Communicable Disease Surveillance in South Africa and LMICs: A Systematic Review of Systems, Challenges, and Integration with Environmental Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Approach
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
- Peer-reviewed articles published from January 2010 to March 2025.
- Articles about communicable disease surveillance
- The review focused on open-access articles only
- Low to middle-income countries, especially African countries.
- Full-text articles in the English language
- Articles focusing on non-communicable diseases.
- Non-peer-reviewed articles and peer-reviewed articles that were published before 2010.
- The articles with limited access or not open-access
- Articles without full-text and written in any language other than English.
2.4. Data Extraction
2.5. Data Analysis
2.6. Quality Assessment (Table S1)
3. Results
3.1. Overview of Study Selection and Characteristics
3.1.1. Study Designs Followed
3.1.2. Sample Sizes
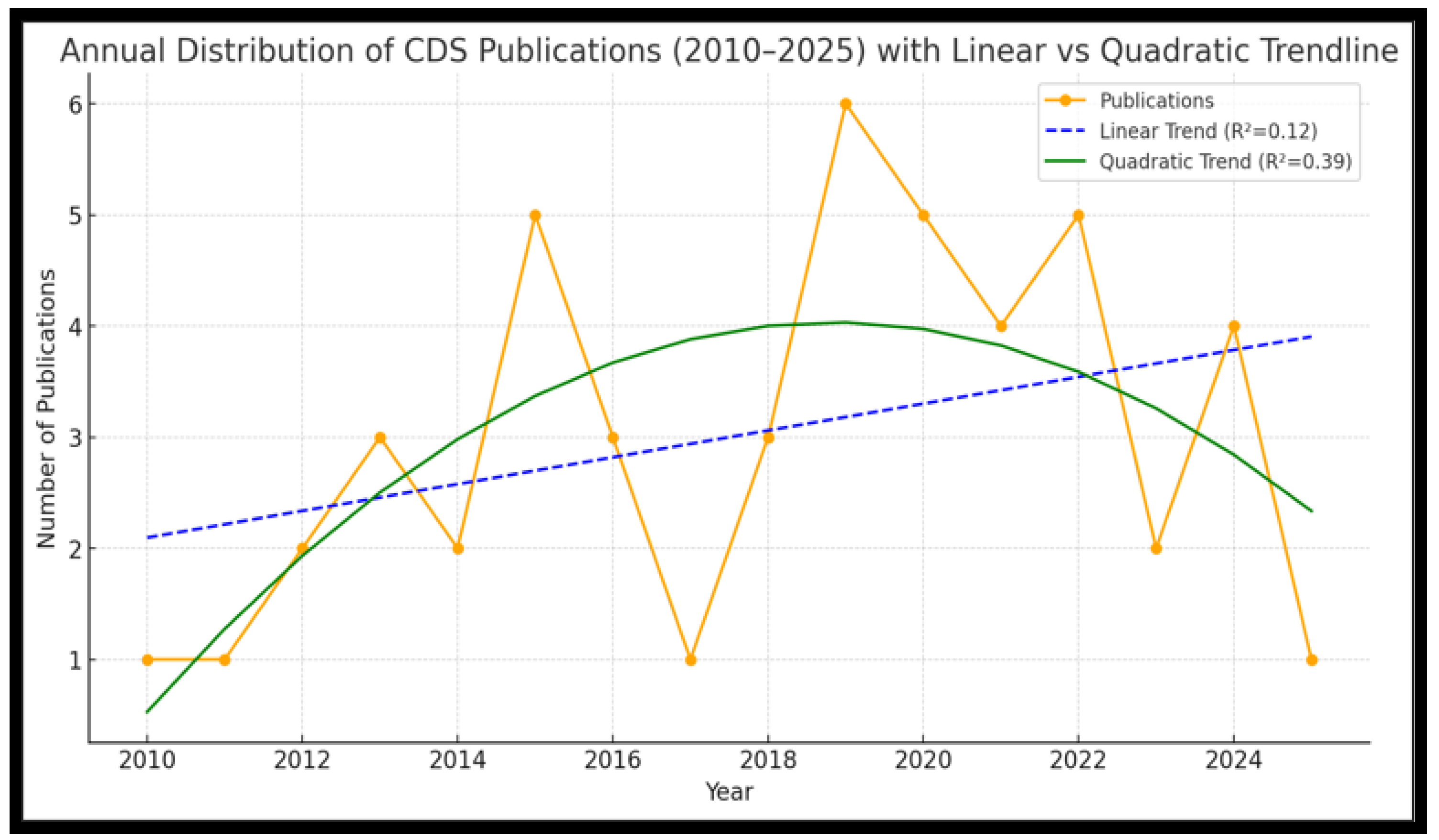
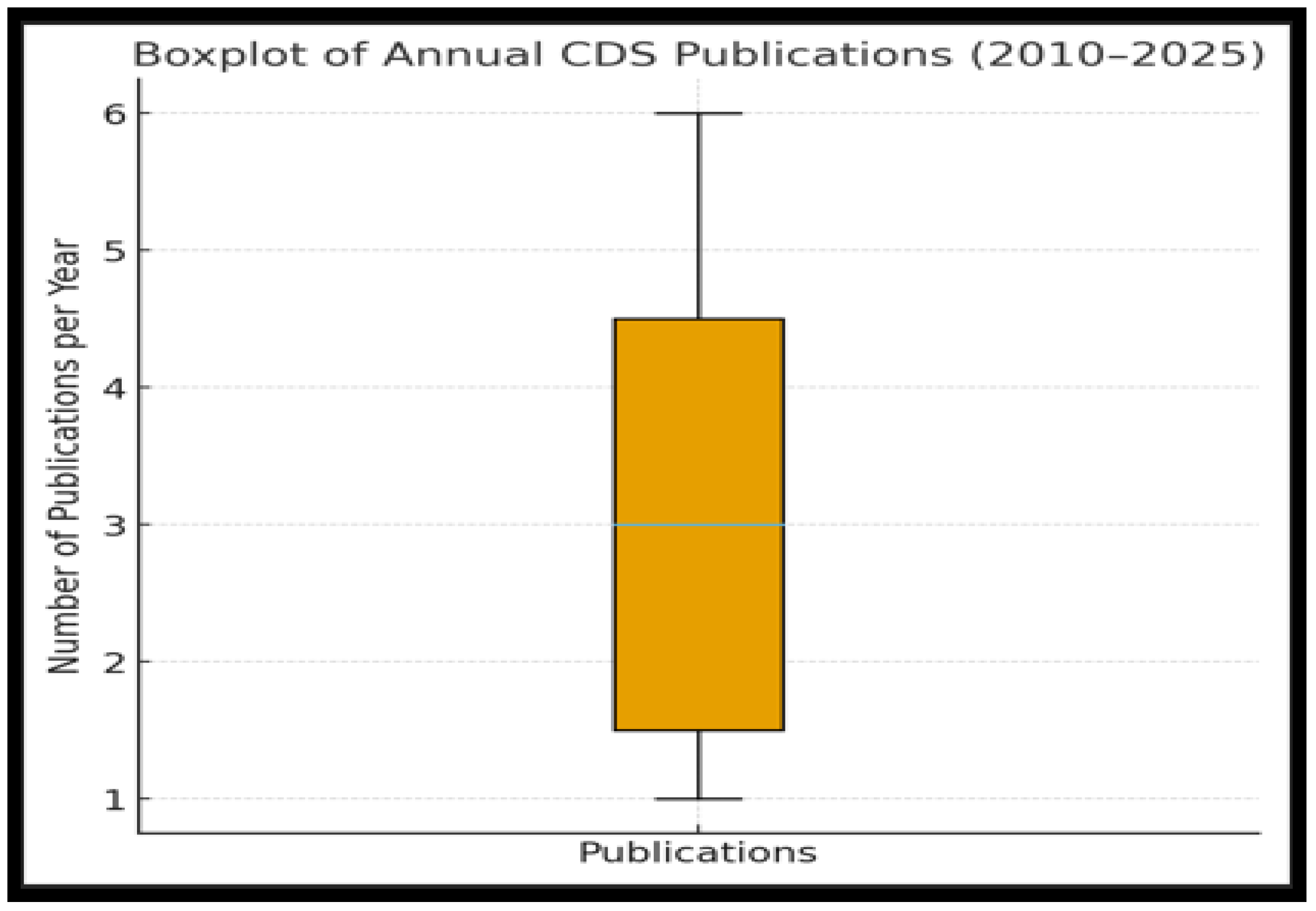
3.2. Publication Trends and Geographic Distribution
3.2.1. Annual Distribution of Publications
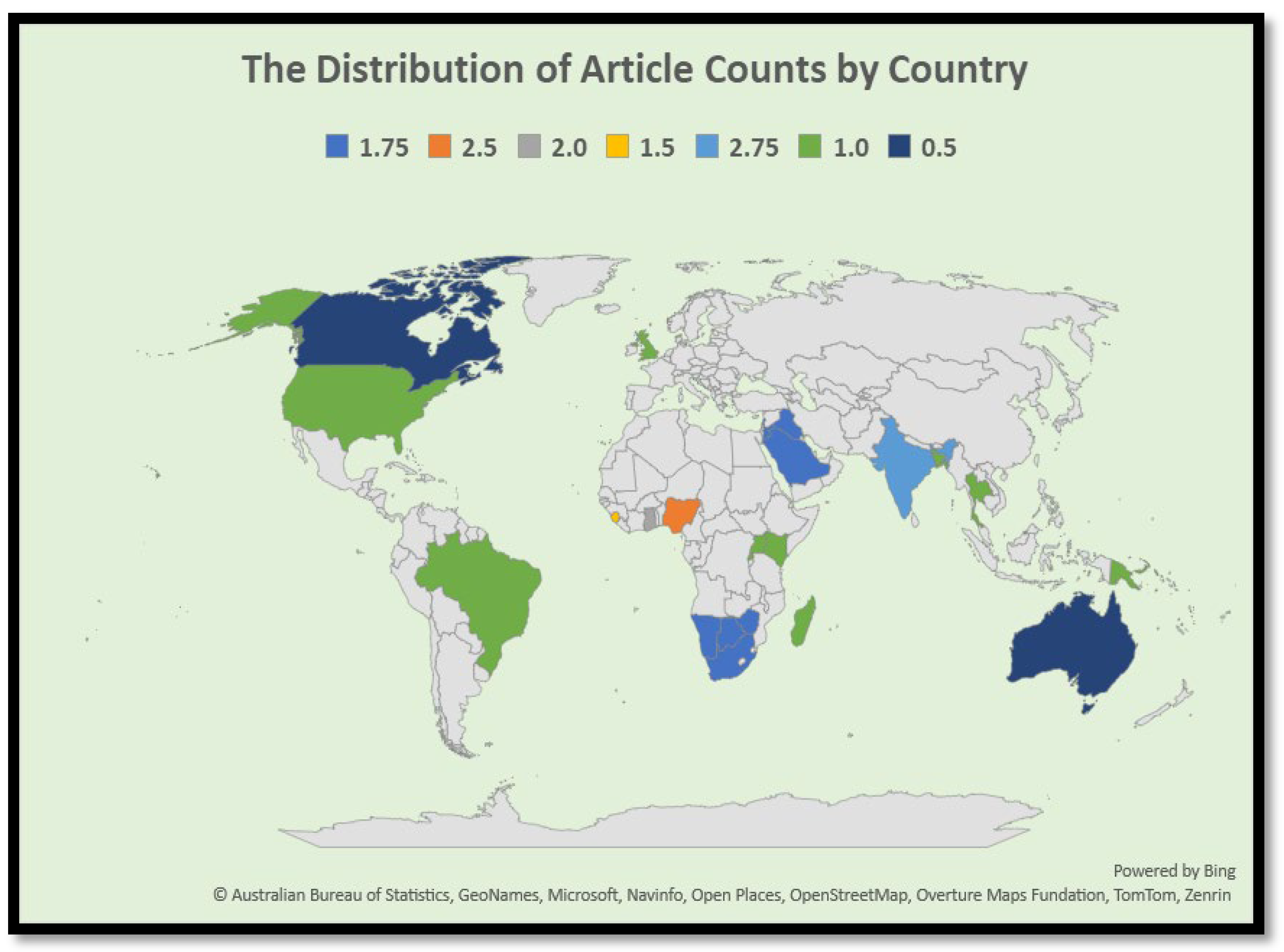
3.2.2. Geographical Distribution of Reviewed Literature
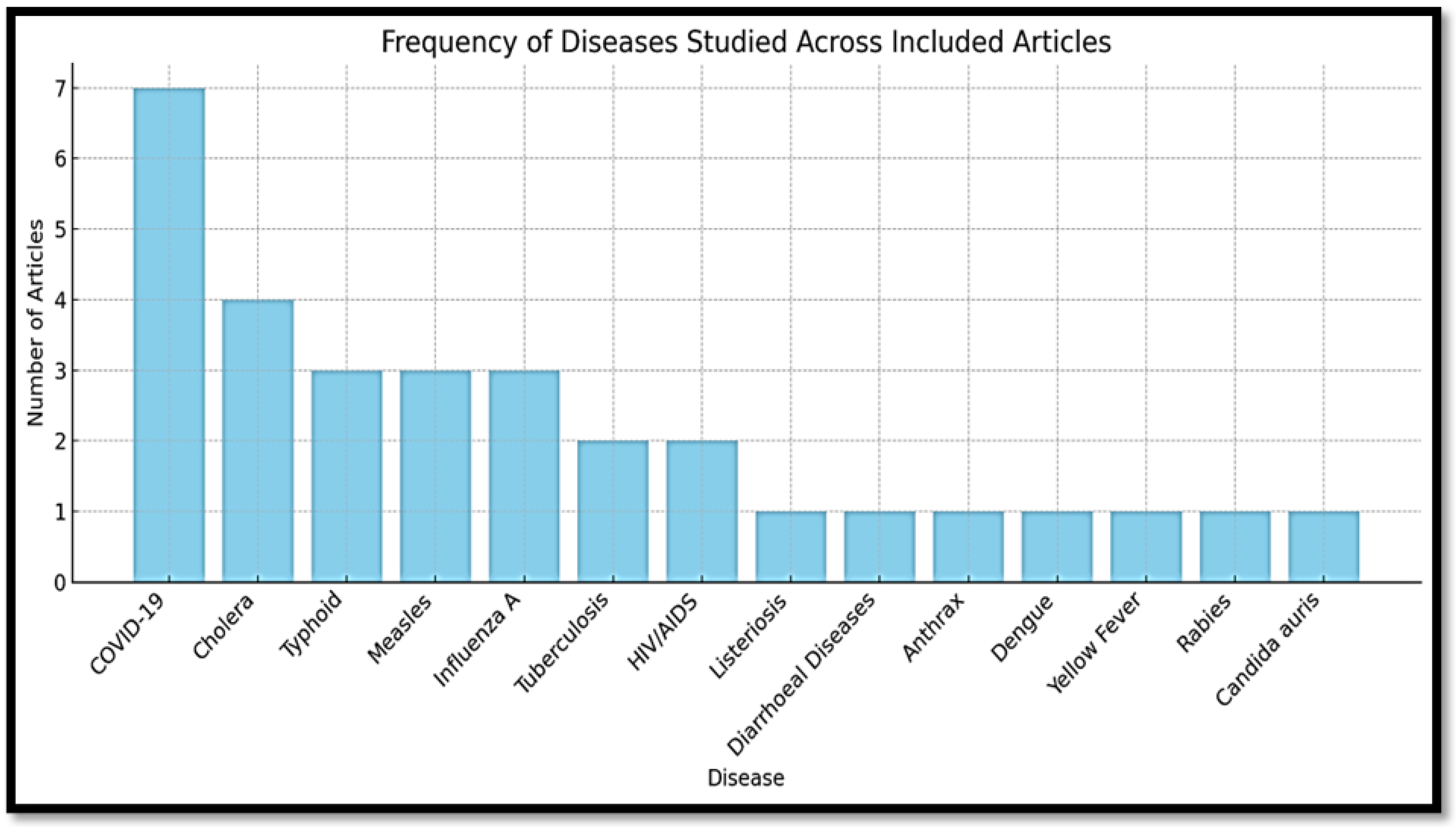
3.3. Communicable Diseases Focus and Surveillance System
3.3.1. Most Frequently Studied Diseases
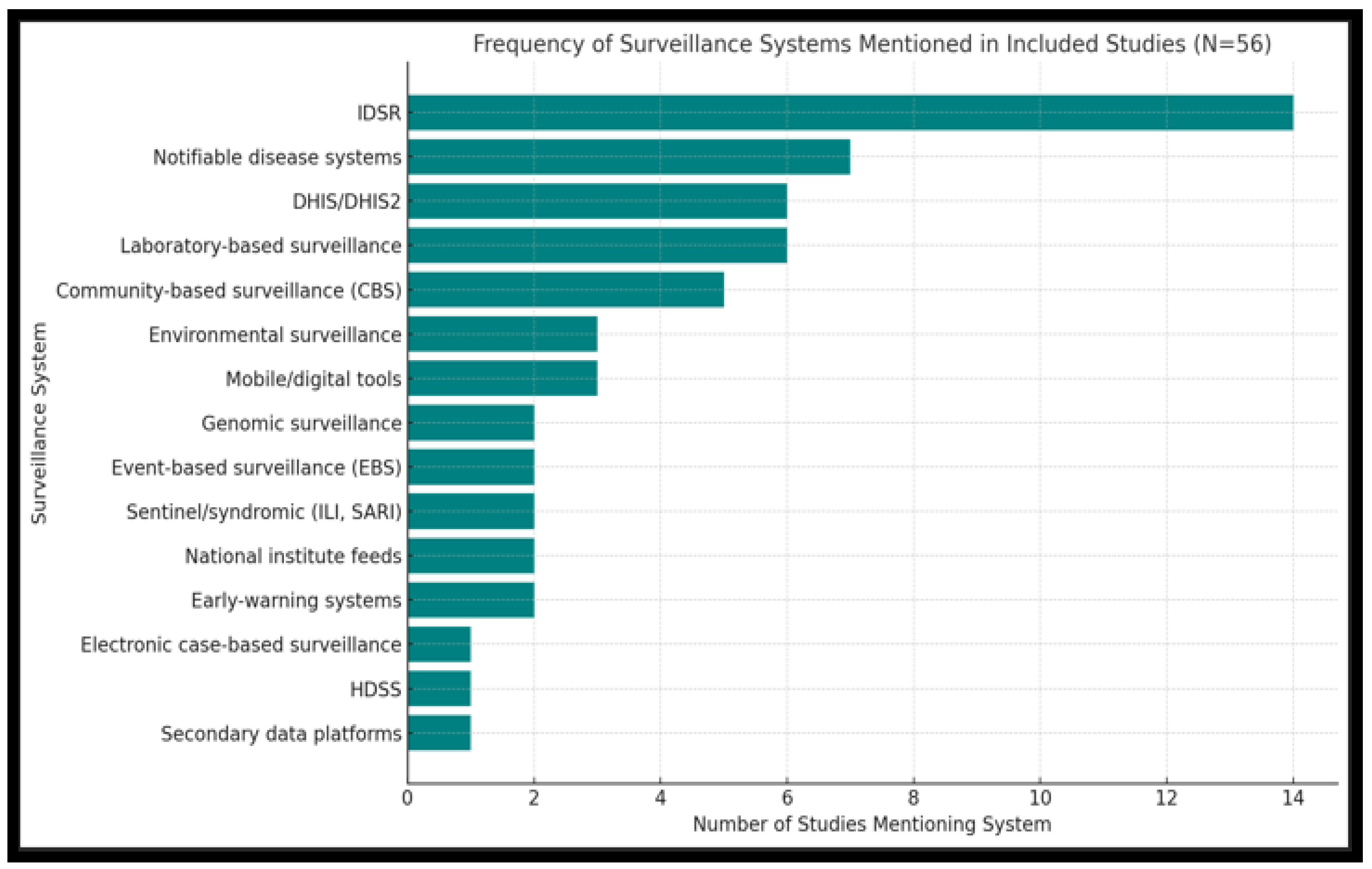
3.3.2. Surveillance Frameworks and Tools Used
3.3.3. Governance and Policy Frameworks
3.4. Comparative Analysis of the Application Process in South Africa vs. Other LMICs
3.4.1. Reporting Processes and Data Flow
3.4.2. Digital Tools and Surveillance Technologies
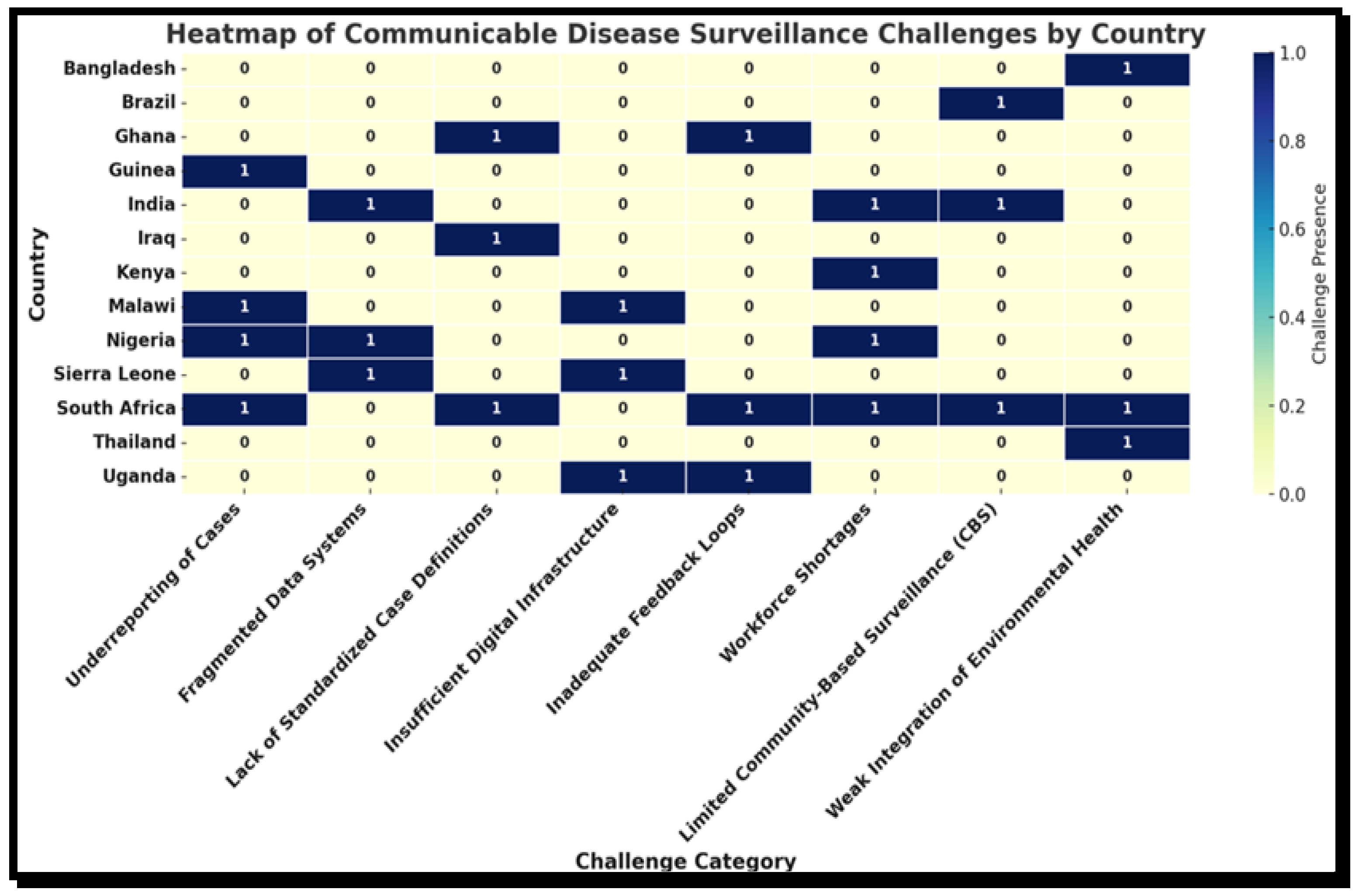
3.5. Challenges or Limitations in the Implementation of Surveillance Systems Across LMICs
3.6. Integration of Environmental Health in Surveillance Systems
3.7. Quality Appraisal and Risk of Bias
4. Discussion
4.1. Summary of Key Findings
4.2. Systemic and Structural Challenges Undermining Disease Surveillance Systems
4.3. Environmental Health Surveillance: A Missing Link
4.4. Methodological Diversity and Its Implications
4.5. Policy and Practice Recommendations
- Strengthen integration between surveillance platforms: National ministries should harmonize vertical programs and IDSR structures to reduce redundancy and ensure comprehensive coverage. Furthermore, develop and adopt national standards for interoperability between surveillance platforms and promote regulatory frameworks aligned with IHR (2005) and Africa CDC protocols [66].
