K.; Lim, J.K.; Seydou, Y.; Carabali, M.; Barro, A.; Dahourou, D.L.; Lee, K.S.; Nikiema, T.; Namkung, S.; Lee, J.-S.; et al. Clinical and epidemiologic characteristics associated with dengue during and outside the 2016 outbreak identified in health facility-based surveillance in Ouagadougou, Burkina Faso. PLoS Negl. Trop. Dis. 2019, 13, e0007882. [Google Scholar] [CrossRef]
- Patterson, J.; Sammon, M.; Garg, M. Dengue, Zika and Chikungunya: Emerging Arboviruses in the New World. West. J. Emerg. Med. 2016, 17, 671–679. [Google Scholar] [CrossRef]
- De Lima Cavalcanti, T.Y.V.; Pereira, M.R.; De Paula, S.O.; Franca, R.F.D.O. A Review on Chikungunya Virus Epidemiology, Pathogenesis and Current Vaccine Development. Viruses 2022, 14, 969. [Google Scholar] [CrossRef]
- ECDE. Chikungunya Virus Disease—Annual Epidemiological Report for 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/chikungunya-virus-disease-annual-epidemiological-report-2022 (accessed on 25 June 2024).
- Karkhah, A.; Nouri, H.R.; Javanian, M.; Koppolu, V.; Masrour-Roudsari, J.; Kazemi, S.; Ebrahimpour, S. Zika virus: Epidemiology, clinical aspects, diagnosis, and control of infection. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 2035–2043. [Google Scholar] [CrossRef] [PubMed]
- Koppolu, V.; Shantha Raju, T. Zika virus outbreak: A review of neurological complications, diagnosis, and treatment options. J. Neurovirol. 2018, 24, 255–272. [Google Scholar] [CrossRef]
- WHO. Yellow Fever—African Region (AFRO). Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON510 (accessed on 25 June 2024).
- Johansson, M.A.; Vasconcelos, P.F.C.; Staples, J.E. The whole iceberg: Estimating the incidence of yellow fever virus infection from the number of severe cases. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 482–487. [Google Scholar] [CrossRef]
- Waggoner, J.J.; Rojas, A.; Pinsky, B.A. Yellow Fever Virus: Diagnostics for a Persistent Arboviral Threat. J. Clin. Microbiol. 2018, 56, e00827-18. [Google Scholar] [CrossRef]
- Wright, D.; Kortekaas, J.; Bowden, T.A.; Warimwe, G.M. Rift Valley fever: Biology and epidemiology. J. Gen. Virol. 2019, 100, 1187–1199. [Google Scholar] [CrossRef]
- Ikegami, T.; Makino, S. The Pathogenesis of Rift Valley Fever. Viruses 2011, 3, 493–519. [Google Scholar] [CrossRef]
- Madani, T.A.; Al-Mazrou, Y.Y.; Al-Jeffri, M.H.; Mishkhas, A.A.; Al-Rabeah, A.M.; Turkistani, A.M.; Al-Sayed, M.O.; Abodahish, A.A.; Khan, A.S.; Ksiazek, T.G.; et al. Rift Valley Fever Epidemic in Saudi Arabia: Epidemiological, Clinical, and Laboratory Characteristics. Clin. Infect. Dis. 2003, 37, 1084–1092. [Google Scholar] [CrossRef]
- Baba, M.; Masiga, D.K.; Sang, R.; Villinger, J. Has Rift Valley fever virus evolved with increasing severity in human populations in East Africa? Emerg. Microbes Infect. 2016, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Serretiell, E.; Astorri, R.; Chianese, A.; Stelitano, D.; Zannella, C.; Folliero, V.; Santella, B.; Galdiero, M.; Franci, G.; Galdiero, M. The emerging tick-borne Crimean-Congo haemorrhagic fever virus: A narrative review. Travel Med. Infect. Dis. 2020, 37, 101871. [Google Scholar] [CrossRef] [PubMed]
