Examining Decomposition and Nitrogen Mineralization in Five Common Urban Habitat Types across Southern California to Inform Sustainable Landscaping
Abstract
1. Introduction
2. Materials and Methods
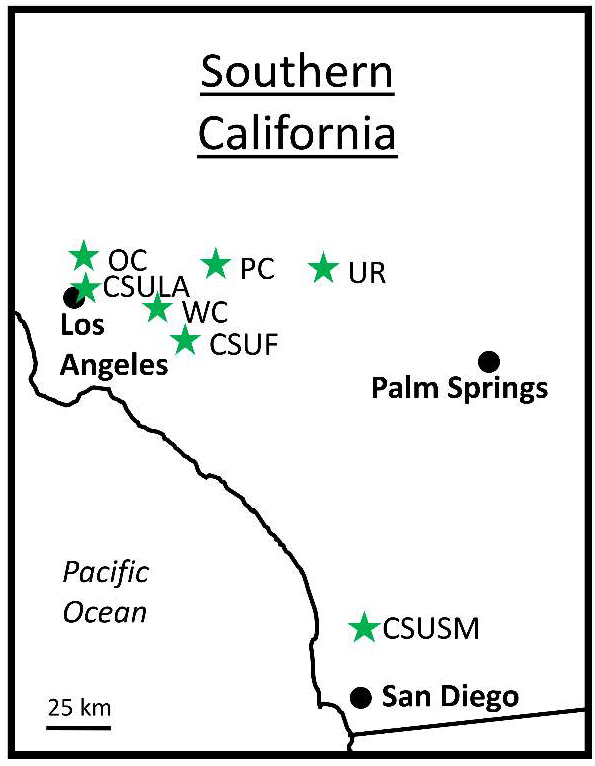
2.1. Site Descriptions
2.2. Litter Decomposition
2.3. Laboratory Analyses
2.4. Data Analyses
3. Results
3.1. Initial Substrate Chemistry
3.2. Landcover Variations in Soil Properties
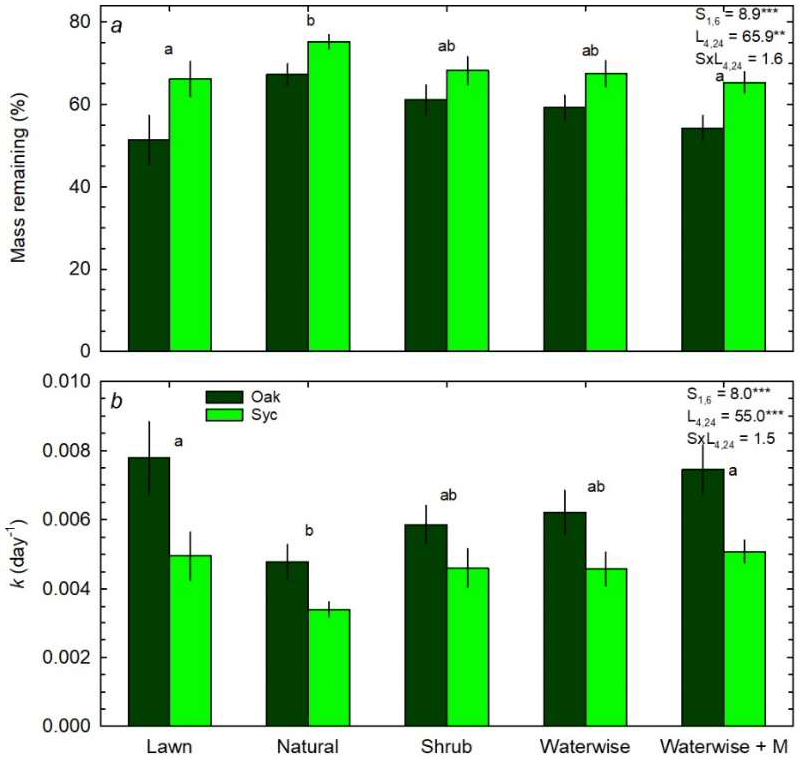
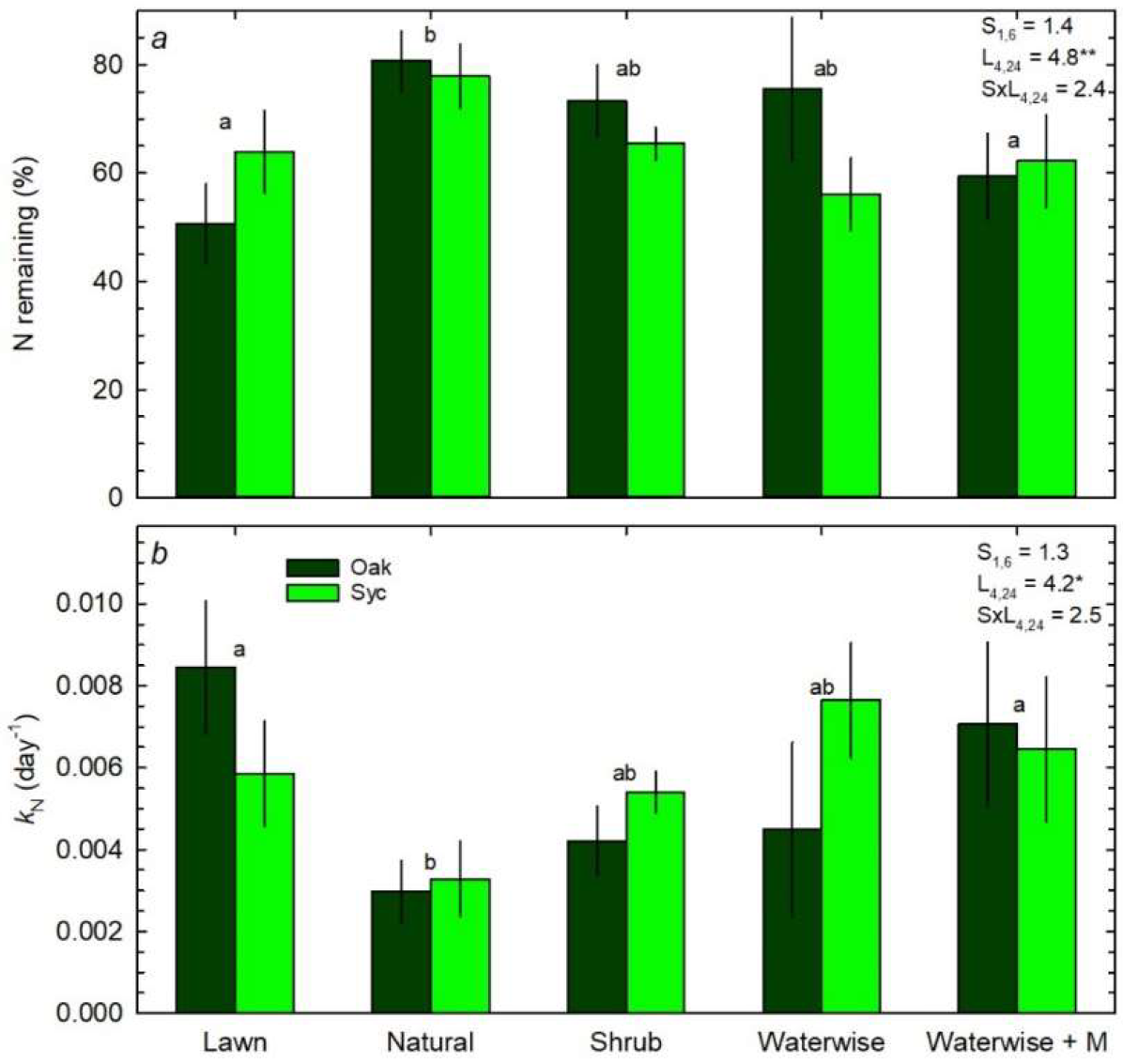
3.3. Litter Decomposition and N Mineralization
4. Discussion
4.1. Effects of Landscape Cover on Mass and N Loss
4.2. Effects of Substrate on Mass and N Loss
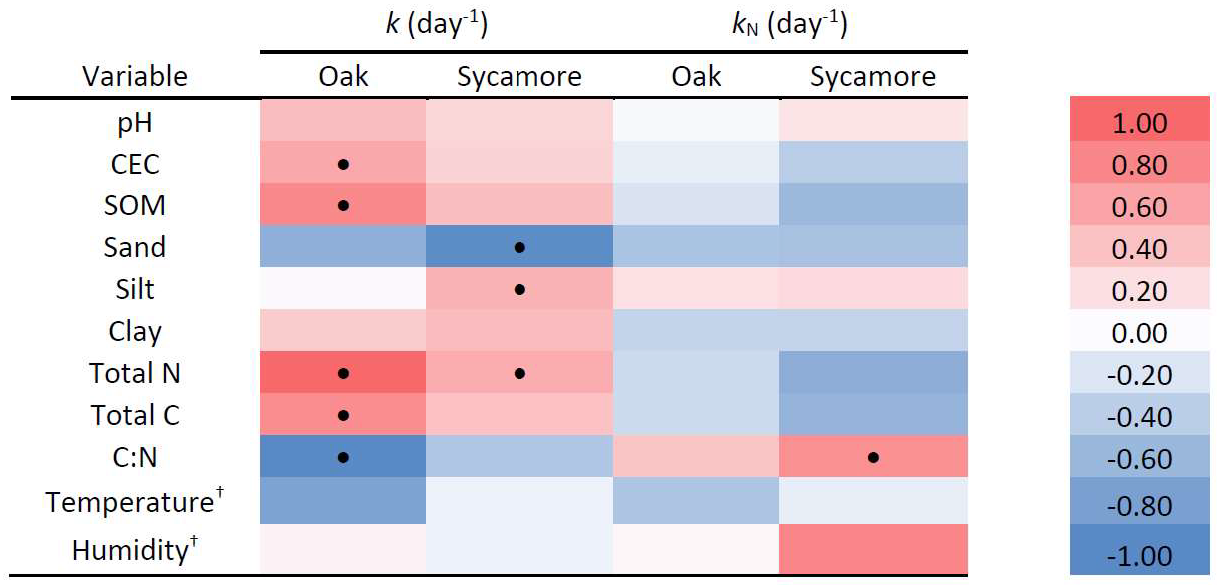
4.3. Relationship between Environmental Variables and Mass and N Loss
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Location and Abiotic Aspects of the Seven College/University Campuses
| Site (College) | Latitude (N) | Longitude (W) | Temperature (°C) | Precipitation (mm) |
|---|---|---|---|---|
| Pomona College | 34°05′52″ | 117°42′43″ | 16.6 | 430.5 |
| Whittier College | 33°58′40″ | 118°01′45″ | 19.7 | 375.4 |
| California State University at Fullerton | 33°52′57″ | 117°53′06″ | 17.4 | 365.8 |
| Occidental College | 34°07′38″ | 118°12′36″ | 18.3 | 436.9 |
| University of Redlands | 34°03′46″ | 117°09′48″ | 17.6 | 344.4 |
| California State University at San Marcos | 33°07′46″ | 117°09′35″ | 17.2 | 332.5 |
| California State University at Los Angeles | 34°04′01″ | 118°10′06″ | 18.3 | 451.4 |
References