- Digitize and decentralize data collection: Investing in DHIS2 and similar mobile reporting platforms can enhance timeliness, particularly in remote areas. However, infrastructure must be accompanied by training and technical support.
- Institutionalize environmental health data collection: Countries should expand the use of WASH indicators, air quality metrics, and climate-linked early warning systems in disease surveillance frameworks.
- Enhance community-level reporting: Community-based surveillance (CBS), when paired with training and supervision, has shown promise for early detection of outbreaks, and should be scaled up [14].
- Ensure sustainable funding and capacity-building: Surveillance is often underfunded and overlooked until emergencies occur. Governments and donors should prioritize long-term financing for training, feedback systems, and performance monitoring.
4.6. Strengths and Limitations of the Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LMIC | Low-Middle Income Countries |
| CDSS | Communicable Disease Surveillance System |
| CDC | Centre for Communicable Disease |
| CBS | Community-Based Surveillance |
| IHR | International Health Regulations 2005 |
| NMC | Notifiable Medical Conditions |
| NMCSS | Notifiable Medical Conditions Surveillance System |
| IDSR | Integrated Disease Surveillance and Response |
| COVID-19 | Coronavirus Disease 2019 |
| DHIS2 | District Health Information System 2 |
| ECOWAS | Economic Community of West African States |
| EHPs | Environmental Health Practitioners |
| HIV/AIDS | Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome |
| KAP | Knowledge, Attitudes, and Perception |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SARS | Severe Acute Respiratory Syndrome |
| SDGs | Sustainable Development Goals |
| WASH | Water, Sanitation, and Hygiene |
| SADC | South African Development Countries |
| WHO | World Health Organization |
| FCRE | Faculty Committee of Research Ethics |
| TB | Tuberculosis |
References
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Wang, L.F. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef]
- Fall, I.S.; Rajatonirina, S.; Yahaya, A.A.; Zabulon, Y.; Nsubuga, P.; Nanyunja, M.; Wamala, J.; Njuguna, C.; Lukoya, C.O.; Alemu, W.; et al. Integrated Disease Surveillance and Response (IDSR) strategy: Current status, challenges and perspectives for the future in Africa. BMJ Glob. Health 2019, 4, e001427. [Google Scholar] [CrossRef]
- Bagherian, H.; Farahbakhsh, M.; Rabiei, R.; Moghaddasi, H.; Asadi, F. National communicable disease surveillance system: A review on information and organizational structures in developed countries. Acta Inform. Med. 2017, 25, 271. [Google Scholar] [CrossRef] [PubMed]
