Core Symptoms of Eating Disorders and Heart Rate Variability: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
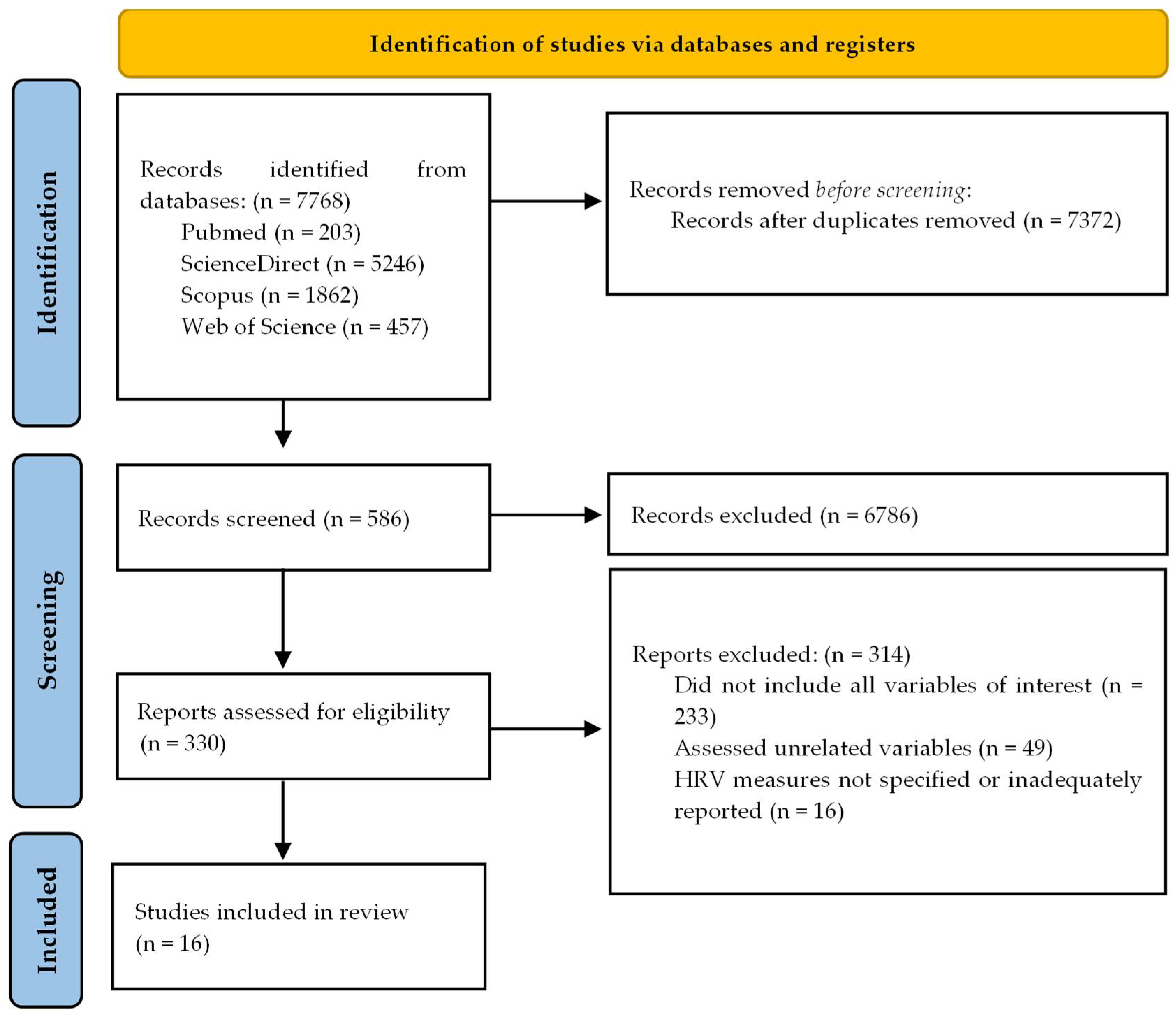
2.2. Study Selection
2.3. Data Extraction
2.4. Risk-of-Bias Assessment
3. Results
3.1. Symptomatology Measurements
3.2. HRV Measurement
3.3. Risk-of-Bias Analysis Results
3.4. Overall Findings
3.4.1. Anorexia Nervosa and Autonomic Regulation
3.4.2. Binge Eating and Emotional Dysregulation
3.4.3. Dietary Restraint and Self Control
3.4.4. Weight Status and Food Cues
3.4.5. Emotional Eating and Locus of Control
4. Discussion
4.1. Practical Implications
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AN | Anorexia Nervosa |
| AN-RT | Anorexia Nervosa Restrictive Type |
| ANS | Autonomic Nervous System |
| BE | Binge Eating |
| BED | Binge Eating Disorder |
| BL | Baseline |
| BMI | Body Mass Index |
| BN | Bulimia Nervosa |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders, 5th Edition |
| EDs | Eating Disorders |
| HC | Healthy Control |
| HF | High Frequency |
| HFn | normalized High Frequency |
| HPA | Hypothalamic–pituitary–adrenal |
| HR | Heart Rate |
| HRV | Heart Rate Variability |
| IBI | Interbeat interval |
| LF | Low Frequency |
| LFn | normalized Low Frequency |
| LF/HF | Ratio of Low Frequency to High Frequency |
| LOC | Loss of Control |
| OBE | Objective Binge Episode |
| pNN50 | Percentage of consecutive NN intervals that differ by more than 50 ms with respect to the total number of NN intervals |
| RMSSD | Root Mean Square of Successive Differences |
| RS | Restrained Scale |
| RSA | Respiratory Sinus Arrhythmia |
| SDANN | Standard Deviation of the Average NN intervals |
| SDNN | Standard Deviation of NN intervals |
| SD rate | Standard Deviation of Heart Rate |
| TSST | Trier Social Stress Test |
| WR | Weight Restriction |
References
- Insel, T.; Cuthbert, B.; Garvey, M.; Heinssen, R.; Pine, D.S.; Quinn, K.; Sanislow, C.; Wang, P. Research Domain Criteria (RDoC): Toward a New Classification Framework for Research on Mental Disorders. Am. J. Psychiatry 2010, 167, 748–751. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 0-89042-555-8. [Google Scholar]
- Treasure, J.; Duarte, T.A.; Schmidt, U. Eating Disorders. Lancet 2020, 395, 899–911. [Google Scholar] [CrossRef]
- Bhattacharya, A.; DeFilipp, L.; Timko, C.A. Feeding and Eating Disorders. In Handbook of Clinical Neurology; Elsevier B.V.: Amsterdam, The Netherlands, 2020; Volume 175, pp. 387–403. ISBN 9780826180520. [Google Scholar]
- Green, M.A.; Miles, L.; Sage, E.; Smith, J.; Carlson, G.; Hogan, K.; Bogucki, J.; Ferenzi, L.; Hartman, E.; Tao, Y.; et al. Cardiac Biomarkers of Disordered Eating as a Function of Diagnostic Subtypes. Eat. Behav. 2020, 39, 101425. [Google Scholar] [CrossRef] [PubMed]
- Herman, C.P.; Polivy, J. Experimental and Clinical Aspects of Restrained Eating; Stunkard, A.J., Ed.; Saunders: Philadelphia, PA, USA, 1980. [Google Scholar]
- Pereira, E.M.; da Silva, K.B.B.; Costa, P.R.d.F.; da Silva, L.E.M.; Nepomuceno, C.M.M.; da Silva, H.B.M.; Belfort, É.S.; Cunha, C.d.M.; de Santana, M.L.P. Restrained Eating Behaviour, Anorexia Nervosa and Food Consumption between Children and Adolescents: A Scoping Review. Br. J. Nutr. 2022, 128, 1565–1586. [Google Scholar] [CrossRef] [PubMed]
