Current Progress on Passiflora caerulea L. In Vitro Culturing
Abstract
1. Introduction
Research Methodology
2. Challenges Related to the Culturing of P. caerulea
2.1. Seed Dormancy and Low Germination Rate
2.2. Contamination Issues
3. In Vitro Micropropagation of P. caerulea
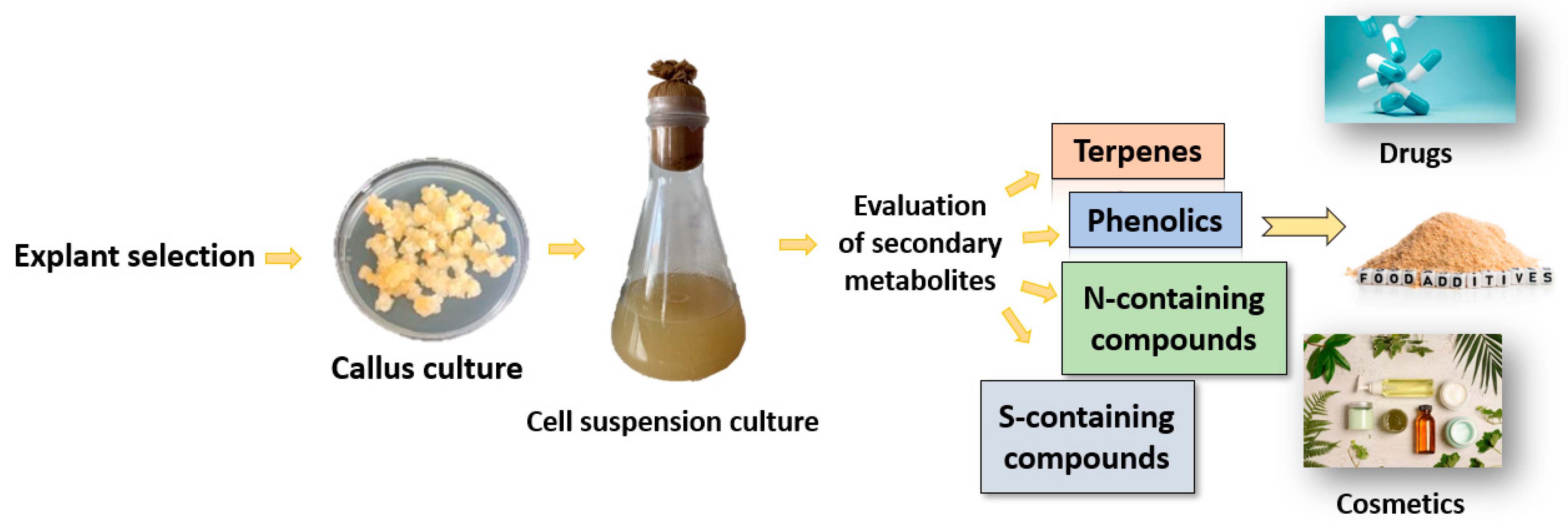
4. Regenerated Plantlets from P. caerulea and Phytochemical Analysis
Future Prospects and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PGRs | Plant growth regulators |
| MS | Murashige and Skoog |
| WPM | Woody plant medium |
| IAA | Indole-3-acetic acid |
| IBA | Indole-3-butyric acid |
| NAA | 1-naphthaleneacetic acid |
| 2,4-D | 2,4-dichlorophenoxyacetic acid |
| iP | Isopentenyl adenine |
| BAP | Benzylaminopurine |
| KIN | Kinetin |
| TDZ | Thidiazuron |
| GRNN | General Regression Neural Networks |
| RF | Random Forests |
| BA | Benzyladenine or BAP—6-Benzylaminopurine |
| GA3 | Gibberellic acid |
| TLC | Thin-layer chromatography |
| HPLC | High-performance liquid chromatography |
| HPTLC | High-performance thin-layer chromatography |
References
- Davis, C.C.; Choisy, P. Medicinal plants meet modern biodiversity science. Curr. Biol. 2024, 34, R158–R173. [Google Scholar] [CrossRef]
- Mishuk, A.U.; Hossain, A.; Karmakar, U.K. Medicinal potential of Passiflora foetida L. plant extracts: Biological and pharmacological activities. J. Integr. Med. 2014, 12, 121–126. [Google Scholar] [CrossRef]
- De Moura, T.; Araújo, F.; Cerqueira, S.; De, R.G. Current topics in medicinal chemistry. Curr. Top. Med. Chem. 2022, 22, 2315–2328. [Google Scholar] [CrossRef]
- Resources, P.G.; De Carlo, A. In vitro biotechnology for conservation and sustainable use of plant genetic resources. Plants 2024, 13, 1897. [Google Scholar] [CrossRef]
- Oros, P.B.; Cătană, C. In vitro plant tissue culture: Means for production of Passiflora species. Int. J. Innov. Approaches Agric. Res. 2020, 4, 505–523. [Google Scholar] [CrossRef]
- Berberich, S.; Snyder, J.; Geneve, R.; Williams, M.A. Growth and flowering response of container grown passion flower cultivars to fertilizer and paclobutrazol. J. Environ. Hortic. 1962, 24, 109–114. [Google Scholar] [CrossRef]
