Targeting Cancer with Paris’ Arrow: An Updated Perspective on Targeting Wnt Receptor Frizzled 7
Abstract
1. Introduction
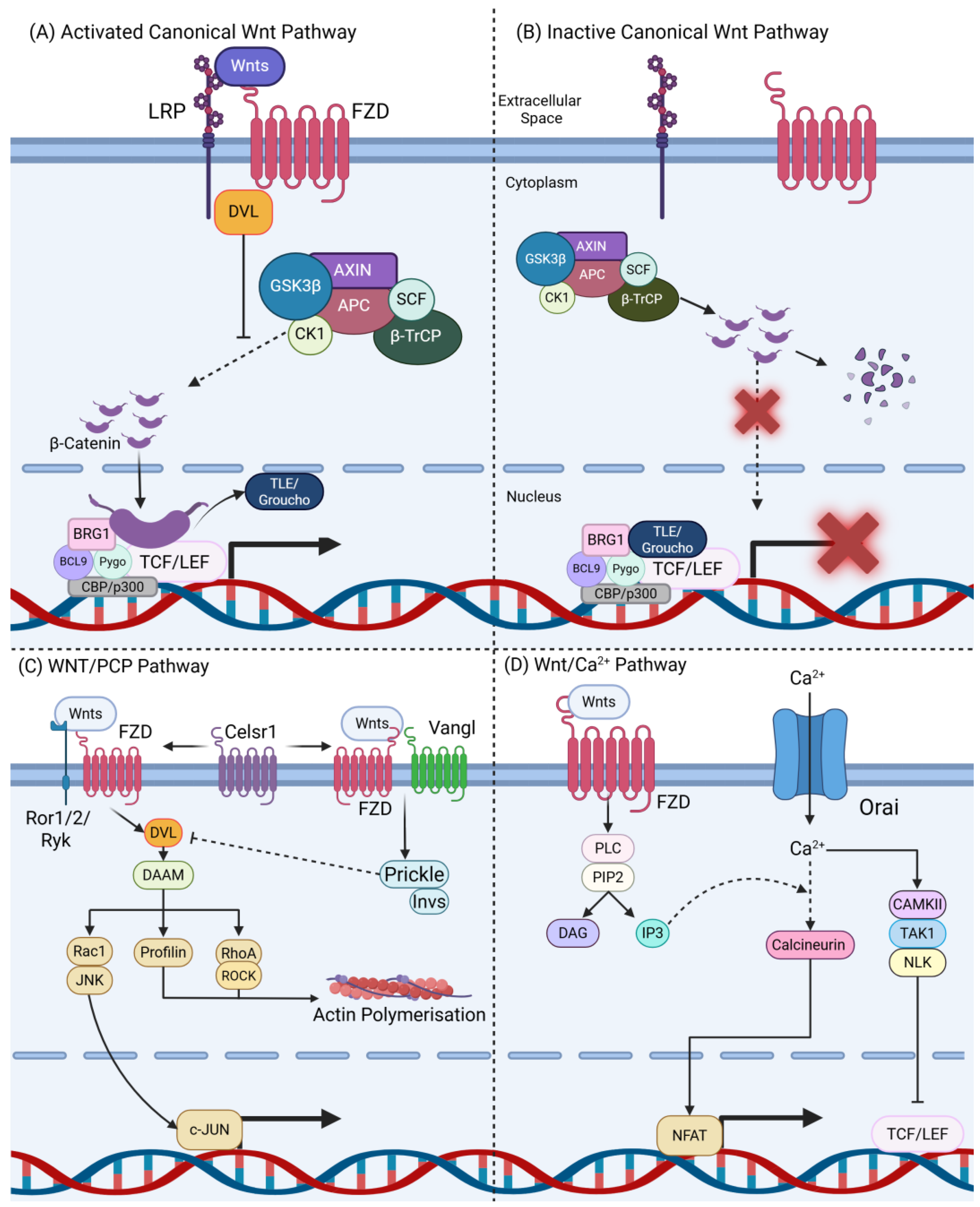
1.1. Overview of the Wnt Signalling Pathway
1.2. Frizzled Receptor 7 (FZD7) in the Wnt Pathway
1.3. Wnt Signalling Dysregulation in Cancer
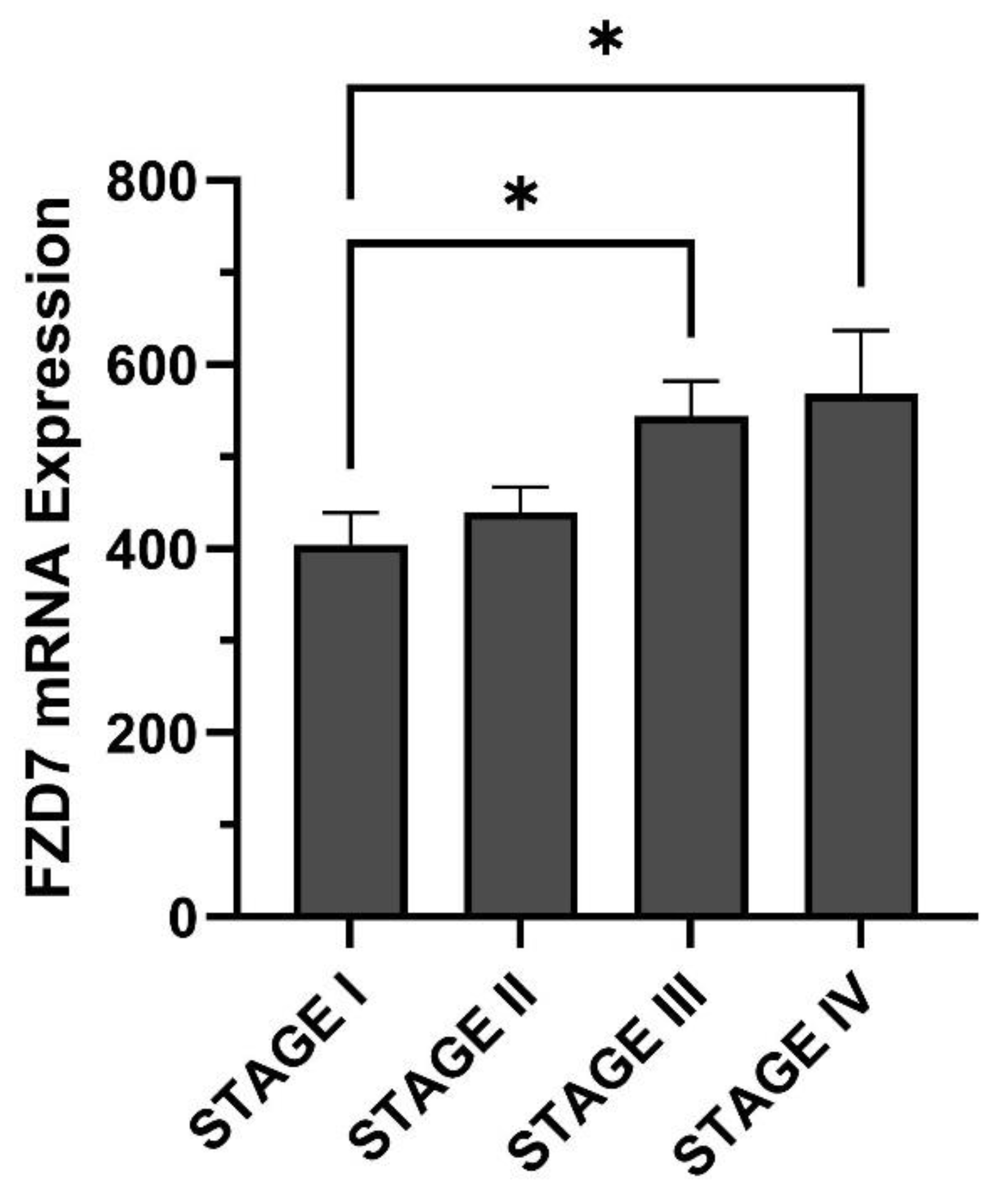
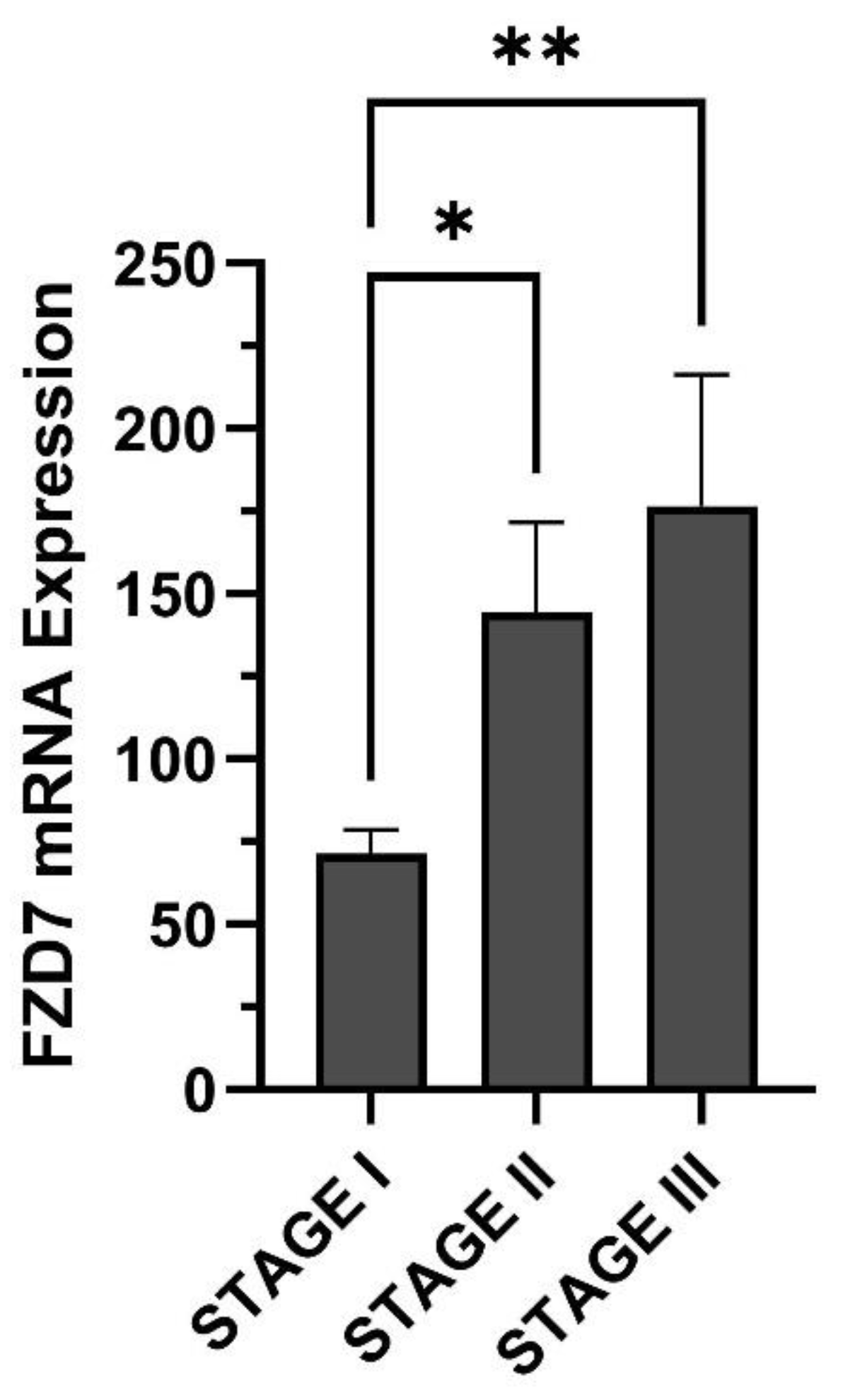
2. Gastrointestinal Cancers
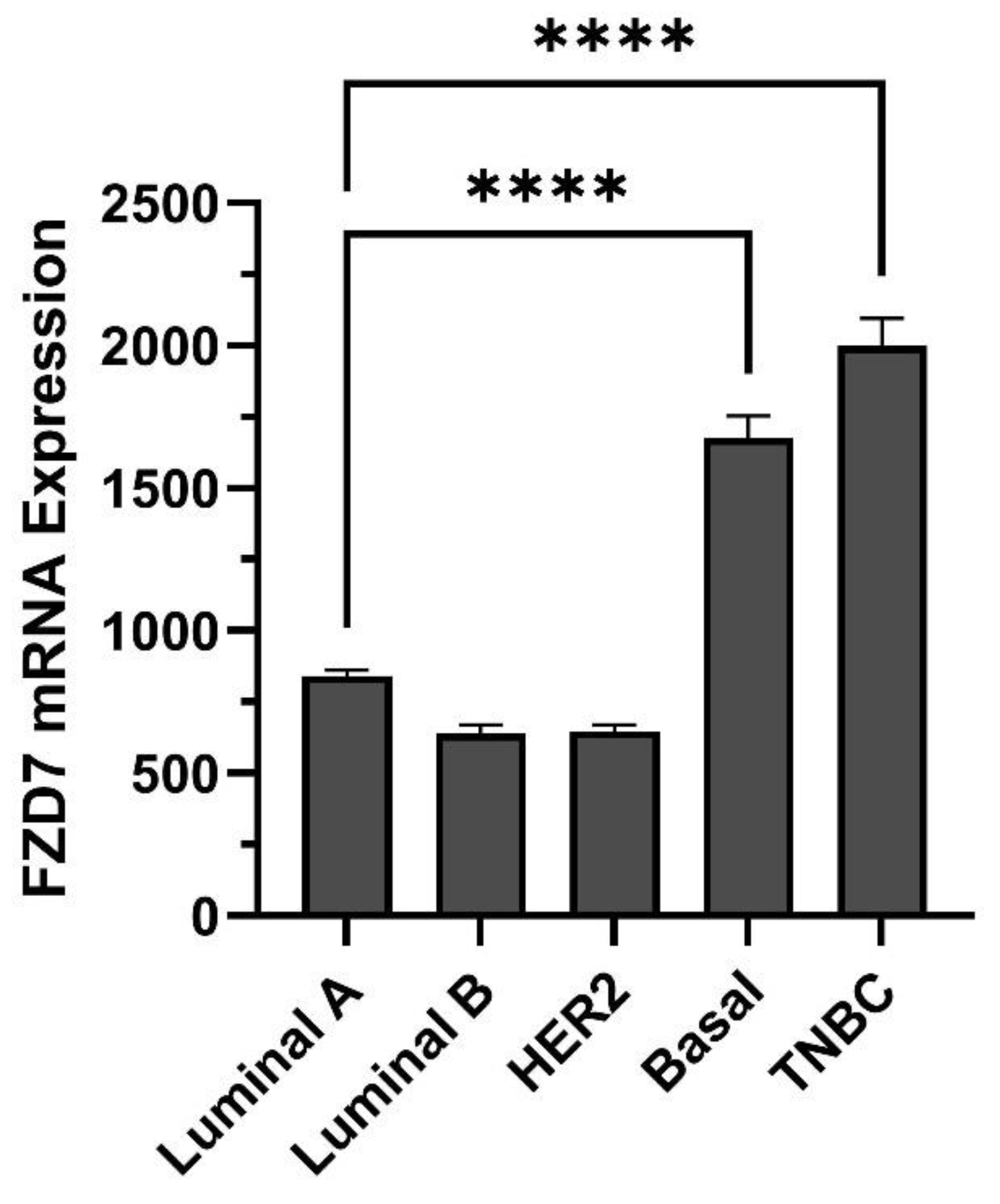
3. Breast Cancer
4. Hepatocellular Carcinoma
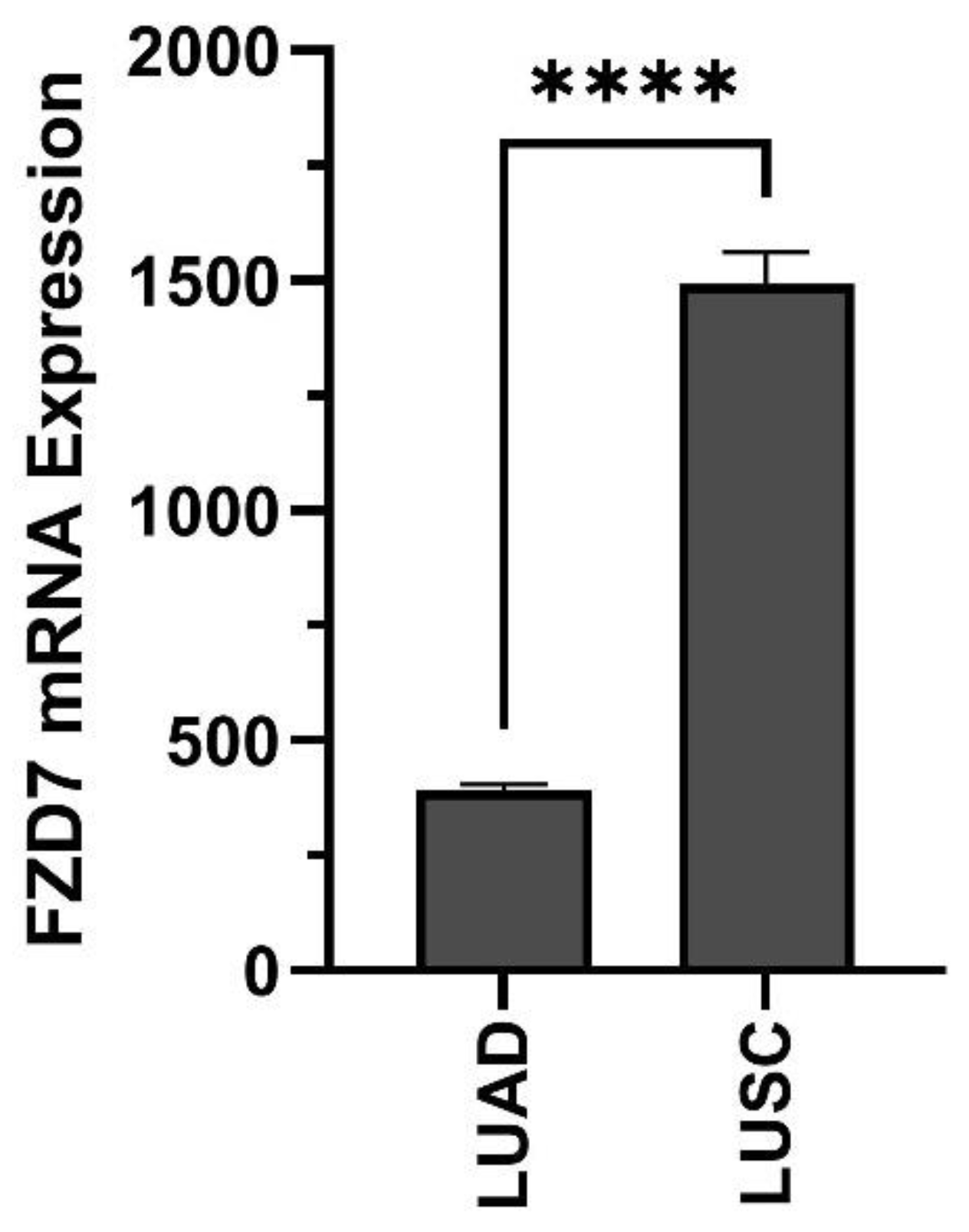
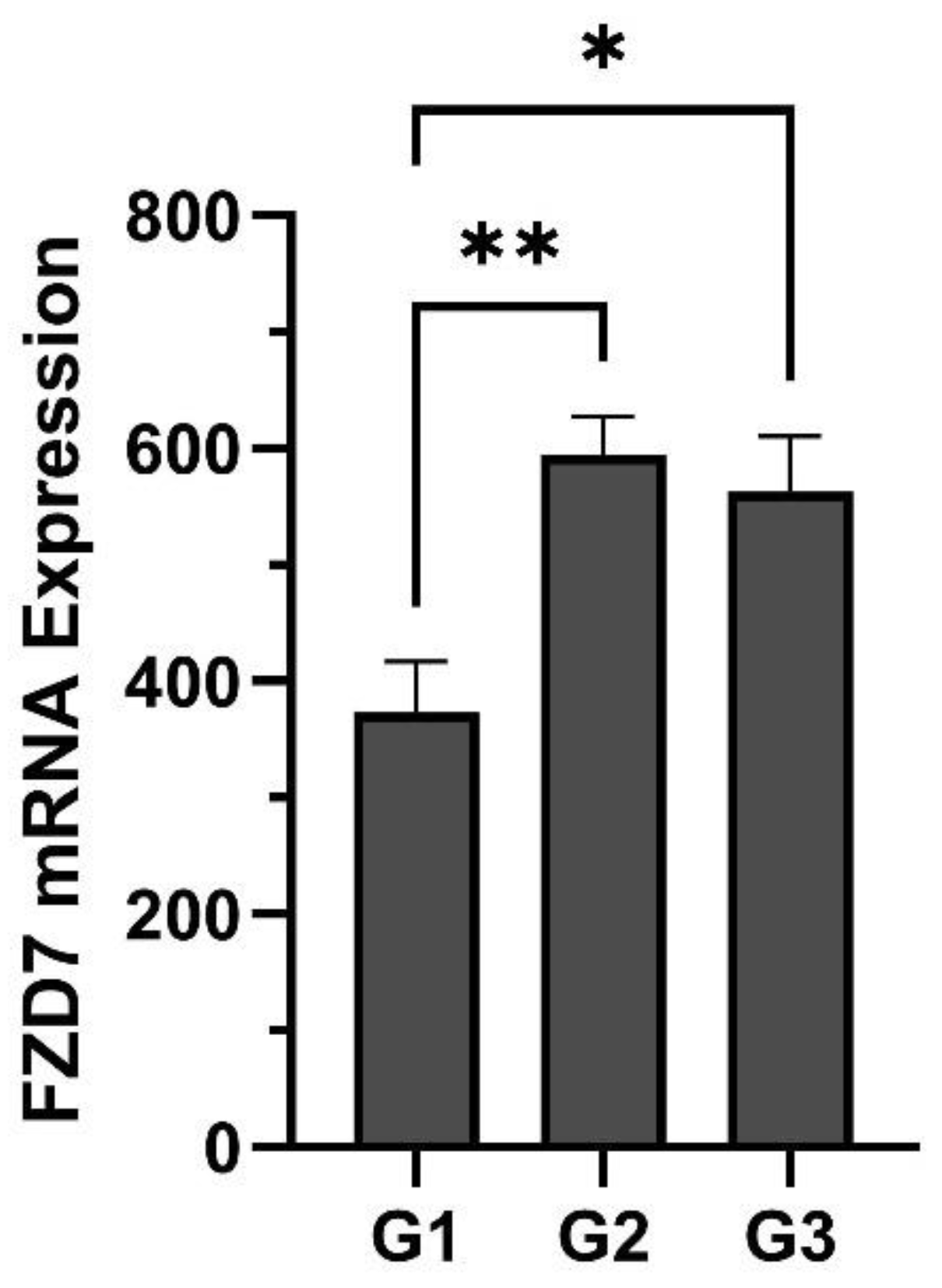
5. Lung Cancer
6. Ovarian Cancer
7. Leukaemia
8. Prostate Cancer
9. Pancreatic Cancer
10. Melanoma
11. Cholangiocarcinoma
12. Renal Cancer
13. Discussion