- Feingold, B.J.; Vegosen, L.; Davis, M.; Leibler, J.; Peterson, A.; Silbergeld, E.K. A Niche for Infectious Disease in Environmental Health: Rethinking the Toxicological Paradigm. Environ. Health Perspect. 2010, 118, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Said, M.D.; Funke, N.; Jacobs, I.; Steyn, M.; Nienaber, S. The Case of Cholera Preparedness, Response and Prevention in the SADC Region: A Need for Proactive and Multi-Level Communication and Co-ordination. Water SA 2011, 37, 559–566. [Google Scholar] [CrossRef]
- Muyembe, C.; Kazonga, E.; Songole, R.S.; Shamisale, K.; Nkole, J.; Haakonde, T.; Kalubula, P. A Systematic Review of the Integrated Disease Surveillance and Response Implementation Among African Countries Between 2010 and 2024. PLoS ONE 2024, 19, e0298939. [Google Scholar] [CrossRef]
- Kaptchouang Tchatchouang, C.-D.; Fri, J.; De Santi, M.; Brandi, G.; Schiavano, G.F.; Amagliani, G.; Ateba, C.N. Listeriosis outbreak in South Africa: A comparative analysis with previously reported cases worldwide. Microorganisms 2020, 8, 135. [Google Scholar] [CrossRef]
- Donadel, M.; Scobie, H.M.; Pastore, R.; Grabovac, V.; Batmunkh, N.; O’Connor, S.; Dahl, B.A.; Murrill, C.S. Comprehensive Vaccine-Preventable Disease Surveillance in the Western Pacific Region: A Literature Review on Integration of Surveillance Functions, 2000–2021. Glob. Health Sci. Pract. 2022, 10, e2200017. [Google Scholar] [CrossRef]
- Jassat, W.; Naicker, N.; Naidoo, S.; Mathee, A. Rodent Control in Urban Communities in Johannesburg, South Africa: From Research to Action. Int. J. Environ. Health Res. 2013, 23, 474–483. [Google Scholar] [CrossRef]
- Muse, E.A.; Karimuribo, E.D.; Misinzo, G.; Mellau, L.S.; Msoffe, P.L.; Gitao, G.C.; Swai, E.S.; Albano, M.O. Epidemiological Investigation into the Introduction and Factors for Spread of Peste Des Petits Ruminants, Southern Tanzania: Proceeding. Onderstepoort J. Vet. Res. 2012, 79, 1–6. [Google Scholar] [CrossRef]
- Andrews, J.R.; Yu, A.T.; Saha, S.; Shakya, J.; Aiemjoy, K.; Horng, L.; Qamar, F.; Garrett, D.; Baker, S.; Saha, S. Environmental surveillance as a tool for identifying high-risk settings for typhoid transmission. Clin. Infect. Dis. 2020, 71, S71–S78. [Google Scholar] [CrossRef]
- Hemingway-Foday, J.J.; Diallo, B.I.; Compaore, S.; Bah, S.; Keita, S.; Diallo, I.T.; Martel, L.D.; Standley, C.J.; Bah, M.B.; Bah, M.; et al. Lessons learned for surveillance system strengthening through capacity building and partnership engagement in post-Ebola Guinea, 2015–2019. Front. Public Health 2022, 10, 715356. [Google Scholar] [CrossRef]
- Mahomed, S.; Archary, M.; Mutevedzi, P.; Mahabeer, Y.; Govender, P.; Ntshoe, G.; Kuhn, W.; Thomas, J.; Olowolagba, A.; Blumberg, L.; et al. An isolated outbreak of diphtheria in South Africa, 2015. Epidemiol. Infect. 2017, 145, 2100–2108. [Google Scholar] [CrossRef]