- Sternheim, L.; Danner, U.; Adan, R.; Van Elburg, A. Drive for Activity in Patients with Anorexia Nervosa. Int. J. Eat. Disord. 2015, 48, 42–45. [Google Scholar] [CrossRef]
- Vannucci, A.; Shomaker, L.B.; Field, S.E.; Sbrocco, T.; Stephens, M.; Kozlosky, M.; Reynolds, J.C.; Yanovski, J.A.; Tanofsky-Kraff, M. History of Weight Control Attempts among Adolescent Girls with Loss of Control Eating. Health Psychol. 2014, 33, 419–423. [Google Scholar] [CrossRef]
- Sonneville, K.R.; Horton, N.J.; Micali, N.; Crosby, R.D.; Swanson, S.A.; Solmi, F.; Field, A.E. Longitudinal Associations between Binge Eating and Overeating and Adverse Outcomes among Adolescents and Young Adults: Does Loss of Control Matter? JAMA Pediatr. 2013, 167, 149–155. [Google Scholar] [CrossRef]
- Tanofsky-Kraff, M.; Yanovski, S.Z.; Schvey, N.A.; Olsen, C.H.; Gustafson, J.; Yanovski, J.A. A Prospective Study of Loss of Control Eating for Body Weight Gain in Children at High Risk for Adult Obesity. Int. J. Eat. Disord. 2009, 42, 26–30. [Google Scholar] [CrossRef]
- Tanofsky-Kraff, M.; Shomaker, L.B.; Olsen, C.; Roza, C.A.; Wolkoff, L.E.; Columbo, K.M.; Raciti, G.; Zocca, J.M.; Wilfley, D.E.; Yanovski, S.Z.; et al. A Prospective Study of Pediatric Loss of Control Eating and Psychological Outcomes. J. Abnorm. Psychol. 2011, 120, 108–118. [Google Scholar] [CrossRef]
- Vögele, C.; Florin, I. Psychophysiological Responses to Food Exposure: An Experimental Study in Binge Eaters. Int. J. Eat. Disord. 1997, 21, 147–157. [Google Scholar] [CrossRef]
- Munakata, Y.; Herd, S.A.; Chatham, C.H.; Depue, B.E.; Banich, M.T.; O’Reilly, R.C. A Unified Framework for Inhibitory Control. Trends Cogn. Sci. 2011, 15, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Bodell, L.P.; Pearson, C.M.; Smith, K.E.; Cao, L.; Crosby, R.D.; Peterson, C.B.; Crow, S.J.; Berg, K.C. Longitudinal Associations between Emotion Regulation Skills, Negative Affect, and Eating Disorder Symptoms in a Clinical Sample of Individuals with Binge Eating. Eat. Behav. 2019, 32, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.; Sullivan, S.; Tchanturia, K.; Treasure, J. Emotional Functioning in Eating Disorders: Attentional Bias, Emotion Recognition and Emotion Regulation. Psychol. Med. 2010, 40, 1887–1897. [Google Scholar] [CrossRef]
- Robinson, A.; Safer, D.L.; Austin, J.L.; Etkin, A. Does Implicit Emotion Regulation in Binge Eating Disorder Matter? Eat. Behav. 2015, 18, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Kenardy, J.; Arnow, B.; Agras, W.S. The Aversiveness of Specific Emotional States Associated with Binge-Eating in Obese Subjects. Aust. New Zealand J. Psychiatry 1996, 30, 839–844. [Google Scholar] [CrossRef]
- Heatherton, T.E.; Baumeister, R.F. Binge Eating as Escape From Self-Awareness. Psychol. Bull. 1991, 110, 86–108. [Google Scholar] [CrossRef]
- Whiteside, U.; Chen, E.; Neighbors, C.; Hunter, D.; Lo, T.; Larimer, M. Difficulties Regulating Emotions: Do Binge Eaters Have Fewer Strategies to Modulate and Tolerate Negative Affect? Eat. Behav. 2007, 8, 162–169. [Google Scholar] [CrossRef]