- Spengler, J.R.; Bente, D.A.; Bray, M.; Burt, F.; Hewson, R.; Korukluoglu, G.; Mirazimi, A.; Weber, F.; Papa, A. Second International Conference on Crimean-Congo Hemorrhagic Fever. Antivir. Res. 2018, 150, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Hawman, D.W.; Feldmann, H. Crimean–Congo haemorrhagic fever virus. Nat. Rev. Microbiol. 2023, 21, 463–477. [Google Scholar] [CrossRef]
- Nimo-Paintsi, S.C.; Mosore, M.; Addo, S.O.; Lura, T.; Tagoe, J.; Ladzekpo, D.; Addae, C.; Bentil, R.E.; Behene, E.; Dafeamekpor, C.; et al. Ticks and prevalence of tick-borne pathogens from domestic animals in Ghana. Parasit. Vectors 2022, 15, 86. [Google Scholar] [CrossRef] [PubMed]
- Gyasi, P.; Yakass, M.B.; Quaye, O. Analysis of dengue fever disease in West Africa. Exp. Biol. Med. 2023, 248, 1850–1863. [Google Scholar] [CrossRef]
- Tarnagda, Z.; Congo, M.; Sagna, T.; Ouédraogo, C.; Nikiéma, V.; Cissé, A.; Sanou, M.A.; Ouédraogo, A.; Ouédraogo, J.B.; Sangaré, L. Outbreak of dengue fever in Ouagadougou, Burkina Faso, 2013. Int. J. Microbiol. Immunol. Res. 2014, 2, 101–108. [Google Scholar]
- Tarnagda, Z.; Cissé, A.; Bicaba, B.W.; Diagbouga, S.; Sagna, T.; Ilboudo, A.K.; Tialla, D.; Lingani, M.; Sondo, K.A.; Yougbaré, I.; et al. Dengue Fever in Burkina Faso, 2016. Emerg. Infect. Dis. 2018, 24, 170–172. [Google Scholar] [CrossRef]
- Im, J.; Ridde, V.; Agnandji, S.T.; Lell, B.; Yaro, S.; Yang, J.S.; Hoinard, D.; Weaver, S.C.; Vanhomwegen, J.; Salje, H.; et al. The epidemiology of dengue outbreaks in 2016 and 2017 in Ouagadougou, Burkina Faso. Heliyon 2020, 6, e04389. [Google Scholar] [CrossRef]
- Lim, J.K.; Ridde, V.; Agnandji, S.T.; Lell, B.; Yaro, S.; Yang, J.S.; Hoinard, D.; Weaver, S.C.; Vanhomwegen, J.; Salje, H.; et al. Seroepidemiological Reconstruction of Long-term Chikungunya Virus Circulation in Burkina Faso and Gabon. J. Infect. Dis. 2022, 227, 261–267. [Google Scholar] [CrossRef]
- Tinto, B.; Kaboré, D.A.; Kania, D.; Kagoné, T.S.; Kiba-Koumaré, A.; Pinceloup, L.; Thaurignac, G.; de Perre, P.V.; Dabire, R.K.; Baldet, T.; et al. Serological Evidence of Zika Virus Circulation in Burkina Faso. Pathogens 2022, 11, 741. [Google Scholar] [CrossRef]
- Yaro, S.; Zango, A.; Rouamba, J.; Diabaté, A.; Dabiré, R.; Kambiré, C.; Tiendrebeogo, S.M.R.; Yonli, T.; Ouango, J.G.; Diagbouga, S.P. « Situation épidémiologique de la fièvre jaune au Burkina Faso de 2003 à 2008. Bull. Société Pathol. Exot. 2010, 103, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.K.; Carabali, M.; Edwards, T.; Barro, A.; Lee, J.-S.; Dahourou, D.; Lee, K.S.; Nikiema, T.; Shin, M.Y.; Bonnet, E.; et al. Estimating the Force of Infection for Dengue Virus Using Repeated Serosurveys, Ouagadougou, Burkina Faso. Emerg. Infect. Dis. 2021, 27, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Ouattara, C.A.; Traore, S.; Sangare, I.; Traore, T.I.; Meda, Z.C.; Savadogo, L.G.B. Spatiotemporal analysis of dengue fever in Burkina Faso from 2016 to 2019. BMC Public Health 2022, 22, 462. [Google Scholar] [CrossRef]