- Kambalame, D.; Yelewa, M.; Iversen, B.G.; Khunga, N.; Macdonald, E.; Nordstrand, K.; Mwale, A.; Muula, A.; Chitsa Banda, E.; Phuka, J.; et al. Factors influencing operationalization of Integrated Disease Surveillance in Malawi. Public Health 2024, 228, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.K. Epidemiologic surveillance for controlling the COVID-19 pandemic: Types, challenges, and implications. J. Infect. Public Health 2020, 13, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Mathee, A.; Wright, C. Environmental Health in South Africa. S. Afr. Health Rev. 2013, 2013, 105–116. Available online: https://hdl.handle.net/10520/EJC161446 (accessed on 29 September 2025).
- Ahmed, W.; Tscharke, B.; Bertsch, P.M.; Bibby, K.; Bivins, A.; Choi, P.; Clarke, L.; Dwyer, J.; Edson, J.; Nguyen, T.M.; et al. SARS-CoV-2 RNA Monitoring in Wastewater as a Potential Early Warning System for COVID-19 transmission in the Community: A Temporal Case Study. Sci. Total Environ. 2021, 761, 144216. [Google Scholar] [CrossRef]
- Mercy, K.; Salyer, S.J.; Mankga, C.; Hedberg, C.; Zondo, P.; Kebede, Y. Establishing an Early Warning Event Management System at Africa CDC. PLoS Digit. Health 2024, 3, e0000564. [Google Scholar] [CrossRef]
- Sa, R.; Garganta, S.; Borges, C.; Correia, M.; Emilio, D.; Rodrigues, A.P. Addressing Influenza Outbreaks in Schools. Eur. J. Public Health 2023, 33, ckad160.1540. [Google Scholar] [CrossRef]
- Bridget, M.; Gebru, G.N.; Odongo, G.S.; Hedberg, C.; Elduma, A.H.; Kanu, J.S.; Bangura, J.; Squire, J.S.; Foster, M.A. Digitalizing disease surveillance: Experience from Sierra Leone. Health Policy Plan. 2025, 40, 85–96. [Google Scholar] [CrossRef]
- Freeman, M.C.; Ogden, S.; Jacobson, J.; Abbott, D.; Addiss, D.G.; Amnie, A.G.; Utzinger, J. Integration of Water, Sanitation, and Hygiene for the Prevention and Control of Neglected Tropical Diseases: A Rationale for Inter-Sectoral Collaboration. PLoS Negl. Trop. Dis. 2013, 7, e2439. [Google Scholar] [CrossRef]
- Kouadio, I.K.; Aljunid, S.; Kamigaki, T.; Hammad, K.; Oshitani, H. Infectious Diseases Following Natural Disasters: Prevention and Control Measures. Expert Rev. Anti-Infect. Ther. 2012, 10, 95–104. [Google Scholar] [CrossRef]