- Gerasimova, A.; Nikolova, K.; Petkova, N.; Ivanov, I.; Dincheva, I.; Tumbarski, Y.; Yanakieva, V.; Todorova, M.; Gentscheva, G.; Gavrilova, A.; et al. Metabolic Profile of Leaves and Pulp of Passiflora caerulea L. (Bulgaria) and Their Biological Activities. Plants 2024, 13, 1731. [Google Scholar] [CrossRef]
- Smruthi, R.; Divya, M.; Archana, K.; Ravi, M. The active compounds of Passiflora spp. and their potential medicinal uses from both in vitro and in vivo evidences. J. Adv. Biomed. Pharm. Sci. 2021, 4, 45–55. [Google Scholar] [CrossRef]
- Silva, C.G. Tissue Culture and Phytochemical Studies of Podophyllum, Diphylleia and Passiflora Species. Bachelor’s Thesis, University of Nottingham, Nottingham, UK, 2000. Available online: http://eprints.nottingham.ac.uk/28994/1/311836.pdf (accessed on 14 April 2025).
- Yi, S.-S. A Composition for Sleep Induction Comprising Passiflora incarnata Extract. KR 102240706B1, 2021. Available online: https://patents.google.com/patent/KR102240706B1/en (accessed on 19 February 2025).
- Feliú-Hemmelmann, K.; Monsalve, F.; Rivera, C. Melissa officinalis and Passiflora caerulea infusion as physiological stress decreaser. Int. J. Clin. Exp. Med. 2013, 6, 444–451. [Google Scholar]
- Nagabhushan Arun, M.; Mahender Kumar, R.; Sreedevi, B.; Padmavathi, G.; Revathi, P.; Pathak, N.; Venkatanna, B. The Rising Threat of Invasive Alien Plant Species in Agriculture. In The Rising Threat of Invasive Alien Plant Species in Agriculture; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Barbault, R.; Atramentowicz, M. Les Invasions Biologiques, une Question de Natures et de Sociétés; Cairn.info: Paris, France, 2010; p. 180. ISBN 978-2-7592-0373-4. [Google Scholar] [CrossRef]
- Silva, C.V.; Oliveira, L.S.; Loriato, V.A.P.; Silva, L.C.; de Campos, J.M.S.; Viccini, L.F.; de Oliveira, E.J.; Otoni, W.C. Organogenesis from Root Explants of Commercial Populations of Passiflora edulis Sims and a Wild Passionfruit Species, P. cincinnata Masters. Plant Cell Tissue Organ Cult. 2011, 107, 407–416. [Google Scholar] [CrossRef]
- Prithviraj, H.S.; Hemanth Kumar, N.K.; Prakasha; Shobha, J. An efficient in vitro regeneration of multiple shoots from leaf explant of Passiflora caerulea L., an important medicinal plant. Int. J. Recent Sci. Res. Res. 2015, 6, 7263–7265. Available online: https://recentscientific.com/sites/default/files/3767.pdf (accessed on 18 May 2025).