- Gilboa-Schechtman, E.; Avnon, L.; Zubery, E.; Jeczmien, P. Emotional Processing in Eating Disorders: Specific Impairment or General Distress Related Deficiency? Depress. Anxiety 2006, 23, 331–339. [Google Scholar] [CrossRef]
- Svaldi, J.; Griepenstroh, J.; Tuschen-Caffier, B.; Ehring, T. Emotion Regulation Deficits in Eating Disorders: A Marker of Eating Pathology or General Psychopathology? Psychiatry Res. 2012, 197, 103–111. [Google Scholar] [CrossRef]
- Polivy, J.; Herman, C.P. Causes of Eating Disorders. Annu. Rev. Psychol. 2002, 53, 187–213. [Google Scholar] [CrossRef]
- Aldao, A.; Nolen-Hoeksema, S.; Schweizer, S. Emotion-Regulation Strategies across Psychopathology: A Meta-Analytic Review. Clin. Psychol. Rev. 2010, 30, 217–237. [Google Scholar] [CrossRef]
- Puhl, R.M.; Wall, M.M.; Chen, C.; Bryn Austin, S.; Eisenberg, M.E.; Neumark-Sztainer, D. Experiences of Weight Teasing in Adolescence and Weight-Related Outcomes in Adulthood: A 15-Year Longitudinal Study. Prev. Med. 2017, 100, 173–179. [Google Scholar] [CrossRef]
- Rojo, L.; Conesa, L.; Bermudez, O.; Livianos, L. Influence of Stress in the Onset of Eating Disorders: Data from a Two-Stage Epidemiologic Controlled Study. Psychosom. Med. 2006, 68, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.C.; Clément, P.F. Binge Eating: Measurement Problems and a Conceptual Model. In The Binge-Purge Syndrome; Springer: New York, NY, USA, 2004. [Google Scholar]
- Jenkins, Z.M.; Eikelis, N.; Phillipou, A.; Castle, D.J.; Wilding, H.E.; Lambert, E.A. Autonomic Nervous System Function in Anorexia Nervosa: A Systematic Review. Front. Neurosci. 2021, 15, 682208. [Google Scholar] [CrossRef]
- Sekaninova, N.; Olexova, L.B.; Visnovcova, Z.; Ondrejka, I.; Tonhajzerova, I. Role of Neuroendocrine, Immune, and Autonomic Nervous System in Anorexia Nervosa-Linked Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 7302. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, S.S.; Kemeny, M.E. Acute Stressors and Cortisol Responses: A Theoretical Integration and Synthesis of Laboratory Research. Psychol. Bull. 2004, 130, 355–391. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, C.; Pirke, K.-M.; Hellhammer, D.H. The ‘Trier Social Stress Test’—A Tool for Investigating Psychobiological Stress Responses in a Laboratory Setting. Neuropsychobiology 1993, 28, 76–81. [Google Scholar] [CrossRef]
- Koo-Loeb, J.H.; Costello, N.; Light, K.C.; Girdler, S.S. Women with Eating Disorder Tendencies Display Altered Cardiovascular, Neuroendocrine, and Psychosocial Profiles. Psychosom. Med. 2000, 62, 539–548. [Google Scholar] [CrossRef]
- Koo-Loeb, J.H.; Pedersen, C.; Girdler, S.S. Blunted Cardiovascular and Catecholamine Stress Reactivity in Women with Bulimia Nervosa. Psychiatry Res. 1998, 80, 13–27. [Google Scholar] [CrossRef]
- Culbert, K.M.; Racine, S.E.; Klump, K.L. Hormonal Factors and Disturbances in Eating Disorders. Curr. Psychiatry Rep. 2016, 18, 65. [Google Scholar] [CrossRef]