- Cox, R.L.; Underwood, E.C. The importance of conserving biodiversity outside of protected areas in Mediterranean ecosystems. PLoS ONE 2011, 6, e14508. [Google Scholar] [CrossRef] [PubMed]
- Cowling, R.M.; Rundel, P.W.; Lamont, B.B.; Arroyo, M.K.; Arianoutsou, M. Plant diversity in Mediterranean-climate regions. Trends Ecol. Evol. 1996, 11, 362–366. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Sala, O.S.; Chapin, F.S., III; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sannwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Underwood, E.C.; Viers, J.H.; Klausmeyer, K.R.; Cox, R.L.; Shaw, M.R. Threats and biodiversity in the Mediterranean biome. Divers. Distrib. 2009, 15, 188–197. [Google Scholar] [CrossRef]
- Riordan, E.C.; Rundel, P.W. Modelling the distribution of a threatened habitat: The California sage scrub. J. Biogeogr. 2009, 36, 2176–2188. [Google Scholar] [CrossRef]
- Pataki, D.E.; Alig, R.J.; Fung, A.S.; Golubiewski, N.E.; Kennedy, C.A.; McPherson, E.G.; Nowak, D.J.; Pouyat, R.V.; Romero, L.P. Urban ecosystems and the North American carbon cycle. Glob. Chang. Biol. 2006, 12, 2092–2102. [Google Scholar] [CrossRef]
- Mitchell, L.E.; Lin, J.C.; Bowling, D.R.; Pataki, D.E.; Strong, C.; Schauer, A.J.; Bares, R.; Bush, S.E.; Stephens, B.B.; Mendoza, D.; et al. Long-term urban carbon dioxide observations reveal spatial and temporal dynamics related to urban characteristics and growth. Proc. Natl. Acad. Sci. USA 2018, 115, 2912–2917. [Google Scholar] [CrossRef]
- Pickett, S.T.A.; Cadenasso, M.L.; Grove, J.M.; Boone, G.G.; Groffman, P.M.; Irwin, E.; Kaushal, S.S.; Marshall, V.; McGrath, B.P.; Nilon, C.H.; et al. Urban ecological systems: Scientific foundations and a decade of progress. J. Environ. Manag. 2011, 92, 331–362. [Google Scholar] [CrossRef]