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Phesse, T.; Flanagan, D.; Vincan, E. Frizzled7: A Promising Achilles’ Heel for Targeting the Wnt Receptor Complex to Treat Cancer. Cancers 2016, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Holzem, M.; Boutros, M.; Holstein, T.W. The origin and evolution of Wnt signalling. Nat. Rev. Genet. 2024, 25, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Yu, J.; Virshup, D.M.; Madan, B. Wnts and the hallmarks of cancer. Cancer Metastasis Rev. 2020, 39, 625–645. [Google Scholar] [CrossRef]
- Qin, K.; Yu, M.; Fan, J.; Wang, H.; Zhao, P.; Zhao, G.; Zeng, W.; Chen, C.; Wang, Y.; Wang, A.; et al. Canonical and noncanonical Wnt signaling: Multilayered mediators, signaling mechanisms and major signaling crosstalk. Genes Dis. 2024, 11, 103–134. [Google Scholar] [CrossRef]
- Stanganello, E.; Scholpp, S. Role of cytonemes in Wnt transport. J. Cell Sci. 2016, 129, 665–672. [Google Scholar] [CrossRef]
- Routledge, D.; Rogers, S.; Ono, Y.; Brunt, L.; Meniel, V.; Tornillo, G.; Ashktorab, H.; Phesse, T.J.; Scholpp, S. The scaffolding protein flot2 promotes cytoneme-based transport of wnt3 in gastric cancer. eLife 2022, 11, e77376. [Google Scholar] [CrossRef]
- Maurice, M.M.; Angers, S. Mechanistic insights into Wnt–β-catenin pathway activation and signal transduction. Nat. Rev. Mol. Cell Biol. 2025, 26, 371–388. [Google Scholar] [CrossRef]
- Nile, A.H.; Mukund, S.; Stanger, K.; Wang, W.; Hannoush, R.N. Unsaturated fatty acyl recognition by Frizzled receptors mediates dimerization upon Wnt ligand binding. Proc. Natl. Acad. Sci. USA 2017, 114, 4147–4152. [Google Scholar] [CrossRef]