- Monteleone, A.M.; Monteleone, P.; Serino, I.; Amodio, R.; Monaco, F.; Maj, M. Underweight Subjects with Anorexia Nervosa Have an Enhanced Salivary Cortisol Response Not Seen in Weight Restored Subjects with Anorexia Nervosa. Psychoneuroendocrinology 2016, 70, 118–121. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Åhs, F.; Fredrikson, M.; Sollers, J.J.; Wager, T.D. A Meta-Analysis of Heart Rate Variability and Neuroimaging Studies: Implications for Heart Rate Variability as a Marker of Stress and Health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.H.; Zhou, L.L.; Wei, Y.Y.; Tang, X.C.; Gao, Y.Q.; Hu, Y.G.; Xu, L.H.; Chen, T.; Liu, H.C.; Li, C.B.; et al. Heart Rate Variability in Patients with Psychiatric Disorders from Adolescence to Adulthood. Gen. Hosp. Psychiatry 2023, 84, 179–187. [Google Scholar] [CrossRef]
- Christensen, K.A.; Feeling, N.R.; Rienecke, R.D. Meta-Analysis and Systematic Review of Resting-State High-Frequency Heart Rate Variability in Binge-Eating Disorder. J. Psychophysiol. 2023, 37, 50–63. [Google Scholar] [CrossRef]
- Svaldi, J.; Caffier, D.; Tuschen-Caffier, B. Emotion Suppression but Not Reappraisal Increases Desire to Binge in Women with Binge Eating Disorder. Psychother. Psychosom. 2010, 79, 188–190. [Google Scholar] [CrossRef]
- Peschel, S.K.V.; Feeling, N.R.; Vögele, C.; Kaess, M.; Thayer, J.F.; Koenig, J. A Systematic Review on Heart Rate Variability in Bulimia Nervosa. Neurosci. Biobehav. Rev. 2016, 63, 78–97. [Google Scholar] [CrossRef]
- Yiu, A.; Christensen, K.; Arlt, J.M.; Chen, E.Y. Distress Tolerance across Self-Report, Behavioral and Psychophysiological Domains in Women with Eating Disorders, and Healthy Controls. J. Behav. Ther. Exp. Psychiatry 2018, 61, 24–31. [Google Scholar] [CrossRef]
- Jarczok, M.N.; Weimer, K.; Braun, C.; Williams, D.W.P.; Thayer, J.F.; Gündel, H.O.; Balint, E.M. Heart Rate Variability in the Prediction of Mortality: A Systematic Review and Meta-Analysis of Healthy and Patient Populations. Neurosci. Biobehav. Rev. 2022, 143, 104907. [Google Scholar] [CrossRef]
- Hilbert, A.; Vögele, C.; Tuschen-Caffier, B.; Hartmann, A.S. Psychophysiological Responses to Idiosyncratic Stress in Bulimia Nervosa and Binge Eating Disorder. Physiol. Behav. 2011, 104, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Messerli-Bürgy, N.; Engesser, C.; Lemmenmeier, E.; Steptoe, A.; Laederach-Hofmann, K. Cardiovascular Stress Reactivity and Recovery in Bulimia Nervosa and Binge Eating Disorder. Int. J. Psychophysiol. 2010, 78, 163–168. [Google Scholar] [CrossRef]
- Friederich, H.C.; Schild, S.; Schellberg, D.; Quenter, A.; Bode, C.; Herzog, W.; Zipfel, S. Cardiac Parasympathetic Regulation in Obese Women with Binge Eating Disorder. Int. J. Obes. 2006, 30, 534–542. [Google Scholar] [CrossRef]
- Green, M.A.; Hallengren, J.J.; Davids, C.M.; Riopel, C.M.; Skaggs, A.K. An Association between Eating Disorder Behaviors and Autonomic Dysfunction in a Nonclinical Population. A Pilot Study. Appetite 2009, 53, 139–142. [Google Scholar] [CrossRef]
- Nederkoorn, C.; Smulders, F.T.Y.; Jansen, A. Cephalic Phase Responses, Craving and Food Intake in Normal Subjects. Appetite 2000, 35, 45–55. [Google Scholar] [CrossRef]
- Udo, T.; Weinberger, A.H.; Grilo, C.M.; Brownell, K.D.; Dileone, R.J.; Lampert, R.; Matlin, S.L.; Yanagisawa, K.; McKee, S.A. Heightened Vagal Activity during High-Calorie Food Presentation in Obese Compared with Non-Obese Individuals—Results of a Pilot Study. Obes. Res. Clin. Pract. 2014, 8, e258–e265. [Google Scholar] [CrossRef] [PubMed]