- Adesoye, T.; Sridhar, M.K.C.; Coker, A.O.; Adejumo, M. Landscaping of residential estates as a mitigation measure to reduce carbon dioxide and temperature levels in Inner Ibadan City, Nigeria. Int. J. Environ. Monit. Anal. 2019, 7, 93–102. [Google Scholar] [CrossRef]
- Chen, S.; Long, H.; Fath, B.D.; Chen, B. Global urban carbon networks: Linking inventory to modeling. Environ. Sci. Technol. 2020, 54, 5790–5801. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, D.J.; Benham, M.; Singh, B.K.; Delgado-Baquerizo, M. Ecosystem properties in urban areas vary with habitat type and settlement age. Plant Soil. 2021, 461, 489–500. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Thomson, A. A global database of soil respiration data. Biogeosciences 2010, 7, 1915–1926. [Google Scholar] [CrossRef]
- Meentemeyer, V. Macroclimate and lignin control of litter decomposition rates. Ecology 1978, 59, 465–472. [Google Scholar] [CrossRef]
- Suárez, E.R.; Fahey, T.J.; Yavitt, J.B.; Groffman, P.M.; Bohlen, P.J. Patterns of litter disappearance in a northern hardwood forest invaded by exotic earthworms. Ecol. Appl. 2006, 16, 154–165. [Google Scholar] [CrossRef]
- Hobbie, S.; Reich, P.; Oleksyn, J.; Ogdahl, M.; Zytkowiak, R.; Hale, C.; Karolewski, P. Tree species effects on decomposition and forest floor dynamics in a common garden. Ecology 2006, 87, 2288–2297. [Google Scholar] [CrossRef]