- Quinn, M.M.; Henneberger, P.K.; Braun, B.; Delclos, G.L.; Fagan, K.; Huang, V.; Knaack, J.L.; Kusek, L.; Lee, S.; Zock, J.P.; et al. Cleaning and Disinfecting Environmental Surfaces in Health Care: Toward an Integrated Framework for Infection and Oanupational Illness Prevention. Am. J. Infect. Control 2015, 43, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Chirisa, I.; Nyamadzawo, L.; Bandauko, E.; Mutsindikwa, N. The 2008/2009 Cholera Outbreak in Harare, Zimbabwe: Case of Failure in Urban Environmental Health and Planning. Rev. Environ. Health 2015, 30, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Mwachui, M.A.; Crump, L.; Hartskeerl, R.; Zinsstag, J.; Hattendorf, J. Environmental and Behavioural Determinants of Leptospirosis Transmission: A Systematic Review. PLoS Negl. Trop. Dis. 2015, 9, e0003843. [Google Scholar] [CrossRef] [PubMed]
- Joseph Wu, T.-S.; Kagoli, M.; Kaasbøll, J.J.; Bjune, G.A. Integrated Disease Surveillance and Response (IDSR) in Malawi: Implementation gaps and challenges for timely alert. PLoS ONE 2018, 13, e0200858. [Google Scholar] [CrossRef]
- Mercogliano, M.; Spatari, G.; Noviello, C.; Di Serafino, F.; Mormile, M.E.; Granvillano, G.; Iagnemma, A.; Mimmo, R.; Schenone, I.; Raso, E. Building evidences in Public Health Emergency Preparedness (“BePHEP” Project)—A systematic review. Int. J. Equity Health 2025, 24, 41. [Google Scholar] [CrossRef]
- Hamalaw, S.A.; Bayati, A.H.; Babakir-Mina, M.; Kiani, M.M.; Takian, A. The lessons of COVID-19 pandemic for communicable diseases surveillance system in Kurdistan Region of Iraq. Health Policy Technol. 2023, 12, 100717. [Google Scholar] [CrossRef]
- Nnebue, C.; Onwasigwe, C.; Adogu, P.; Adinma, E. Challenges of data collection and disease notification in Anambra State, Nigeria. Trop. J. Med. Res. 2014, 17, 1–6. [Google Scholar] [CrossRef]
- Meierkord, A.; Körner-Nahodilová, L.; Gotsche, C.; Baruch, J.; Briesemeister, V.; Correa-Martinez, C.; Hanefeld, J. Strengthening disease surveillance capacity at national level across five countries: A qualitative study. Public Health 2024, 233, 115–120. [Google Scholar] [CrossRef]
- Lafond, K.E.; Dalhatu, I.; Shinde, V.; Ekanem, E.E.; Ahmed, S.; Peebles, P.; Kudumu, M.; Bynum, M.; Salami, K.; Okeibunor, J. Notifiable disease reporting among public sector physicians in Nigeria: A cross-sectional survey to evaluate possible barriers and identify best sources of information. BMC Health Serv. Res. 2014, 14, 568. [Google Scholar] [CrossRef] [PubMed]
- Lebelo, K.; Van Wyk, R. Communicable Disease Surveillance in the City of Ekurhuleni: Environmental Health Practitioners’ Perceptions. In Proceedings of the 2019 Open Innovations (Oi), Cape Town, South Africa, 2–4 October 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 371–376. [Google Scholar] [CrossRef]
- McCarthy, K.; Quan, V. Communicable Diseases Surveillance and Outbreak Investigation in South Africa. S. Afr. Health Rev. 2018, 2018, 87–98. Available online: https://hdl.handle.net/10520/EJC-144db1444e (accessed on 29 September 2025).