- Ridde, V.; Agier, I.; Bonnet, E.; Carabali, M.; Dabiré, K.R.; Fournet, F.; Ly, A.; Meda, I.B.; Parra, B. Presence of three dengue serotypes in Ouagadougou (Burkina Faso): Research and public health implications. Infect. Dis. Poverty 2016, 5, 23. [Google Scholar] [CrossRef]
- Tinto, B.; Kania, D.; Kagone, T.S.; Dicko, A.; Traore, I.; de Rekeneire, N.; Bicaba, B.W.; Hien, H.; Van de Perre, P.; Simonin, Y.; et al. Circulation du virus de la dengue en Afrique de l’Ouest: Une problématique émergente de santé publique. Médecine/Sciences 2022, 38, 152–158. [Google Scholar] [CrossRef]
- Hien, A.S.; Sangare, I.; Ouattara, E.L.P.; Sawadogo, S.P.; Soma, D.D.; Maiga, H.; Diabate, A.; Bonnet, E.; Ridde, V.; Fournet, F.; et al. Chikungunya (Togaviridae) and dengue 2 (Flaviviridae) viruses detected from Aedes aegypti mosquitoes in Burkina Faso by qRT-PCR technique: Preliminary results and perspective for molecular characterization of arbovirus circulation in vector populations. Front. Trop. Dis. 2022, 3, 920224. [Google Scholar] [CrossRef]
- Dahourou, L.D.; Akio, S.; Savadogo, M.; Yougbaré, B.; Ouoba, L.B.; Tapsoba, A.S.R.; Zerbo, L.H.; Ilboudo, A.K.; Abga, R.L.; Traoré, A.; et al. Serological evidence and factors associated with Crimean–Congo haemorrhagic fever in sheep in Burkina Faso. Vet. Med. Sci. 2024, 10, e1322. [Google Scholar] [CrossRef] [PubMed]
- Bukbuk, D.N.; Dowall, S.D.; Lewandowski, K.; Bosworth, A.; Baba, S.S.; Varghese, A.; Watson, R.J.; Bell, A.; Atkinson, B.; Hewson, R. Serological and Virological Evidence of Crimean-Congo Haemorrhagic Fever Virus Circulation in the Human Population of Borno State, Northeastern Nigeria. PLoS Negl. Trop. Dis. 2016, 10, e0005126. [Google Scholar] [CrossRef] [PubMed]
- El Ghassem, A.; Apolloni, A.; Vial, L.; Bouvier, R.; Bernard, C.; Khayar, M.S.; Ahmed, M.C.; Fausther-Bovendo, H.; Beyit, A.D.; Yahya, B.; et al. Risk factors associated with Crimean-Congo hemorrhagic fever virus circulation among human, livestock and ticks in Mauritania through a one health retrospective study. BMC Infect. Dis. 2023, 23, 764. [Google Scholar] [CrossRef] [PubMed]
- Sankhe, S.; Talla, C.; Thiam, M.S.; Faye, M.; Barry, M.A.; Diarra, M.; Dia, M.; Ndiaye, O.; Sembene, P.M.; Diop, B.; et al. Seroprevalence of Crimean-Congo Hemorrhagic Fever Virus and Rift Valley Fever Virus in human population in Senegal from October to November 2020. IJID Reg. 2023, 7, 216–221. [Google Scholar] [CrossRef]
- Bosworth, A.; Ghabbari, T.; Dowall, S.; Varghese, A.; Fares, W.; Hewson, R.; Zhioua, E.; Chakroun, M.; Tiouiri, H.; Jemaa, M.B. Serologic evidence of exposure to Rift Valley fever virus detected in Tunisia. New Microbes New Infect. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Sadeuh-Mba, S.A.; Wansi, G.M.Y.; Demanou, M.; Gessain, A.; Njouom, R. Serological evidence of rift valley fever Phlebovirus and Crimean-Congo hemorrhagic fever orthonairovirus infections among pygmies in the east region of Cameroon. Virol. J. 2018, 15, 63. [Google Scholar] [CrossRef]