- Segerstrom, S.C.; Nes, L.S. Heart Rate Variability Reflects Self-Regulatory Strength, Effort, and Fatigue. Psychol. Sci. 2007, 18, 275. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; Clark, J.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, b2535. [Google Scholar] [CrossRef]
- Bottera, A.R.; Mancuso, C.J.; Kambanis, P.E.; De Young, K.P. Examining Heart Rate Variability as an Indicator of Top-down Inhibitory Control over Emotions and Eating Behaviors among Individuals with and without Binge Eating. Appetite 2021, 159, 105071. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Juarascio, A.; Manasse, S.; Minassian, A.; Risbrough, V.; Afari, N. Heart Rate Variability and Emotion Regulation among Individuals with Obesity and Loss of Control Eating. Physiol. Behav. 2019, 199, 73–78. [Google Scholar] [CrossRef]
- Het, S.; Vocks, S.; Wolf, J.M.; Herpertz, S.; Wolf, O.T. Treatment-Resistant Blunted HPA Activity, but Reversible Cardiovascular Stress Reactivity in Young Women with Eating Disorders. Front. Psychiatry 2020, 11, 726. [Google Scholar] [CrossRef] [PubMed]
- Juarascio, A.S.; Crochiere, R.J.; Tapera, T.M.; Palermo, M.; Zhang, F. Momentary Changes in Heart Rate Variability Can Detect Risk for Emotional Eating Episodes. Appetite 2020, 152, 104698. [Google Scholar] [CrossRef]
- Mehak, A.; Wilson, S.; Racine, S.E. A Psychophysiological Investigation of Mechanisms Underlying “Feeling Fat” in Women with and Without Binge Eating. Int. J. Eat. Disord. 2024, 57, 2393–2401. [Google Scholar] [CrossRef]
- Ortmann, J.; Schulz, A.; Lutz, A.P.C.; van Dyck, Z.; Vögele, C. Cardiac Interoceptive Processing and Emotional Experience in Binge Eating Behavior: Neural Evidence of Disengagement from Bodily Sensations. Appetite 2025, 208, 107948. [Google Scholar] [CrossRef]
- Ranzenhofer, L.M.; Engel, S.G.; Crosby, R.D.; Haigney, M.; Anderson, M.; McCaffery, J.M.; Tanofsky-Kraff, M. Real-Time Assessment of Heart Rate Variability and Loss of Control Eating in Adolescent Girls: A Pilot Study. Int. J. Eat. Disord. 2016, 49, 199–203. [Google Scholar] [CrossRef]
- Ranzenhofer, L.M.; Solhjoo, S.; Crosby, R.D.; Kim, B.H.; Korn, R.; Koorathota, S.; Lloyd, E.C.; Walsh, B.T.; Haigney, M.C. Autonomic Indices and Loss-of-Control Eating in Adolescents: An Ecological Momentary Assessment Study. Psychol. Med. 2023, 53, 4742–4750. [Google Scholar] [CrossRef]
- Rommel, D.; Nandrino, J.L.; De Jonckheere, J.; Swierczek, M.; Dodin, V.; Logier, R. Maintenance of Parasympathetic Inhibition Following Emotional Induction in Patients with Restrictive Type Anorexia Nervosa. Psychiatry Res. 2015, 225, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.N.; Faulkner, L.M.; Shank, L.M.; Schvey, N.A.; Loch, L.K.; Haynes, H.E.; Bloomer, B.F.; Moursi, N.A.; Fatima, S.; Te-Vazquez, J.A.; et al. Heart Rate Variability and Laboratory-Based Loss-of-Control Eating in Children and Adolescents. Nutrients 2022, 14, 4027. [Google Scholar] [CrossRef] [PubMed]
- Paysal, J.; Thireau, J.; Terral, D.; Rochette, E.; Obert, P.; Merlin, E.; Nottin, S. Cardiac Remodeling and Its Determinants in Anorexia Nervosa Adolescents: Impact of Weight Recovery. Children 2022, 9, 458. [Google Scholar] [CrossRef]