- Gómez i Prat, J.; Alguacil, H.M.; Pequeño Saco, S.; Ouaarab Essadek, H.; Montero i Garcia, J.; Catasús i Llena, O.; Mendioroz Peña, J. Implementation of a community-based public model for the prevention and control of communicable diseases in migrant communities in Catalonia. Trop. Med. Infect. Dis. 2023, 8, 446. [Google Scholar] [CrossRef] [PubMed]
- Kleynhans, J.; Treurnicht, F.K.; Cohen, C.; Vedan, T.; Seleka, M.; Maki, L.; Von Gottberg, A.; Mccarthy, K.; Ramkrishna, W.; Mcmorrow, M.; et al. Outbreak of influenza A in a boarding school in South Africa, 2016. Pan Afr. Med. J. 2019, 33, 42. [Google Scholar] [CrossRef]
- Tavoschi, L.; Vroling, H.; Madeddu, G.; Babudieri, S.; Monarca, R.; Vonk Noordegraaf-Schouten, M.; Beer, N.; GomesDias, J.; Hendrich, D.; Oordt-Speets, A. Active Case Finding for Communicable Diseases in Prison Settings: Increasing Testing Coverage and Uptake Among the Prison Population in the European Union/European Economic Area. Epidemiol. Rev. 2018, 40, 105–120. [Google Scholar] [CrossRef]
- Leventhal, A.; Ramlawi, A.; Belbiesi, A.; Sheikh, S.; Haddadin, A.; Husseini, S.; Abdeen, Z.; Cohen, D. Enhanced Surveillance for Detection and Management of Infectious Diseases: Regional Collaboration in the Middle East. Emerg. Health Threat. J. 2013, 6, 19955. [Google Scholar] [CrossRef]
- Grover, E.; Hossain, M.K.; Uddin, S.; Venkatesh, M.; Ram, P.K.; Dreibelbis, R. Comparing the Behavioural Impact of a Nudge-Based Handwashing Intervention to High-Intensity Hygiene Education: A Cluster-Randomised Trial in Rural Bangladesh. Trop. Med. Int. Health 2018, 23, 10–25. [Google Scholar] [CrossRef]
- Jemiluyi, O.O.; Bank-Ola, R.F. The Burden of Infectious Diseases: A Trend Appraisal in Sub-Saharan Africa Regional Trade Blocs. J. Sci. Res. Med. Biol. Sci. 2021, 2, 104–122. [Google Scholar] [CrossRef]
- Govender, N.P.; Avenant, T.; Brink, A.; Chibabhai, V.; Cleghorn, J.; Du Toit, B.; Govind, C.; Lewis, E.; Lowman, W.; Mahlangu, H. Federation of Infectious Diseases Societies of Southern Africa guideline: Recommendations for the detection, management and prevention of healthcare-associated Candida auris colonisation and disease in South Africa. S. Afr. J. Infect. Dis. 2019, 34, 163. [Google Scholar] [CrossRef]
- Budgell, E.; Cohen, A.L.; Mcanerney, J.; Walaza, S.; Madhi, S.A.; Blumberg, L.; Dawood, H.; Kahn, K.; Tempia, S.; Venter, M. Evaluation of two influenza surveillance systems in South Africa. PLoS ONE 2015, 10, e0120226. [Google Scholar] [CrossRef]
- Liu, Q.; Deng, J.; Yan, W.; Qin, C.; Du, M.; Wang, Y.; Zhang, S.; Liu, M.; Liu, J. Burden and Trends of Infectious Disease Mortality Attributed to Air Pollution, Unsafe Water, Sanitation, And Hygiene, and Non-Optimal Temperature Globally and in Different Socio-Demographic Index Regions. Glob. Health Res. Policy 2024, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, S.L.; Page, N.A.; Thomas, J.; Madhi, S.A.; Mutevedzi, P.; Myburgh, N.; Herrera, C.; Groome, M.J. Diarrhoeal Diseases in Soweto, South Africa, 2020: A Cross-Sectional Community Survey. BMC Public Health 2021, 21, 1431. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, L.M.; Stephen, M.; Okudo, I.; Kitgakka, S.M.; Mamadu, I.N.; Njali, I.F.; Oladele, S.; Ojo, O.; Ihekweazu, C.; Lasuba, C.L.P. A Rapid Assessment of The Implementation of Integrated Disease Surveillance and Response System in Northeast Nigeria, 2017. BMC Public Health 2020, 20, 600. [Google Scholar] [CrossRef] [PubMed]
- Stewart-Ibarra, A.M.; Romero, M.; Hinds, A.Q.; Lowe, R.; Mahon, R.; Van Meerbeeck, C.J.; Rollock, L.; St. Ville, S.; Ryan, S.J.; Borbor-Cordova, M.J.; et al. Co-developing Climate Services for Public Health: Stakeholder Needs and Perceptions for the Prevention and Control of Aedes-Transmitted Diseases in the Caribbean. PLoS Negl. Trop. Dis. 2019, 13, e0007772. [Google Scholar] [CrossRef]
- Ng’etich, A.K.S.; Voyi, K.; Kirinyet, R.C.; Mutero, C.M. A Systematic Review on Improving Implementation of the Revitalised Integrated Disease Surveillance and Response System in the African Region: A Health Workers’ Perspective. PLoS ONE 2021, 16, e0248998. [Google Scholar] [CrossRef]
- Mcgowan, C.R.; Takahashi, E.; Romig, L.; Bertram, K.; Kadir, A.; Cummings, R.; Cardinal, L.J. Community-based surveillance of infectious diseases: A systematic review of drivers of success. BMJ Glob. Health 2022, 7, e009934. [Google Scholar] [CrossRef]
- Brown, J.; Hayashi, M.A.; Eisenberg, J.N. The Critical Role of Compliance in Delivering Health Gains from Environmental Health Interventions. Am. J. Trop. Med. Hyg. 2019, 100, 777. [Google Scholar] [CrossRef]
- Islam, M.S.; Rahman, K.M.; Sun, Y.; Qureshi, M.O.; Abdi, I.; Chughtai, A.A.; Seale, H. Current Knowledge of COVID-19 and Infection Prevention and Control Strategies in Healthcare Settings: A Global Analysis. Infect. Control. Hosp. Epidemiol. 2020, 41, 1196–1206. [Google Scholar] [CrossRef]
- Hanin, M.C.; Queenan, K.; Savic, S.; Karimuribo, E.; Rüegg, S.R.; Häsler, B. A One Health Evaluation of The Southern African Centre for Infectious Disease Surveillance. Front. Vet. Sci. 2018, 5, 33. [Google Scholar] [CrossRef]
- Rasmussen, S.L.; Sahay, S. Engaging with uncertainty: Information practices in the context of disease surveillance in Burkina Faso. Inf. Organ. 2021, 31, 100366. [Google Scholar] [CrossRef]
- Agenbag, M.H.; Human, I.S.; Schutte, D. Local Government Environmental Health Services: Fundamentals for Effective Municipal Service Delivery and Preventive Health Outcomes. J. New Gener. Sci. 2022, 20, 40–54. Available online: https://hdl.handle.net/10520/ejc-newgen-v20-n2-a4 (accessed on 29 September 2025). [CrossRef]
- Mousazadeh, M.; Naghdali, Z.; Rahimian, N.; Hashemi, M.; Paital, B.; Al-Qodah, Z.; Mukhtar, A.; Karri, R.R.; Mahmoud, A.E.; Emamjomeh, M.M.; et al. Management of Environmental Health to Prevent an Outbreak of COVID-19: A Review. In Environmental and Health Management of Novel Coronavirus Disease (COVID-19); Academic Press: Cambridge, MA, USA, 2021; pp. 235–267. [Google Scholar] [CrossRef]
- Girdler-Brown, B.V. Evaluation of the notifiable diseases surveillance system in South Africa. Int. J. Infect. Dis. 2017, 59, 139–140. [Google Scholar] [CrossRef]