- Hobbie, S.; Baker, L.; Buyarski, C.; Nidzgorski, D.; Finlay, J. Decomposition of tree leaf litter on pavement: Implications for urban water quality. Urban Ecosyst. 2013, 17, 369–385. [Google Scholar] [CrossRef]
- Tresch, S.; Frey, D.; Le Bayon, R.C.; Zanetta, A.; Rasche, F.; Fliessbach, A.; Moretti, M. Litter decomposition driven by soil fauna, plant diversity and soil management in urban gardens. Sci. Total Environ. 2019, 658, 1614–1629. [Google Scholar] [CrossRef]
- Pouyat, V.; Szlavecz, K.; Yesilonis, I.D.; Groffman, P.M.; Schwarz, K. Chemical, Physical, and Biological Characteristics of Urban Soils. In Urban Ecosystem Ecology; Aitkenhead-Peterson, J., Volder, A., Eds.; Urban Ecosystem Ecology; American Society of Agronomy: Madison, WI, USA, 2010; pp. 119–152. [Google Scholar]
- Radeloff, V.C.H.; Kramer, D.P.; Mockrin, H.A.; Alexandre, M.H.; Massada, P.M.B.; Butsic, A.; Hawbaker, V.; Martinuzzi, T.J.; Syphard, S.; Stewart, A.D.; et al. The 1990–2010 Wildland-Urban Interface of the Conterminous United States-Geospatial Data. 2017. Available online: https://www.fs.usda.gov/rds/archive/Catalog/RDS-2015-0012-2 (accessed on 15 August 2022). [CrossRef]
- Radeloff, V.C.; Helmers, D.P.; Kramer, H.A.; Mockrin, M.H.; Alexandre, P.M.; Bar-Massada, A.; Butsic, V.; Hawbaker, T.J.; Martinuzzi, S.; Syphard, A.D.; et al. Rapid growth of the US wildland-urban interface raises wildfire risk. Proc. Natl. Acad. Sci. USA 2018, 115, 3314–3319. [Google Scholar] [CrossRef]
- Johnson, H.; McGhee, E.; Mejia, M.C. California’s Population Public Policy Institute of California. Available online: https://www.ppic.org/publication/californias-population/ (accessed on 2 April 2022).