- Jafari, M.; Daneshvar, M.H.; Lotfi, A. Control of in vitro contamination of Passiflora caerulea using sodium hypochlorite. Indo-Am. J. Agric. Vet. Sci. 2016, 4, 7–16. [Google Scholar]
- Faria, G.A.; Oliveira, C.P.M.; Lopes, B.G.; Rocha, P.S.; Peron, G.M.; Souza, K.S.; Garcia, C.K.; Furlani Junior, E.; Cavichioli, J.C.; Felizardo, L.M. Establishment of a Protocol for In Vitro Propagation of Passiflora caerulea. Res. Soc. Dev. 2020, 9, e157997158. [Google Scholar] [CrossRef]
- Ożarowski, M. Influence of the Physico-Chemical Factors, Plant Growth Regulators, Elicitors and Type of Explants on Callus Cultures of Medicinal Climbers of Passiflora L. Herba Pol. 2011, 57, 58–75. [Google Scholar]
- Mendiondo, G.M.; Amela García, M.T. Germination of stored and scarified seeds of Passiflora caerulea L. (Passifloraceae). Plant Biosyst. 2009, 143, 369–376. [Google Scholar] [CrossRef]
- Phartyal, S.S.; Baskin, J.M.; Baskin, C.C.; Thapliyal, R.C. Physical dormancy in seeds of Dodonaea viscosa (Sapindaceae) from India. Seed Sci. Res. 2005, 15, 59–61. [Google Scholar] [CrossRef]
- Upretee, P.; Bandara, M.S.; Tanino, K.K. The role of seed characteristics on water uptake preceding germination. Seeds 2024, 3, 559–574. [Google Scholar] [CrossRef]
- Ożarowski, M.; Thiem, B. Progress in micropropagation of Passiflora spp. to produce medicinal plants: A mini-review. Rev. Bras. Farmacogn. 2013, 23, 937–947. [Google Scholar] [CrossRef]
- Rezazadeh, A.; Bailey, M.; Sarkhosh, A. Passion fruit problems in the home landscape. Univ. Fla. EDIS 2020, 2020, 6. [Google Scholar] [CrossRef]
- Espinosa, C.A.; César, L.; Garza, A.P.; García, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef]
- Hasnain, A.; Atif, S.; Naqvi, H.; Ayesha, S.I.; Khalid, F.; Ellahi, M.; Abbas, A.; Adamski, R.; Markowska, D. Plants in vitro propagation with its applications in food, pharmaceuticals and cosmetic industries: Current scenario and future approaches. Front. Plant Sci. 2022, 13, 1009395. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.M.; Nakhooda, M. Clonal and Micropropagation. In Encyclopedia of Applied Plant Sciences, 2nd ed.; Thomas, B., Murray, B.G., Murphy, D.J., Eds.; Academic Press: Oxford, UK, 2017; pp. 428–432. ISBN 978-0-12-394808-3. [Google Scholar] [CrossRef]
- Ahmad, N.; Strnad, M. (Eds.) Meta-Topolin: A Growth Regulator for Plant Biotechnology and Agriculture, 1st ed.; Springer: Singapore, 2021; Volume XIII, p. 339. [Google Scholar] [CrossRef]
- Micheli, M.; Prosperi, F.; Facchin, S.; Fernandes, D. Safeguard of plant germplasm through the in vitro culture. Hortic. Int. J. 2020, 4, 50–52. [Google Scholar] [CrossRef]
- Fernando, J.A.; Lu, M.; Appezzato-da-Glo, S.R.M. New insights into the in vitro organogenesis process: The case of Passiflora. Plant Cell Tissue Organ Cult. 2007, 91, 37–44. [Google Scholar] [CrossRef]