- Schmalbach, I.; Herhaus, B.; Pässler, S.; Runst, S.; Berth, H.; Wolff, S.; Schmalbach, B.; Petrowski, K. Autonomic Nervous System Response to Psychosocial Stress in Anorexia Nervosa: A Cross-Sectional and Controlled Study. Front. Psychol. 2021, 12, 649848. [Google Scholar] [CrossRef]
- Billeci, L.; Tonacci, A.; Brunori, E.; Raso, R.; Calderoni, S.; Maestro, S.; Morales, M.A. Autonomic Nervous System Response during Light Physical Activity in Adolescents with Anorexia Nervosa Measured by Wearable Devices. Sensors 2019, 19, 2820. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C.; Huang, W.L.; Liu, C.Y.; Tseng, M.M.C.; Yang, C.C.H.; Kuo, T.B.J. Heart Rate Variability Reactivity to Food Image Stimuli Is Associated with Body Mass Index. Appl. Psychophysiol. Biofeedback 2021, 46, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Geisler, F.C.M.; Kleinfeldt, A.; Kubiak, T. Restrained Eating Predicts Effortful Self-Control as Indicated by Heart Rate Variability during Food Exposure. Appetite 2016, 96, 502–508. [Google Scholar] [CrossRef]
- Peschel, S.K.; Feeling, N.R.; Vögele, C.; Kaess, M. A Meta-Analysis on Resting State High-Frequency Heart Rate Variability in Bulimia Nervosa. Eur. Eat. Disord. Rev. J. Eat. Disord. Assoc. 2016, 24, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Watford, T.; Braden, A.; O’Brien, W. Resting State Heart Rate Variability in Clinical and Subthreshold Disordered Eating: A Meta-Analysis. Int. J. Eat. Disord. 2020, 53, 1021–1033. [Google Scholar] [CrossRef]
- Mather, M. Is There a Maximum Desirable Heart Rate Variability? Neurosci. Biobehav. Rev. 2021, 128, 87–89. [Google Scholar] [CrossRef]
- Meule, A.; Freund, R.; Skirde, A.K.; Vögele, C.; Kübler, A. Heart Rate Variability Biofeedback Reduces Food Cravings in High Food Cravers. Appl. Psychophysiol. Biofeedback 2012, 37, 241–251. [Google Scholar] [CrossRef]
- Bomba, M.; Corbetta, F.; Gambera, A.; Nicosia, F.; Bonini, L.; Neri, F.; Tremolizzo, L.; Nacinovich, R. Heart Rate Variability in Adolescents with Functional Hypothalamic Amenorrhea and Anorexia Nervosa. Psychiatry Res. 2014, 215, 406–409. [Google Scholar] [CrossRef]
- Di Cola, G.; Jacoangeli, F.; Jacoangeli, F.; Lombardo, M.; Iellamo, F. Cardiovascular Disorders in Anorexia Nervosa and Potential Therapeutic Targets. Intern. Emerg. Med. 2014, 9, 717–721. [Google Scholar] [CrossRef]
- Malcolm, A.; Phillipou, A. Current Directions in Biomarkers and Endophenotypes for Anorexia Nervosa: A Scoping Review. J. Psychiatr. Res. 2021, 137, 303–310. [Google Scholar] [CrossRef]
| Study | Sample Characterization | Diagnosis Criteria | ED Symptoms | Main HRV Results | Effect Size | ||||
|---|---|---|---|---|---|---|---|---|---|
| Type | N | % Female | Age (M ± SD) | BMI (M kg/m2 ± SD) | |||||
| Billeci et al. [65] | Adolescent girls | 40 | AN-RT (n = 23) | DSM-IV and DSM-5 criteria: AN-RT | Physical activity | AN-RT: RMSSD/SDNN stable; LFn ↑ slightly during task, ↓ at recovery; HFn ↓ during task, ↑ at recovery; LF/HF ↑ during task HC: RMSSD/SDNN ↑ during task, ↓ at recovery; LFn ↓; HFn ↑; LF/HF stable | Moderate to large | ||
| 100% | 15.2 ± 1.9 | 15.7 ± 1.6 | |||||||