- Stein, E.D.; Dark, S.; Longcore, T.; Hall, N.; Beland, M.; Grossinger, R.; Casanova, J.; Sutula, M. Historical Ecology and Landscape Change of the San Gabriel River and Floodplain; Southern California Coastal Water Research Project Technical Report: Costa Meas, CA, USA, 2007. [Google Scholar]
- Rundel, P.W. Sage scrub. In Terrestrial Vegetation of California; Barbour, M.G., Keeler Wolf, T., Schoenherr, A.A., Eds.; University of California Press: Berkeley, CA, USA, 2007; pp. 208–228. [Google Scholar]
- Talluto, M.V.; Suding, K.N. Historical change in coastal sage scrub in Southern Californica, USA in relation to fire frequency and air pollution. Landsc. Ecol. 2008, 23, 803–815. [Google Scholar] [CrossRef]
- Cox, R.D.; Preston, K.L.; Johnson, R.F.; Minnich, R.A.; Allen, E.B. Influence of landscape-scale variables on vegetation conversion to exotic annual grassland in southern California, USA. Glob. Ecol. Conserv. 2014, 2, 190–203. [Google Scholar] [CrossRef]
- Wheeler, M.M.; Dipman, M.M.; Adams, T.A.; Runia, A.V.; Robbins, C.R.; Meyer, W.M., III. Carbon and nitrogen storage in California sage scrub and non-native grassland habitats. J. Arid Environ. 2016, 129, 119–125. [Google Scholar] [CrossRef]
- Caspi, T.; Estrada, L.; Dowling, A.V.; Su, E.; Leshchinskiy, M.; Cavalcanti, A.R.O.; Crane, E.J.; Meyer, W.M., III. Carbon and nitrogen in the topsoils of Inceptisols and Mollisols under native sage scrub and non-native grasslands in southern California. Geoderma Reg. 2018, 14, e00172. [Google Scholar] [CrossRef]
- Caspi, T.; Hartz, L.A.; Soto Villa, A.E.; Loesberg, J.A.; Robins, C.R.; Meyer, W.M., III. Impacts of invasive annuals on soil carbon and nitrogen storage in southern California depend on the identity of the invader. Ecol. Evol. 2016, 9, 4980–4993. [Google Scholar] [CrossRef] [PubMed]
- Dipman, M.M.; Meyer, W.M., III. Type conversion from native California sage scrub to non-native grassland accelerates decomposition processes. Appl. Soil Ecol. 2019, 144, 68–71. [Google Scholar] [CrossRef]
- Mauritz, M.; Lipson, D.A. Plant community composition alters moisture and temperature sensitivity of soil respiration in semi-arid shrubland. Oecologia 2021, 197, 1003–1015. [Google Scholar] [CrossRef]
- Vauramo, S.; Setälä, H. Decomposition of labile and recalcitrant litter types under different plant communities in urban soils. Urban Ecosyst. 2011, 14, 59–70. [Google Scholar] [CrossRef]
- Berg, B. Decomposition patterns for foliar litter: A theory for influencing factors. Soil Biol. Biochem. 2014, 78, 222–232. [Google Scholar] [CrossRef]
- Aerts, R. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: A triangular relationship. Oikos 1997, 79, 439–449. [Google Scholar] [CrossRef]
- Miller, A.E.; Schimel, J.P.; Meixner, T.; Sickman, J.O.; Melack, J.M. Episodic rewetting enhances carbon and nitrogen release from chaparral soils. Soil Biol. Biochem. 2005, 37, 2195–2204. [Google Scholar] [CrossRef]
- Jenerette, G.D.; Chatterjee, A. Soil metabolic pulses: Water, substrate, and biological regulation. Ecology 2012, 93, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Keuskamp, J.A.; Dingemans, B.J.J.; Lehtinen, T.; Sarneel, J.M.; Hefting, M.M. Tea bag index: A novel approach to collect uniform decomposition data across ecosystems. Methods Ecol. Evol. 2013, 4, 1070–1075. [Google Scholar] [CrossRef]