- Aparecida, C.; Brito, R.; Faria, D.; Pinheiro, P.; Carvalho, D.; Izabel, A.; Fealho, I.; Carvalho, D.; Monteiro, E.; Matos, D.; et al. In vitro regeneration of triploid plants from mature endosperm culture of commercial passionfruit (Passiflora edulis Sims). Sci. Hortic. 2018, 238, 408–415. [Google Scholar] [CrossRef]
- Rathod, H.P.; Pohare, M.; Bhor, S.; Jadhav, K.P.; Batule, B.S. In vitro micropropagation of blue passion flower (Passiflora caerulea L.). Trends Biosci. 2015, 7, 3079–3082. [Google Scholar]
- Jafari, M.; Daneshvar, M.H.; Lotfi, A. In vitro shoot proliferation of Passiflora caerulea L. via cotyledonary node and shoot tip explants. J. Biotechnol. Comput. Biol. Bionanotechnol. 2017, 98, 113–119. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, Y.; Fu, Q.; Li, S.; Sun, X.; Wang, Y.; Yu, M.; Qin, D.; Huo, J.; Zhu, C. Plant Regeneration via Somatic Embryogenesis and Indirect Organogenesis in Blue Honeysuckle (Lonicera caerulea L.). Horticulturae 2023, 9, 996. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Nhut, D.T.; Khiet, B.L.T.; Thi, N.N.; Thuy, D.T.T.; Duy, N.; Hai, N.T.; Huyen, P.X. High Frequency Shoot Formation of Yellow Passion Fruit (Passiflora edulis F. flavicarpa) via Thin Cell Layer (TCL) Technology. In Protocols for Micropropagation of Woody Trees and Fruits; Jain, S.M., Häggman, H., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 417–426. ISBN 978-1-4020-6352-7. [Google Scholar] [CrossRef]
- Vieira, L.M.; Rocha, D.I.; Taquetti, M.F. In vitro plant regeneration of Passiflora setacea D.C. (Passifloraceae): The influence of explant type, growth regulators, and incubation conditions. In Vitr. Cell. Dev. Biol. Plant 2014, 50, 738–745. [Google Scholar] [CrossRef]
- Monteiro, A.D.A.; Higashi, E.N.; Gonçalves, A.N.; Rodriguez, A.P.M. A Novel Approach for the Definition of the Inorganic Medium Components for Micropropagation of Yellow Passionfruit (Passiflora edulis Sims f. flavicarpa Deg.). In Vitr. Cell. Dev. Biol. Plant 2000, 36, 527–531. [Google Scholar] [CrossRef]
- Id, M.J.; Daneshvar, M.H. Machine learning-mediated Passiflora caerulea callogenesis optimization. PLoS ONE 2024, 19, e0292359. [Google Scholar] [CrossRef]
- Jafari, M.; Daneshvar, M.H. Prediction and optimization of indirect shoot regeneration of Passiflora caerulea using machine learning and optimization algorithms. BMC Biotechnol. 2023, 23, 27. [Google Scholar] [CrossRef]
- Antognoni, F.; Zheng, S.; Pagnucco, C.; Baraldi, R.; Poli, F.; Biondi, S. Induction of flavonoid production by UV-B radiation in Passiflora quadrangularis callus cultures. Fitoterapia 2007, 78, 345–352. [Google Scholar] [CrossRef]
- Rocha, D.I.; Vieira, L.M. Somatic embryogenesis of a wild passion fruit species Passiflora cincinnata Masters: Histocytological and histochemical evidences. Protoplasma 2012, 249, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Daneshvar, M.H.; Jafari, S.; Hesami, M. Machine learning-assisted in vitro rooting optimization in Passiflora caerulea. Forests 2022, 13, 2020. [Google Scholar] [CrossRef]