| HC (n = 17) | |||||||||
| 100% | 15.7 ± 2.1 | 21.7 ± 2.8 | |||||||
| Bottera et al. [53] | Males and females with or without OBE | 86 | OBE+ (n = 34) | DSM-5 criteria: Modified EDDS to assess past-month and lifetime OBE and EDE-Q | Emotional regulation and BE | ↑ RMSSD linked to greater guilt after OBE ↓ parasympathetic activity during emotion regulation while eating | Small | ||
| 73.50% | 22.26 ± 5.89 | 24.01 ± 5.36 | |||||||
| OBE- (n = 52) | |||||||||
| 74.50% | 20.48 ± 2.92 | 23.27 ± 3.95 | |||||||
| Chang et al. [66] | General non-clinical sample individuals | 99 | 61.61% | 28.77 ± 6.04 | 23.06 ± 4.59 | BITE and EDEQ | Reactivity to food | No SDNN/HF response to food cues; LF/HF reactive to high-caloric vs. neutral stimuli and inversely correlated with symptom severity | Not reported |
| Geisler et al. [67] | Undergraduate students | 111 | 77% | 23.06 ± 4.50 | Ego depletion (n = 55) 23.67 ± 3.88 | German Restraint Scale | Restrained eating, self-control in response to food cues | ↑ RMSSD linked to eating restriction; restraint score predicted HRV post-ego depletion only | Moderate |
| Non-ego depletion (n = 56) 23.19 ± 2.52 | |||||||||
| Godfrey et al. [54] | Adults, BMI >30 kg/m2, ≥4 LOC/overeating episodes in 4 weeks, PSS ≥2 | 28 | 78.6% | 41.1 ± 16.5 | BED (n = 15) 37.5 ± 5.1 | DSM-5: EDE-Q (LOC and overeating episodes, past 4 weeks), DEBQ and BES for self-reported ED symptoms | Emotional regulation, stress response, and BE severity | Post-stress: RMSSD ↓ trend; SDNN stable; LFn/LF-HF ↑ during stress; HFn ↓ (math vs. rest); no link between HRV and overeating/LOC | Moderate |
| No BED (n = 13) 39.4 ± 5.6 | |||||||||
| Het et al. [55] | AN/BN female patients (pre-/post-treatment) | 50 | ED (n = 13) | BMI pre-treatment: 17.2 ± 0.80 | DSM-5: EDE-Q | Emotional regulation and stress response | ED > HC in negative affect (pre/post); post-TSST, HF blunted in ED | Not reported | |
| 100% | 21 ± 1.3 | ||||||||
| HC (n = 22) | |||||||||
| 100% | 23 ± 1.1 | 21.9 ± 0.60 | |||||||
| Jenkins et al. [28] | Female participants with AN and AN-WR | 37 | AN (n = 10) | DSM-5: Current AN diagnosis; AN-WR = past AN diagnosis, BMI >18.5 for ≥12 months | Weight regain | AN: ↓ HR and ↓ HRV (SDNN, SD rate); LF/HF ↔ across groups; no HRV differences in AN-WR vs. HC | Moderate to large | ||
| 100% | 31.64 ± 11.25 | 15.57 ± 1.69 | |||||||
| AN-WR (n = 17) | |||||||||
| 100% | 25.07 ± 4.87 | 21.64 ± 2.14 | |||||||
| HC (n = 10) | |||||||||
| 100% | 27.44 ± 6.07 | 21.40 ± 1.84 | |||||||
| Juarascio et al. [56] | Participants with clinically significant emotional eating (≥4 episodes/28 days) | 21 | 85.7% | 34.05 ± 14.41 | Initial BMI: 27.79 ± 6.92 | Unspecified: EOQ | Emotional eating | ↓ SDANN/RMSSD and ↑ SDNN linked to ↑ emotional eating; HF/LF showed greater fluctuation pre-episode vs. control | Moderate to large |
| Mehak et al. [57] | Women with ≥1 OBE in past 3 months | 82 | BE (n = 41) | Unspecified: Adapted SCID-5-RV and ED examination; ≥1 OBE in past 3 months | Emotional regulation and somatic experience of being overweight | No effects on RMSSD, RSA, or HF | Not reported | ||
| 100% | 24.73 ± 6.95 | 26.24 ± 6.73 | |||||||
| HC (n = 41) | |||||||||