- Dorendorf, J.; Wilken, A.; Eschenbach, A.; Jensen, K. Urban-induced changes in tree leaf litter accelerate decomposition. Ecol. Process. 2015, 4, 1. [Google Scholar] [CrossRef]
- Austin, A.T.; Vivanco, L. Plant litter decomposition in a semiarid ecosystem controlled by photodegradation. Nature 2006, 442, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Berhe, A.A. Effect of litterbags on rate of organic substrate decomposition along soil depth and geomorphic gradients. J. Soils Sediments 2013, 13, 629–640. [Google Scholar] [CrossRef]
- Latifmanesha, H.; Deng, A.; Li, L.; Chen, Z.; Zheng, Y.; Bao, X.; Zheng, C.; Zhang, W. How incorporation depth of corn straw affects straw decomposition rate and C&N release in the wheat-corn cropping system. Agric. Ecosyst. Environ. 2020, 300, 107000. [Google Scholar]
- Berenstecher, P.; Araujo, P.I.; Austin, A.T. Worlds apart: Location above-or below-ground determines plant litter decomposition in a semi-arid Patagonian steppe. J. Ecol. 2021, 109, 2885–2896. [Google Scholar] [CrossRef]
- Blair, J.M. Nitrogen, sulfur and phosphorus dynamics in decomposing deciduous leaf litter in the southern Appalachians. Soil Biol. Biochem. 1988, 20, 693–701. [Google Scholar] [CrossRef]
- Pouyat, R.V.; McDonnell, M.J.; Pickett, S.T.A. Litter decomposition and nitrogen mineralization in oak stands along an urban–rural land use gradient. Urban Ecosyst. 1997, 1, 117–131. [Google Scholar] [CrossRef]
- Pavao-Zuckerman, M.A.; Coleman, D.C. Decomposition of chestnut oak (Quercus prinus) leaves and nitrogen mineralization in an urban environment. Biol. Fertil. Soils 2005, 41, 343–349. [Google Scholar] [CrossRef]
- Olson, J.S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Monograph in Applied Statistics and Probability 57; Chapman & Hall: New York, NY, USA, 1993. [Google Scholar]
- Schlesinger, W.H.; Hasey, M.M. Decomposition of chaparral shrub foliage: Losses of organic and inorganic constituents from deciduous and evergreen leaves. Ecology 1981, 62, 762–774. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Bernhardt, E.S. Biogeochemistry: An Analysis of Global Change, 3rd ed.; Elsevier: Oxford, UK, 2013. [Google Scholar]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of the soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Pouyat, R.V.; Yesilonis, I.D.; Nowak, D.J. Carbon storage by urban soils in the United States. J. Environ. Q. 2006, 35, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Pouyat, R.V.; Yesilonis, I.D.; Golubiewski, N.E. A comparison of soil organic carbon stocks between residential turf grass and native soil. Urban Ecosyst. 2009, 12, 45–62. [Google Scholar] [CrossRef]
- Jenerette, G.D.; Wu, J.; Grimm, N.B.; Hope, D. Points, patches, and regions: Scaling soil biogeochemical patterns in an urbanized arid ecosystem. Glob. Chang. Biol. 2006, 12, 1532–1544. [Google Scholar] [CrossRef]
- Huang, R.; Lan, T.; Song, X.; Li, J.; Ling, J.; Deng, O.; Wang, C.-Q.; Gao, X.; Li, Q.-Q.; Tang, X.; et al. Soil labile organic carbon impacts C:N:P stoichiometry in urban park green spaces depending on vegetation types and time after planting. Appl. Soil Ecol. 2021, 163, 103926. [Google Scholar] [CrossRef]
- Sylvain, Z.A.; Wall, D.H. Linking soil biodiversity and vegetation: Implications for a changing planet. Am. J. Bot. 2011, 98, 517–527. [Google Scholar] [CrossRef]