- Oros, P.B.; Catana, C.; Gocan, T.; Moldovan, G.; Székely-Varga, Z. Influence of culture substrates and biostimulators on Passiflora rooting. Horticulture 2020, 77, 12. [Google Scholar] [CrossRef]
- Busilacchi, C.; Sapio, D. Field culture of micropropagated Passiflora caerulea L. histological and chemical studies. Bol. Latinoam. Caribe Plant. Med. Aromaticas. 2008, 7, 257–263. [Google Scholar] [CrossRef]
- Garmidolova, A.; Halkoglu-Hristova, P.; Georgiev, V. Exploring the multifunctionality of Passiflora caerulea L.: From traditional remedies to modern applications. Appl. Sci. 2025, 15, 3251. [Google Scholar] [CrossRef]
- Nawaz, M.S.; Ali, A.; Shahani, A.; Khalid, M.N. Genomic Assisted Crop Breeding Approaches for Designing Future Crops to Combat Food Production Challenges. Bio-Sci. Rev. J. 2022, 2022, 1. [Google Scholar] [CrossRef]
- Anand, A.; Subramanian, M.; Kar, D. Breeding Techniques to Dispense Higher Genetic Gains. Front. Plant Sci 2023, 13, 1076094. [Google Scholar] [CrossRef]
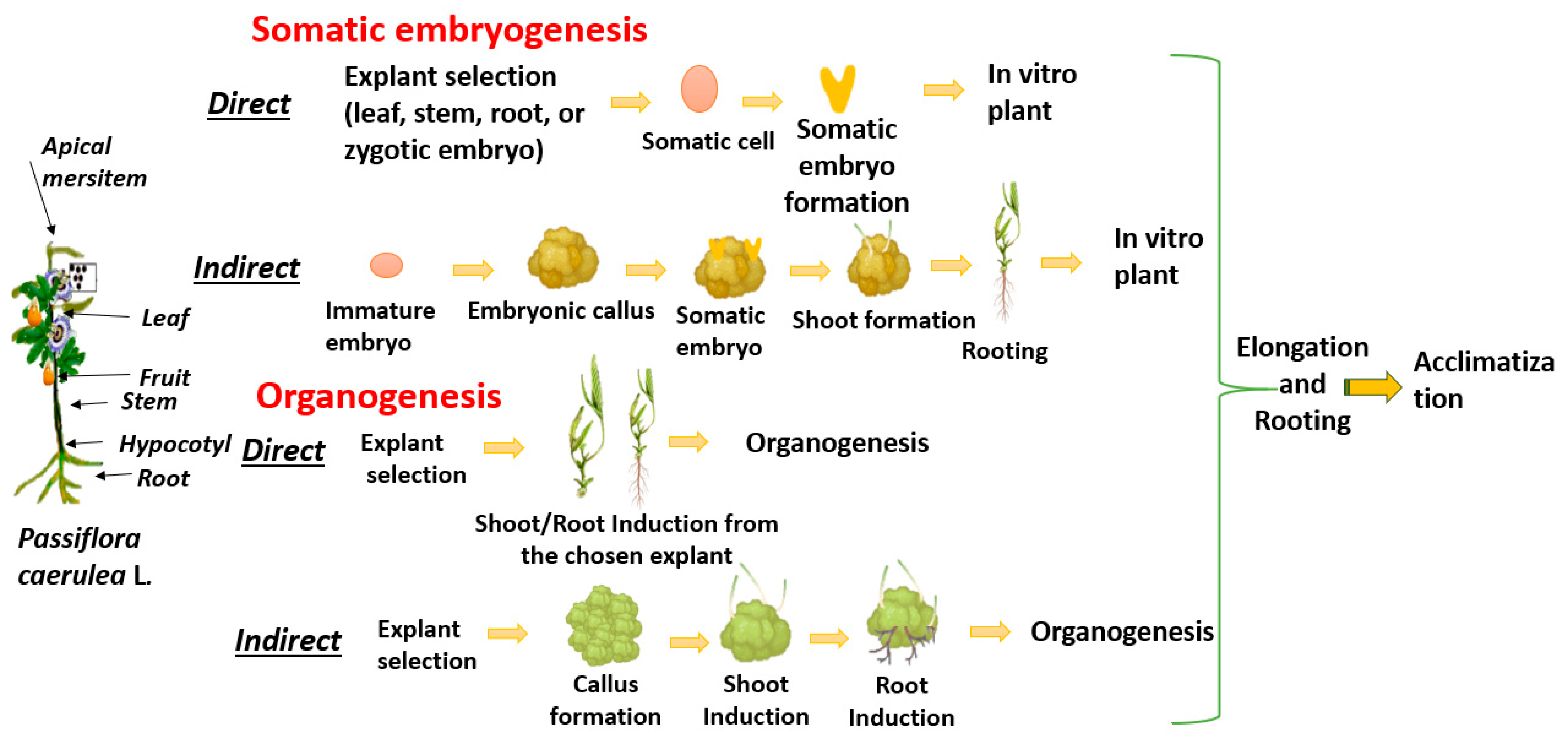
| Method | Formation | Direct/Indirect | Result |
|---|---|---|---|
| Direct Organogenesis | Shoots/roots directly from explant | No callus formation | New plant organs |
| Direct Somatic Embryogenesis | Embryos from somatic cells | No callus formation | Embryo-like structures |
| Callogenesis | Formation of callus | Explant to callus | Intermediate stage |
| Indirect Organogenesis | Shoots/roots from callus | Callus stage present | New plant organs |
| Indirect Somatic Embryogenesis | Embryos from callus | Callus stage present | Embryo-like structures |
| Explant Tissue | Culture Medium and Plant Growth Regulators (mg/mL) | Response | Reference |
|---|---|---|---|
| Leaf segments | MS + (0.5) NAA + (0.5) IAA + (0.5) 2,4-D + (0.5) KIN + (1) BAP | Shoot induction + callus formation | [31] |
| MS + (1) KIN + (2) BAP | Shoot induction | ||
| MS + (1) KIN + (2) BAP + (0.4) IAA | Shoot multiplication | ||
| MS + (2) 2,4-D + (0.8) IAA + (1) KIN + (2) BAP | Callus fully developed | ||
| MS + (1) NAA + (0.5) IBA + (0.5) IAA | Root induction | ||
| Leaf segments on abaxial or adaxial surface | MS + (0.5–4) BAP MS | Shoot induction | [15] |
| MS + (0.5–3) BAP + (0.5–1) NAA | Shoot + root induction | ||
| MS + (0.25–3) IBA + NAA (0.25–3) | Root induction | ||
| Shoot tips, cotyledonary node | MS + (0.5, 1, or 1.5) BAP + (0.05, 0.1 or 0.15) IBA | Shoot induction Root induction | [16] |
| MS + (0.25, 0.5 or 1) TDZ + (0.025, 0.05 or 0.1) IBA | |||
| MS + (1 or 2) KIN + (0.1 or 0.2) IBA | |||
| MS + (0.5, 1 or 2) IBA | Root induction Regenerated plants | ||
| MS + (0.5, 1 or 2) NAA | |||
| MS + (0.5, 1 or 2) IAA | |||
| Nodal segments | MS without PGR’s | Regenerated plants | [17] |
| ½ MS without PGRs MS |
| Scope of Research | Rooting/ Elongation/ Acclimatization | Phytochemical Composition | Findings on Regenerated Plantlets/Organogenesis |
|---|---|---|---|