- Périssé, A.R.S.; Souza-Santos, R.; Duarte, R.; Santos, F.; de Andrade, C.R.; Rodrigues, N.C.P.; Schramm, J.M.D.A.; da Silva, E.D.; Jacobson, L.D.S.V.; Lemos, M.C.F.; et al. Zika, dengue and chikungunya population prevalence in Rio de Janeiro city, Brazil, and the importance of seroprevalence studies to estimate the real number of infected individuals. PLoS ONE 2020, 15, e0243239. [Google Scholar] [CrossRef]
- Sissoko, D.; Moendandze, A.; Malvy, D.; Giry, C.; Ezzedine, K.; Solet, J.L.; Pierre, V. Seroprevalence and Risk Factors of Chikungunya Virus Infection in Mayotte, Indian Ocean, 2005-2006: A Population-Based Survey. PLoS ONE 2008, 3, e3066. [Google Scholar] [CrossRef]
- Muniz, P.T.; de Souza, V.A.F.; Pannuti, C.S.; Sperança, M.A.; Terzian, A.C.B.; Nogueira, M.L.; Yamamura, A.M.Y.; Freire, M.S.; da Silva, N.S.; Malafronte, R.S.; et al. Risk Factors for Dengue Virus Infection in Rural Amazonia: Population-based Cross-sectional Surveys. Am. J. Trop. Med. Hyg. 2008, 79, 485–494. [Google Scholar] [CrossRef]
- Soghaier, M.A.; Mahmood, S.F.; Pasha, O.; Azam, S.I.; Karsani, M.M.; Elmangory, M.M.; Elmagboul, B.A.; Okoued, S.I.; Shareef, S.M.; Khogali, H.S.; et al. Factors associated with dengue fever IgG sero-prevalence in South Kordofan State, Sudan, in 2012: Reporting prevalence ratios. J. Infect. Public Health 2014, 7, 54–61. [Google Scholar] [CrossRef]
- Salje, H.; Cauchemez, S.; Alera, M.T.; Rodriguez-Barraquer, I.; Thaisomboonsuk, B.; Srikiatkhachorn, A.; Lago, C.B.; Villa, D.; Klungthong, C.; Tac-An, I.A.; et al. Reconstruction of 60 Years of Chikungunya Epidemiology in the Philippines Demonstrates Episodic and Focal Transmission. J. Infect. Dis. 2016, 213, 604–610. [Google Scholar] [CrossRef]
- Chan, K.R.; Chan, K.R.; Ismail, A.A.; Thergarajan, G.; Raju, C.S.; Yam, H.C.; Rishya, M.; Sekaran, S.D. Serological cross-reactivity among common flaviviruses. Front. Cell. Infect. Microbiol. 2022, 12, 975398. [Google Scholar] [CrossRef]
- Da Silva, P.G.; Reis, J.A.S.D.; Rodrigues, M.N.; Da Silva Ardaya, Q.; Mesquita, J.R. Serological Cross-Reactivity in Zoonotic Flaviviral Infections of Medical Importance. Antibodies 2023, 12, 18. [Google Scholar] [CrossRef]
- Endale, A.; Medhin, G.; Darfiro, K.; Kebede, N.; Legesse, M. Magnitude of Antibody Cross-Reactivity in Medically Important Mosquito-Borne Flaviviruses: A Systematic Review. Infect. Drug Resist. 2021, 14, 4291–4299. [Google Scholar] [CrossRef] [PubMed]
- Koraka, P.; Zeller, H.; Niedrig, M.; Osterhaus, A.D.M.E.; Groen, J. Reactivity of serum samples from patients with a flavivirus infection measured by immunofluorescence assay and ELISA. Microbes Infect. 2002, 4, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Souza, N.C.S.E.; Félix, A.C.; De Paula, A.V.; Levi, J.E.; Pannuti, C.S.; Romano, C.M. Evaluation of serological cross-reactivity between yellow fever and other flaviviruses. Int. J. Infect. Dis. 2019, 81, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Kam, Y.-W.; Pok, K.-Y.; Eng, K.E.; Tan, L.-K.; Kaur, S.; Lee, W.W.L.; Leo, Y.-S.; Ng, L.-C.; Ng, L.F.P. Sero-Prevalence and Cross-Reactivity of Chikungunya Virus Specific Anti-E2EP3 Antibodies in Arbovirus-Infected Patients. PLoS Negl. Trop. Dis. 2015, 9, e3445. [Google Scholar] [CrossRef] [PubMed]