- Abdala-Roberts, L.; Galmán, A.; Petry, W.K.; Covelo, F.; de la Fuente, M.; Glauser, G.; Moreira, X. Interspecific variation in leaf functional and defensive traits in oak species and its underlying climatic drivers. PLoS ONE 2018, 13, e0202548. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From tropics to tundra: Global convergence in plant functioning. Proc. Natl. Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef]
- John, G.P. The structural and compositional basis of leaf traits and their influence on plant water use and drought tolerance. Ph.D. Thesis, University of California Los Angeles, Los Angeles, CA, USA, 2017. [Google Scholar]
- John, G.P.; Scoffoni, C.; Buckley, T.N.; Villar, R.; Poorter, H.; Sack, L. The anatomical and compositional basis of leaf mass per area. Ecol. Lett. 2017, 20, 412–425. [Google Scholar] [CrossRef]
- Mauffette, Y.; Oechel, W.C. Seasonal variation in leaf chemistry of the coast live oak Quercus agrifolia and implications for the California oak moth Phryganidia californica. Oecologia 1989, 79, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.P.; Schaeffer, S.M. Microbial control over carbon cycling in soil. Front. Microbiol. 2012, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.; Mohan, M. Litter decomposition in forest ecosystems: A review. Energy Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- Binkley, D.; Hart, S. The components of nitrogen availability assessments in forest soils. Adv. Soil Sci. 1989, 10, 57–112. [Google Scholar]
- Fierer, N.; Bradford, M.; Jackson, R. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Goldfarb, K.C.; Karaoz, U.; Hanson, C.A.; Santee, C.A.; Bradford, M.A.; Treseder, K.K.; Wallenstein, M.D.; Brodie, E.L. Differential growth responses of soil bacterial taxa to carbon substrates of varying chemical recalcitrance. Front. Microbiol. 2011, 2, 1–10. [Google Scholar] [CrossRef]
- de Carvalho, C.A.; Raposo, M.; Pinto-Gomes, C.; Matos, R. Native or Exotic: A Bibliographical Review of the Debate on Ecological Science Methodologies: Valuable Lessons for Urban Green Space Design. Land 2022, 11, 1201. [Google Scholar] [CrossRef]
- Ge, B.; Mehring, A.S.; Levin, L.A. Urbanization alters belowground invertebrate community structure in semi-arid regions: A comparison of lawns, biofilters and sage scrub. Landsc. Urban Plan 2019, 192, 103664. [Google Scholar] [CrossRef]
- Staubus, W.J.; Boyd, E.S.; Adams, T.A.; Spear, D.M.; Dipman, M.M.; Meyer, W.M., III. Ant communities in native sage scrub, non-native grassland, and suburban habitats in Los Angeles County, USA: Conservation implications. J. Insect Conserv. 2015, 19, 669–680. [Google Scholar] [CrossRef]
- Staubus, W.J.; Bird, S.; Meadors, S.; Meyer, W.M., III. Distributions of invasive arthropods across heterogeneous urban landscapes in Southern California: Aridity as a key component of ecological resistance. Insects 2019, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.J.; Li, E.; Bahlai, C.A.; Meineke, E.K.; McGlynn, T.P.; Brown, B.V. Local-and landscape-scale variables shape insect diversity in an urban biodiversity hot spot. Ecol. Appl. 2020, 30, e02089. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, K.S.; Tomlinson, S.; Dixon, K.W.; Bateman, P.W.; Menz, M.H. Urban native vegetation remnants support more diverse native bee communities than residential gardens in Australia’s southwest biodiversity hotspot. Biol. Conserv. 2022, 265, 109408. [Google Scholar] [CrossRef]