| Direct organogenesis and histological examination and TLC analysis | Elongation: not indicated Rooting: on MS medium Acclimatization: 100% success | TLC of flavonoid fraction showed nine bands. Individual compounds were not identified. Chromatographic profiles of plantlets closely matched those of the donor plants | Shoot buds emerged directly from proliferative tissues without an intermediate callus phase. Approximately 70% of explants successfully regenerated shoots. |
| Direct and indirect organogenesis, with HPLC and HPTLC-based phytochemical profiling of callus | - | Vitexin, isovitexin, rutin, chlorogenic acid, and rosmarinic acid were identified in callus by HPLC | Transferring of internodal explants on MS medium supplemented with BA with GA3 and MS with 2,4-D resulted in 100% callus formation. The highest regeneration rate (106.4%) and bud index (3.1) were observed in stem-derived callus cultured on MS with 4.4 μM BA and 2.88 μM GA3. Callus culture was initiated on MS supplemented with 2.0 mg/L 2,4-D. |
| Organogenesis via both direct and indirect pathways | Elongation: not indicated | - | Culturing P. caerulea on MS medium with BA (4.4 μM) produced up to 16 shoots per explant, often in association with callus formation. |
| Direct organogenesis in liquid rotary culture | Rooting: 100% on MS Acclimatization: 100% | - | Using MS medium enriched with 2,4-D (18.1 μM) in a rotary liquid system, an average of three shoots per explant was achieved, with an overall regeneration efficiency of 90%. |
| Both organogenesis types and HPLC analysis of regenerated plantlets | Elongation: not indicated Rooting: 100% on MS Acclimatization: not indicated | Isovitexin (major compound), chlorogenic acid, rutin, hyperoside, vitexin, luteolin, apigenin, and rosmarinic acid, with no alkaloids detected by HPLC in morphogenic callus | Morphogenic callus formed on MS with BA, while non-morphogenic callus appeared on MS with 2,4-D. The best shoot induction was observed in leaf-derived callus on MS with 8.8 μM BA, and in petiole-derived callus on MS with 4.4 μM BA, yielding three shoots per explant. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halkoglu-Hristova, P.; Garmidolova, A.; Yaneva, T.; Georgiev, V. Current Progress on Passiflora caerulea L. In Vitro Culturing. Sci 2025, 7, 90. https://doi.org/10.3390/sci7030090
Halkoglu-Hristova P, Garmidolova A, Yaneva T, Georgiev V. Current Progress on Passiflora caerulea L. In Vitro Culturing. Sci. 2025; 7(3):90. https://doi.org/10.3390/sci7030090
Chicago/Turabian StyleHalkoglu-Hristova, Pervin, Alexandra Garmidolova, Teodora Yaneva, and Vasil Georgiev. 2025. "Current Progress on Passiflora caerulea L. In Vitro Culturing" Sci 7, no. 3: 90. https://doi.org/10.3390/sci7030090
APA StyleHalkoglu-Hristova, P., Garmidolova, A., Yaneva, T., & Georgiev, V. (2025). Current Progress on Passiflora caerulea L. In Vitro Culturing. Sci, 7(3), 90. https://doi.org/10.3390/sci7030090