| Dengue Virus IgG ELISA | Chikungunya IgG ELISA | Zika Virus IgG (Capture) ELISA | Yellow Fever Virus IgG ELISA | Human Rift Valley Fever Virus IgG ELISA | Human Crimean-Congo Hemorrhagic Fever Virus IgG ELISA | |
|---|---|---|---|---|---|---|
| Manufacturer | DRG Diagnostic, Marburg, Germany | DRG Diagnostic, Marburg, Germany | DRG Diagnostic, Marburg, Germany | DRG Diagnostic, Marburg, Germany | Abbexa LTD, Cambridge, United Kingdom | Abbexa LTD, Cambridge, United Kingdom |
| Diagnostic Sensitivity | 100% | 98.7% | 96.0% | >98% | - | - |
| Diagnostic Specificity | 100% | 100% | 99.6% | >98% | - | - |
| Cross-reactivity | No cross-reactivity in IgG-positive samples for HSV1/2, Rubella, EBV, CMV, VZV, TBE, RSV, Parvovirus | No evidence of false-positive results due to cross-reactivity. Cross-reactivity with antibodies against other alpha viruses cannot be excluded | No evidence of false-positive results due to cross-reactivity | Cross-reactivity has been observed with DENV and ZIKV IgG-positive specimens | - | - |
| Characteristics | n | % |
|---|---|---|
| Sex | ||
| Female | 711 | 39.3 |
| Male | 1097 | 60.7 |
| Age (in years) | ||
| <20 | 88 | 4.9 |
| 20–29 | 446 | 24.7 |
| 30–39 | 687 | 38.0 |
| 40–49 | 367 | 20.3 |
| ≥50 | 220 | 12.2 |
| Residence region | ||
| Hauts Bassins | 1244 | 68.8 |
| Cascades | 192 | 10.6 |
| Sud-Ouest | 232 | 12.8 |
| Boucle du Mouhoun | 140 | 7.7 |
| Characteristics | Total | Anti-DENV IgG | Anti-CHIKV IgG | Anti-ZIKV IgG | Anti-CCHFV IgG | Anti-RVFV | Anti-YFV IgG | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive n (%) | p | Positive n (%) | p | Positive n (%) | p | Positive n (%) | p | Positive n (%) | p | Positive n (%) | p | ||
| Seroprevalence | N = 1808 | 1363 (75.4) | 556 (30.7) | 53 (2.9) | 20 (1.1) | 21 (1.2) | 19 (1.1) | ||||||
| Age (in years) | <0.001 | <0.001 | 0.540 | 0.483 | 0.185 | 0.141 | |||||||
| <20 | 88 | 24 (27.3) | 10 (11.4) | 0 | 0 | 1 (1.1) | 0 | ||||||
| 20–29 | 446 | 294 (65.9) | 111 (24.9) | 7 (1.6) | 4 (0.9) | 3 (0.7) | 3 (0.7) | ||||||
| 30–39 | 687 | 530 (77.1) | 229 (33.3) | 17 (2.5) | 6 (0.9) | 5 (0.7) | 4 (0.6) | ||||||
| 40– 49 | 367 | 316 (86.1) | 122 (33.2) | 20 (5.4) | 6 (1.6) | 8 (2.2) | 7 (1.9) | ||||||
| ≥50 | 220 | 199 (90.5) | 84 (38.2) | 9 (4.1) | 4 (1.8) | 4 (1.8) | 5 (2.3) | ||||||
| Sex | 0.029 | 0.026 | 0.167 | 0.601 | 0.434 | 0.096 | |||||||
| Female | 711 | 516 (72.6) | 240 (33.8) | 16 (2.2) | 9 (1.3) | 10 (1.4) | 11 (1.5) | ||||||
| Male | 1097 | 847 (77.2) | 316 (28.8) | 37 (3.4) | 11 (1.0) | 11 (1.0) | 8 (0.7) | ||||||
| Residence region | 0.002 | 0.055 | 0.353 | 0.496 | 0.092 | 0.193 | |||||||
| Boucle du Mouhoun | 140 | 103 (73.6) | 42 (30.0) | 2 (1.4) | 0 | 0 | 0 | ||||||
| Hauts Bassins | 1244 | 919 (73.9) | 363 (29.2) | 35 (2.8) | 14 (1.1) | 14 (1.1) | 13 (1.0) | ||||||
| Cascades | 192 | 143 (74.5) | 74 (38.5) | 9 (4.7) | 2 (1.0) | 1 (0.5) | 1 (0.5) | ||||||
| Sud-Ouest | 232 | 198 (85.3) | 77 (33.2) | 7 (3.0) | 4 (1.7) | 6 (2.6) | 5 (2.2) | ||||||
| Characteristics | Anti-DENV IgG | Anti-CHIKV IgG | ||||||
|---|---|---|---|---|---|---|---|---|