| Variable | Oak | n | Sycamore | n | Statistic (p) |
|---|---|---|---|---|---|
| Leaf N | 1.51 ± 0.04 | 11 | 1.15 ± 0.05 | 11 | t20 = 6.2 (p < 0.001) |
| Leaf C | 46.3 ± 0.3 | 11 | 43.8 ± 0.3 | 11 | t20 = 5.5 (p < 0.001) |
| C:N | 30.7 ± 1.9 | 11 | 38.7 ± 1.6 | 11 | t20 = −4.6 (p < 0.001) |
| Soluble C | 47.3 ± 3.5 | 11 | 35.8 ± 5.6 | 6 | Bootstrap (NS) |
| Holocellulose C | 13.7 ± 3.3 | 11 | 12.7 ± 6.4 | 6 | Bootstrap (NS) |
| Lignin C | 39.0 ± 5.6 | 11 | 51.3 ± 10.8 | 6 | Bootstrap (NS) |
| Lignin:N | 26.3 ± 4.5 | 11 | 46.4 ± 8.5 | 6 | Bootstrap (p < 0.05) |
| Habitat Type | pH | CEC (meq/100 g) | SOM (%) | Sand (%) | Silt (%) | Clay (%) | Total N (%) | Total C (%) | C:N |
|---|---|---|---|---|---|---|---|---|---|
| Lawn | 7.2 ± 0.2 a | 19.7 + 2.5 a | 5.5 ± 1.3 ab | 62 ± 5 | 25 ± 3 | 13 ± 2 | 0.27 ± 0.06 a | 2.8 ± 0.7 a | 10.3 ± 0.6 |
| Natural | 6.3 ± 0.3 b | 10.9 + 2.7 b | 2.6 ± 0.2 a | 69 ± 4 | 19 ± 2 | 11 ± 2 | 0.11 ± 0.02 b | 1.3 ± 0.2 b | 15.3 ± 3.7 |
| Shrub | 7.5 ± 0.2 a | 14.7 + 3.2 a | 4.4 ± 1.3 ab | 68 ± 3 | 20 ± 2 | 12 ± 1 | 0.17 ± 0.06 b | 2.7 ± 0.9 a | 16.2 ± 1.8 |
| Waterwise | 7.5 ± 0.1 a | 14.7 + 4.1 a | 2.7 ± 0.6 a | 64 ± 5 | 22 ± 3 | 13 ± 2 | 0.11 ± 0.03 b | 1.4 ± 0.4 b | 18.5 ± 5.3 |
| Waterwise + Mulch | 7.4 ± 0.1 a | 22.7 ± 4.1 a | 5.7 ± 1.2 b | 64 ± 3 | 23 ± 2 | 13 ± 1 | 0.27 ± 0.07 a | 3.8 ± 0.9 a | 14.7 ± 1.0 |
| F4,24 | 6.41 | 3.74 | 4.01 † | 1.08 | 1.36 | 0.53 | 3.88 † | 3.92 † | 2.58 † |
| p-value | 0.001 | 0.02 | 0.01 | NS | NS | NS | 0.02 | 0.01 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vourlitis, G.L.; van der Veen, E.L.; Cangahuala, S.; Jaeger, G.; Jensen, C.; Fissore, C.; Wood, E.M.; Abraham, J.K.; Whittemore, K.S.; Slaven, E.; et al. Examining Decomposition and Nitrogen Mineralization in Five Common Urban Habitat Types across Southern California to Inform Sustainable Landscaping. Urban Sci. 2022, 6, 61. https://doi.org/10.3390/urbansci6030061
Vourlitis GL, van der Veen EL, Cangahuala S, Jaeger G, Jensen C, Fissore C, Wood EM, Abraham JK, Whittemore KS, Slaven E, et al. Examining Decomposition and Nitrogen Mineralization in Five Common Urban Habitat Types across Southern California to Inform Sustainable Landscaping. Urban Science. 2022; 6(3):61. https://doi.org/10.3390/urbansci6030061
Chicago/Turabian StyleVourlitis, George L., Emma Lousie van der Veen, Sebastian Cangahuala, Garrett Jaeger, Colin Jensen, Cinzia Fissore, Eric M. Wood, Joel K. Abraham, Kevin S. Whittemore, Elijah Slaven, and et al. 2022. "Examining Decomposition and Nitrogen Mineralization in Five Common Urban Habitat Types across Southern California to Inform Sustainable Landscaping" Urban Science 6, no. 3: 61. https://doi.org/10.3390/urbansci6030061
APA StyleVourlitis, G. L., van der Veen, E. L., Cangahuala, S., Jaeger, G., Jensen, C., Fissore, C., Wood, E. M., Abraham, J. K., Whittemore, K. S., Slaven, E., VanOverbeke, D., Blauth, J., Braker, E., Karnovsky, N., & Meyer, W. M., III. (2022). Examining Decomposition and Nitrogen Mineralization in Five Common Urban Habitat Types across Southern California to Inform Sustainable Landscaping. Urban Science, 6(3), 61. https://doi.org/10.3390/urbansci6030061