- Musoke, D.; Namata, C.; Lubega, G.B.; Niyongabo, F.; Gonza, J.; Chidziwisano, K.; Nalinya, S.; Nuwematsiko, R.; Morse, T. The Role of Environmental Health in Preventing Antimicrobial Resistance in Low-And Middle-Income Countries. Environ. Health Prev. Med. 2021, 26, 100. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, S.; Twyman, A.; de Kraker, M.E.; Rehse, A.P.C.; Tartari, E.; Toledo, J.P.; Cassini, A.; Pittet, D.; Allegranzi, B. The First WHO Global Survey on Infection Prevention and Control in Health-Care Facilities. Lancet Infect. Dis. 2022, 22, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Isere, E.E.; Fatiregun, A.A.; Ajayi, I.O. An overview of disease surveillance and notification system in Nigeria and the roles of clinicians in Disease Outbreak Prevention and Control. Niger. Med. J. 2015, 56, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Makinde, O.A.; Odimegwu, C.O. Compliance with Disease Surveillance and Notification by Private Health Providers in South-West Nigeria. Pan Afr. J. 2020, 35, 114. [Google Scholar] [CrossRef]
- Meyer, R.; Wright, C.; Rother, H.A. Assessment of SADC Countries’ National Adaptation Planning Health Impacts Inclusion: A Thorough Review. Ann. Glob. Health 2024, 90, 57. [Google Scholar] [CrossRef]
- Onwe, F.I.; Okedo-Alex, I.N.; Akamike, I.C.; Igwe-Okomiso, D.O. Vertical Disease Programs and Their effect on Integrated Disease Surveillance and Response: Perspectives of Epidemiologists and Surveillance Officers in Nigeria. Trop. Dis. Travel Med. Vaccines 2021, 7, 28. [Google Scholar] [CrossRef]
- Sasie, S.D.; Ayano, G.; Mamo, F.; Azage, M.; Spigt, M. Assessing the performance of the integrated disease surveillance and response systems: A systematic review of global evidence. Public Health 2024, 231, 71–79. [Google Scholar] [CrossRef]
- Mudambo, K. Outbreaks and Epidemics of Malaria in SADC. In Epidemics and the Health of African Nations; Mapungubwe Institute for Strategic Reflection: Johannesburg, South Africa, 2019; pp. 107–142. Available online: https://library.oapen.org/bitstream/handle/20.500.12657/38243/9780639995618.pdf?sequence=1#page=134 (accessed on 30 September 2025).
- Hamalaw, S.A.; Bayati, A.H.; Babakir-Mina, M.; Benvenuto, D.; Fabris, S.; Guarino, M.; Giovanetti, M.; Ciccozzi, M. Assessment of Core and Support Functions of the Communicable Disease Surveillance System in the Kurdistan Region of Iraq. J. Med. Virol. 2022, 94, 469–479. [Google Scholar] [CrossRef]
- Mghamba, J.M.; Talisuna, A.O.; Suryantoro, L.; Saguti, G.E.; Muita, M.; Bakari, M.; Rusibamayila, N.; Ally, M.; Bernard, J.; Banda, R.; et al. Developing a multisectoral national action plan for health security (NAPHS) to implement the International Health Regulations (IHR 2005) in Tanzania. BMJ Glob. Health 2018, 3, e000600. [Google Scholar] [CrossRef]
- Jayatilleke, K. Challenges in Implementing Surveillance Tools of High-Income Countries (Hics) in Low Middle Income Countries (LMICs). Curr. Treat. Options Infect. Dis. 2020, 12, 191–201. [Google Scholar] [CrossRef]
- Mercy, K.; Balajee, A.; Numbere, T.; Ngere, P.; Simwaba, D.; Kebede, Y. Africa CDC’s blueprint to enhance early warning surveillance: Accelerating implementation of event-based surveillance in Africa. J. Public Health Afr. 2023, 14, 4. [Google Scholar] [CrossRef]




| General Keywords | Specific Keywords | Regional Keywords | Combining Keywords |
|---|---|---|---|
| Diseases | Emerging and re-emerging communicable diseases | Emerging and re-emerging communicable diseases in South Africa | Surveillance and prevention of communicable diseases in LMICs |
| Disease Surveillance | Communicable disease surveillance system | Communicable disease surveillance in LMICs | LMICs’ efforts in surveillance of communicable diseases |
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Phenomenon of interest | Communicable disease surveillance systems and strategies. | Non-communicable diseases and other articles not focusing on communicable diseases |
| Geographical area of interest/Population | Low and middle-income countries Worldwide, including African countries and South Africa. | High-income countries, including those without such classification and not from the African continent, |
| Period of publication | January 2010 to March 2025. | Any other articles published before 2004 or more than 2025 years ago will be excluded. |
| Language | English | Articles written in languages other than English will not be considered. |
| Study Type | Articles | Books, and any other reports, editorial, commentaries published not as articles, and those without access to full articles. |
| Study Design | Review, Exploratory, guideline, case study. | Any published papers without a clear study design will not be included. |
| Country | Surveillance Strategy/Framework | Integration with National/Regional Systems | Real-Time Data Use | Community Involvement | Key Limitations |
|---|---|---|---|---|---|
| South Africa | The National Notifiable Medical Conditions (NMC) system integrates IDSR elements. | Integrated with SADC and Africa CDC; aligns with IHR | Partial (improving with NMC digital platform) | Moderate—through environmental health practitioners | Fragmented municipal capacity, unequal digital access |
| Nigeria | Integrated Disease Surveillance and Response (IDSR) | Aligned with Africa CDC and WHO-AFRO IDSR guidelines | Some real-time reporting (DHIS2) | Strong community-based surveillance (CBS) is active | Inconsistent reporting, poor infrastructure |
| Kenya | IDSR + Electronic Disease Surveillance System (eDEWS) | Integrated with the East African regional response system | Strong—real-time alerts through eDEWS | Strong—community health volunteers trained | Funding gaps, training inconsistencies, and under-resourced surveillance units. |
| India | Integrated Disease Surveillance Programme (IDSP) | National-level integration, minimal international links | Moderate—uses a web-based reporting system | Limited—mostly facility-based surveillance | Overburdened system, data underreporting |
| Brazil | InfoGripe and SINAN (disease notification systems) | Integrated with PAHO; complies with IHR | Strong—especially respiratory syndromes | Moderate—CHWs play some roles in surveillance | Political instability, data transparency issues |
| Governance and Policy Dimension | South Africa | Other LMICs |
|---|---|---|
| Legal and Policy Framework | Governed by the National Health Act and Regulations Relating to Notifiable Medical Conditions (NMCs), clear legal mandate for surveillance and reporting. | Often guided by WHO frameworks (e.g., IDSR, IHR 2005), but national legislation may be weak or inconsistently enforced. |
| Institutional Coordination | Multi-tiered coordination across national, provincial, and local levels; integration with environmental health and municipal health services. | Variable coordination; often hampered by weak local governance and limited intersectoral collaboration. |