- Dijksterhuis, J.P.; Baljinnyam, B.; Stanger, K.; Sercan, H.O.; Ji, Y.; Andres, O.; Rubin, J.S.; Hannoush, R.N.; Schulte, G. Systematic mapping of WNT-FZD protein interactions reveals functional selectivity by distinct WNT-FZD pairs. J. Biol. Chem. 2015, 290, 6789–6798. [Google Scholar] [CrossRef]
- Bous, J.; Kinsolving, J.; Grätz, L.; Scharf, M.M.; Voss, J.H.; Selcuk, B.; Adebali, O.; Schulte, G. Structural basis of frizzled 7 activation and allosteric regulation. Nat. Commun. 2024, 15, 7422. [Google Scholar] [CrossRef] [PubMed]
- Čapek, D.; Smutny, M.; Tichy, A.-M.; Morri, M.; Janovjak, H.; Heisenberg, C.-P. Light-activated Frizzled7 reveals a permissive role of non-canonical wnt signaling in mesendoderm cell migration. eLife 2019, 8, e42093. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Ha, A.; Santos, A.J.M.; Lo, Y.-H.; van Unen, V.; Miao, Y.; Tomaske, M.; Guzman, V.K.; Alwahabi, S.; Yuan, J.J.; et al. FZD5 controls intestinal crypt homeostasis and colonic Wnt surrogate agonist response. Dev. Cell 2024, 60, 342–351.e5. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, D.J.; Phesse, T.J.; Barker, N.; Schwab, R.H.M.; Amin, N.; Malaterre, J.; Stange, D.E.; Nowell, C.J.; Currie, S.A.; Saw, J.T.S.; et al. Frizzled7 Functions as a Wnt Receptor in Intestinal Epithelial Lgr5+ Stem Cells. Stem Cell Rep. 2015, 4, 759–767. [Google Scholar] [CrossRef]
- Flanagan, D.J.; Barker, N.; Nowell, C.; Clevers, H.; Ernst, M.; Phesse, T.J.; Vincan, E. Loss of the Wnt receptor frizzled 7 in the mouse gastric epithelium is deleterious and triggers rapid repopulation in vivo. DMM Dis. Models Mech. 2017, 10, 971–980. [Google Scholar] [CrossRef]
- Flanagan, D.; Barker, N.; Ernst, M.; Vincan, E.; Phesse, T. The function of lgr5+ cells in the gastric antrum does not require fzd7 or myc in vivo. Biomedicines 2019, 7, 50. [Google Scholar] [CrossRef]
- Flanagan, D.J.; Barker, N.; Di Costanzo, N.S.; Mason, E.A.; Gurney, A.; Meniel, V.S.; Koushyar, S.; Austin, C.R.; Ernst, M.; Pearson, H.B.; et al. Frizzled-7 is required for Wnt signaling in gastric tumors with and without APC mutations. Cancer Res. 2019, 79, 970–981. [Google Scholar] [CrossRef]
- Boulter, L.; Guest, R.V.; Kendall, T.J.; Wilson, D.H.; Wojtacha, D.; Robson, A.J.; Ridgway, R.A.; Samuel, K.; Van Rooijen, N.; Barry, S.T.; et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J. Clin. Investig. 2015, 125, 1269–1285. [Google Scholar] [CrossRef]
- Ye, C.; Xu, M.; Lin, M.; Zhang, Y.; Zheng, X.; Sun, Y.; Deng, Y.; Pan, J.; Xu, Z.; Lu, X.; et al. Overexpression of FZD7 is associated with poor survival in patients with colon cancer. Pathol. Res. Pract. 2019, 215, 152478. [Google Scholar] [CrossRef]