| 100% | 24.83 ± 7.65 | 23.35 ± 3.95 | |||||||
| Ortmann et al. [58] | Women with recurrent BE vs. without BE or ED history | 56 | BE (n = 28) | DSM-5: EDI-2, EDIP-Q, and DEBQ | Emotional regulation in BE | BE: ↓ HR, ↑ HFn; ↑ negative affect vs. HC | Moderate to large | ||
| 100% | 35.2 ± 16.5 | 29.2 ± 8.0 | |||||||
| HC (n = 28) | |||||||||
| 100% | 32.9 ± 14.1 | 24.2 ± 4.2 | |||||||
| Parker et al. [62] | Non-clinical youth, BMI >5th percentile, good health | 209 | LOC-E (n = 19) | Unspecified: EDE interview (child version for <13 y) | LOC | RMSSD/pNN50/HF ↔ LOC severity ↑ HRV → ↑ perceived LOC (recent group only) | Not reported | ||
| 73.7% | 13.7 ± 2.7 | 0.88 ± 1.02 (z-score) | |||||||
| No LOC-E (n = 19) | |||||||||
| 52.6% | 12.5 ± 2.7 | 0.49 ± 1.02 (z-score) | |||||||
| Paysal et al. [63] | AN patients without and with WR | 46 | AN without WR | DSM-5: AN | Weight regain | AN without WR: ↑ parasympathetic activity (↑ RMSSD, pNN50, HF), stable LF; ↑ SDNN vs. AN-WR and HC | Moderate to large | ||
| (n = 26) | |||||||||
| 100% | 13.9 ± 1.6 | 13.9 ± 1.6 | |||||||
| AN-WR | |||||||||
| (n = 10) | |||||||||
| 100% | 15.7 ± 1.9 | 16.9 ± 2.2 | |||||||
| HC (n = 33): | |||||||||
| 100% | 14.1 ± 2.0 | 19.2 ± 2.3 | |||||||
| Ranzenhofer et al. [59] | Females, BMI ≥85th percentile, ≥2 LOC episodes/month | 17 | 100% | 14.77 ± 1.55 | 2.17 ± 0.48 (z-score) | Unspecified: EDE | Emotional regulation and LOC | ↑ HR and ↓ RMSSD predicted ↑ LOC; HRV lower before high- vs. low-LOC episodes | Small to moderate |
| Ranzenhofer et al. [60] | Adolescents, BMI >70th percentile, ≥2 LOC episodes/month | 24 | 66.7% | 15.6 ± 1.7 | 30.4 ± 5.6 | Unspecified: EDE | Emotional regulation and LOC | ↑ HR (30 min pre-eating) → ↑ LOC ratings; RMSSD/pNN50 ↓ with ↑ LOC | Small |
| Rommel et al. [61] | AN-RT patients (BMI ≈15) | 40 | AN-RT (n = 16): | DSM-IV: AN-RT | Emotional regulation | AN-RT: ↓ HF, ↑ WT-HRV during emotional induction | Large | ||
| 100% | 19.5 | 15.2 | |||||||
| HC (n = 24) | |||||||||
| 100% | 19 | 21 | |||||||
| Schmalbach et al. [64] | Patients with AN | 38 | AN (n = 19) | DSM-IV: AN | Weight regain and stress response | AN: ↑ stress appraisal; ↓ overall HRV reactivity (↓ SDNN, LF; ↑ HF); SDNN ↑ at rest (low-stress subgroup); blunted HR/LF response | Moderate to large | ||
| 89.5% | 26.05 ± 5.49 | 18.70 ± 3.30 | |||||||
| HC (n = 19) | |||||||||
| 89.5% | 24.21 ± 5.54 | 24.23 ± 3.04 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávila, A.; SanMiguel, N.; Serrano, M.A. Core Symptoms of Eating Disorders and Heart Rate Variability: A Systematic Review. Sci 2025, 7, 89. https://doi.org/10.3390/sci7030089
Ávila A, SanMiguel N, Serrano MA. Core Symptoms of Eating Disorders and Heart Rate Variability: A Systematic Review. Sci. 2025; 7(3):89. https://doi.org/10.3390/sci7030089
Chicago/Turabian StyleÁvila, Aitana, Noemí SanMiguel, and Miguel A. Serrano. 2025. "Core Symptoms of Eating Disorders and Heart Rate Variability: A Systematic Review" Sci 7, no. 3: 89. https://doi.org/10.3390/sci7030089
APA StyleÁvila, A., SanMiguel, N., & Serrano, M. A. (2025). Core Symptoms of Eating Disorders and Heart Rate Variability: A Systematic Review. Sci, 7(3), 89. https://doi.org/10.3390/sci7030089