| Surveillance Strategy | Utilizes both syndromic and case-based surveillance; aligned with national priorities and regional initiatives. | Predominantly follows IDSR with adaptations; reliance on vertical programs in some contexts. |
| Digital Systems and Data Management | Implemented DHIS2 and electronic NMC platforms for real-time data reporting and analysis. | Increasing adoption of DHIS2 and other tools, but implementation is uneven and often lacks interoperability. |
| Human Resource Capacity | Stronger institutional capacity, though workforce shortages and uneven training persist. | Commonly affected by a limited public health workforce and inadequate training in surveillance functions. |
| Integration with International Frameworks | Aligned with IHR and Africa CDC strategies, contributes to SADC regional efforts. | Generally aligned with IDSR and IHR but limited regional collaboration in many countries. |
| Key Challenges | Implementation gaps at the subnational level, fragmented reporting, and resource constraints. | Governance fragmentation, underfunding, political instability, and weak enforcement of surveillance policies. |
| Theme | South Africa | Nigeria | Kenya | India | Brazil |
|---|---|---|---|---|---|
| Governance | Decentralized | Centralized | Hybrid | National | Federal |
| Framework | NMC, DHIS2 | IDSR | eDEWS | IDSP | SINAN |
| Real-time data use | Moderate | Low | High | Medium | High |
| Integration with EH | Limited | Moderate | Strong | Low | Moderate |
| Challenge Category | Description | Countries Most Frequently Affected | Example Citation from Review |
|---|---|---|---|
| Underreporting of Cases | Failure to capture and notify all relevant disease cases, particularly at subnational levels. | South Africa, Nigeria, Malawi, Guinea | [10,13] |
| Fragmented Data Systems | Parallel or vertical systems are not integrated into the national surveillance architecture. | Nigeria, India, Sierra Leone | [21,31] |
| Lack of Standardized Case Definitions | Inconsistent interpretation of diseases across health facilities. | South Africa, Iraq, Ghana | [15,48] |
| Insufficient Digital Infrastructure | Limited access to reliable internet, computers, or mobile-based tools. | Malawi, Sierra Leone, Uganda | [14,20] |
| Inadequate Feedback Loops | Poor flow of analyzed data back to local levels, weakening decision-making. | Uganda, Ghana, South Africa | [9,18] |
| Workforce Shortages | Lack of trained personnel in surveillance, particularly at the municipal or district level. | Nigeria, Kenya, India, South Africa | [30,31] |
| Limited Community-Based Surveillance (CBS) | Minimal involvement of community health workers or EHPs in early warning systems. | South Africa, India, Brazil | [29,32] |
| Weak Integration of Environmental Health | Environmental indicators not linked to communicable disease trends. | South Africa, Bangladesh, Thailand | [17,42] |
| Country | Environmental Monitoring Used | EH Practitioner Involvement | WASH Indicators Included? | Remarks |
|---|---|---|---|---|
| South Africa | Limited pilot use of wastewater surveillance (e.g., COVID-19) | Moderate—Mostly through municipal EHPs | Partially—WASH data collected but not fully linked | Some local-level integration efforts, but national systems remain fragmented |
| Bangladesh | Environmental sampling during the COVID-19 response | Low | Yes—Focus on water quality | EH indicators are included in the response, but not institutionalized long-term. |
| Thailand | Climate-sensitive modelling and environmental risk alerts | Low | No | Emerging models under public health R&D, not integrated into CDSS |
| India | Urban sanitation data used in selected districts | Low to moderate | Yes—Water, sanitation, waste | Integration is pilot-based and varies by state |
| Brazil | Zoonotic and environmental surveillance is partially integrated | Moderate—CHWs and sanitation officials involved | Partially | Some success in aligning environmental data with infectious disease alerts |
| Uganda | No structured environmental monitoring system | Low | No | EH functions are siloed from national surveillance systems |
| Kenya | Vector surveillance and waste monitoring in urban areas | Moderate | Yes | EH data is more integrated due to the One Health strategy |
| Identified Challenges | Future Directions |
|---|---|
| Limited resources for surveillance, particularly in low-income settings. | Strengthening surveillance systems through funding or resources, and capacity building. Increasing financial and infrastructural support for disease surveillance programs. |
| Insufficient integration of national disease surveillance systems. | Enhancing Multisectoral Collaborations to Integrate Surveillance Efforts. Enhancing data integration across all involved sectors. |
| Barriers to adopting innovative tools, such as GIS, in disease prevention programs. | Adapting interventions to emerging health threats through research and innovation, and expanding research on the impact of robust surveillance on reducing disease burden. |
| Generally, fragmented policies exist in the surveillance and prevention of communicable diseases. | Establishing uniform policies and enforcement mechanisms. Promoting public awareness and community involvement. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malebana, L.F.; Sepadi, M.M.; Mokgobu, M.I. Communicable Disease Surveillance in South Africa and LMICs: A Systematic Review of Systems, Challenges, and Integration with Environmental Health. Trop. Med. Infect. Dis. 2025, 10, 314. https://doi.org/10.3390/tropicalmed10110314
Malebana LF, Sepadi MM, Mokgobu MI. Communicable Disease Surveillance in South Africa and LMICs: A Systematic Review of Systems, Challenges, and Integration with Environmental Health. Tropical Medicine and Infectious Disease. 2025; 10(11):314. https://doi.org/10.3390/tropicalmed10110314
Chicago/Turabian StyleMalebana, Ledile Francina, Maasago Mercy Sepadi, and Matlou Ingrid Mokgobu. 2025. "Communicable Disease Surveillance in South Africa and LMICs: A Systematic Review of Systems, Challenges, and Integration with Environmental Health" Tropical Medicine and Infectious Disease 10, no. 11: 314. https://doi.org/10.3390/tropicalmed10110314
APA StyleMalebana, L. F., Sepadi, M. M., & Mokgobu, M. I. (2025). Communicable Disease Surveillance in South Africa and LMICs: A Systematic Review of Systems, Challenges, and Integration with Environmental Health. Tropical Medicine and Infectious Disease, 10(11), 314. https://doi.org/10.3390/tropicalmed10110314