| cOR # (95% CI) | p | aOR * (95% CI) | p | cOR # (95% CI) | p | aOR * (95% CI) | p | |
| Age (in years) | ||||||||
| <20 | 1 | - | 1 | - | 1 | - | 1 | - |
| 20–29 | 5.1 (3.1–8.6) | 0.001 | 5.4 (3.2–9.2) | 0.001 | 2.5 (1.3–5.4) | 0.005 | 2.6 (1.3–5.3) | 0.005 |
| 30–39 | 8.9 (5.4–15.0) | 0.001 | 9.4 (5.6–15.6) | 0.001 | 3.8 (2.0–8.0) | 0.001 | 3.9 (1.9–7.7) | 0.001 |
| 40–49 | 16.3 (9.4–28.9) | 0.001 | 16.9 (9.8–30.2) | 0.001 | 3.8 (1.9–8.1) | 0.001 | 3.8 (1.9–7.7) | 0.001 |
| ≥50 | 24.6 (13.1–48.5) | 0.001 | 27.3 (14.5–54.0) | 0.001 | 4.7 (2.4–10.2) | 0.001 | 4.8 (2.3–9.8) | 0.001 |
| Sex | ||||||||
| Female | 1 | 1 | 1 | 1 | ||||
| Male | 1.3 (1.0–1.6) | 0.02 | 1.2 (1.0–1.6) | 0.01 | 0.8 (0.6–1.0) | 0.02 | 0.8 (0.6–1.0) | 0.030 |
| Residence region | ||||||||
| Boucle du Mouhoun | 1 | 1 | 1 | 1 | ||||
| Cascades | 1.0 (0.6–1.7) | 0.85 | 1.0 (0.6–1.6) | 0.97 | 1.5 (0.9–2.3) | 0.13 | 1.4 (0.9–2.3) | 0.13 |
| Hauts-Bassins | 1.0 (0.7–1.5) | 0.95 | 1.1 (0.7–1.6) | 0.89 | 1.0 (0.6–1.4) | 0.79 | 1.0 (0.6–1.4) | 0.83 |
| Sud-Ouest | 2.1 (1.2–3.5) | 0.006 | 2.3 (1.3–4.0) | 0.003 | 1.1 (0.7–1.8) | 0.54 | 1.2 (0.7–1.8) | 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanou, A.M.; Nikiéma, A.S.; Sausy, A.; Diendéré, J.; Ouattara, M.N.G.; Badiel, A.B.S.; Bonkoungou, I.; Ouédraogo, H.G.; Hübschen, J.M. Serologic Evidence of Circulation of Six Arboviruses (Dengue Virus, Chikungunya Virus, Zika Virus, Rift Valley Virus, Yellow Fever Virus, Crimean-Congo Hemorrhagic Fever Virus) in Four Regions of Burkina Faso, West Africa. Trop. Med. Infect. Dis. 2025, 10, 345. https://doi.org/10.3390/tropicalmed10120345
Sanou AM, Nikiéma AS, Sausy A, Diendéré J, Ouattara MNG, Badiel ABS, Bonkoungou I, Ouédraogo HG, Hübschen JM. Serologic Evidence of Circulation of Six Arboviruses (Dengue Virus, Chikungunya Virus, Zika Virus, Rift Valley Virus, Yellow Fever Virus, Crimean-Congo Hemorrhagic Fever Virus) in Four Regions of Burkina Faso, West Africa. Tropical Medicine and Infectious Disease. 2025; 10(12):345. https://doi.org/10.3390/tropicalmed10120345
Chicago/Turabian StyleSanou, Armel Moumouni, Achille Sindimbasba Nikiéma, Aurélie Sausy, Jeoffray Diendéré, Mathuola Nina Genéviève Ouattara, Arielle Bettina Sandra Badiel, Isidore Bonkoungou, Henri Gautier Ouédraogo, and Judith M. Hübschen. 2025. "Serologic Evidence of Circulation of Six Arboviruses (Dengue Virus, Chikungunya Virus, Zika Virus, Rift Valley Virus, Yellow Fever Virus, Crimean-Congo Hemorrhagic Fever Virus) in Four Regions of Burkina Faso, West Africa" Tropical Medicine and Infectious Disease 10, no. 12: 345. https://doi.org/10.3390/tropicalmed10120345
APA StyleSanou, A. M., Nikiéma, A. S., Sausy, A., Diendéré, J., Ouattara, M. N. G., Badiel, A. B. S., Bonkoungou, I., Ouédraogo, H. G., & Hübschen, J. M. (2025). Serologic Evidence of Circulation of Six Arboviruses (Dengue Virus, Chikungunya Virus, Zika Virus, Rift Valley Virus, Yellow Fever Virus, Crimean-Congo Hemorrhagic Fever Virus) in Four Regions of Burkina Faso, West Africa. Tropical Medicine and Infectious Disease, 10(12), 345. https://doi.org/10.3390/tropicalmed10120345