- Koushyar, S.; Meniel, V.S.; Phesse, T.J.; Pearson, H.B. Exploring the Wnt Pathway as a Therapeutic Target for Prostate Cancer. Biomolecules 2022, 12, 309. [Google Scholar] [CrossRef]
- Zeng, C.M.; Chen, Z.; Fu, L. Frizzled Receptors as Potential Therapeutic Targets in Human Cancers. Int. J. Mol. Sci. 2018, 19, 1543. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Nan, Y.; Wang, X.; Zhang, G.; Zong, S.; Kong, X. MicroRNA-613 inhibits proliferation and invasion of renal cell carcinoma cells through targeting FZD7. Mol. Med. Rep. 2017, 16, 4279–4286. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Chen, C.; Xu, W.; Xu, H.; Zheng, J.; Gu, Y. Downregulation of Frizzled-7 induces the apoptosis of hepatocellular carcinoma cells through inhibition of NF-κB. Oncol. Lett. 2018, 15, 7693–7701. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Y.; Zhao, C. Fzd7/Wnt7b signaling contributes to stemness and chemoresistance in pancreatic cancer. Cancer Med. 2021, 10, 3332–3345. [Google Scholar] [CrossRef]
- Schiffgens, S.; Wilkens, L.; Brandes, A.A.; Meier, T.; Franceschi, E.; Ermani, M.; Hartmann, C.; Erol Sandalcioglu, I.; Dumitru, C.A. Sex-specific clinicopathological significance of novel (Frizzled-7) and established (MGMT, IDH1) biomarkers in glioblastoma. Oncotarget 2016, 7, 55169. [Google Scholar] [CrossRef]
- Cao, T.-T.; Xiang, D.; Liu, B.-L.; Huang, T.-X.; Tan, B.-B.; Zeng, C.-M.; Wang, Z.-Y.; Ming, X.-Y.; Zhang, L.-Y.; Jin, G.; et al. FZD7 is a novel prognostic marker and promotes tumor metastasis via WNT and EMT signaling pathways in esophageal squamous cell carcinoma. Oncotarget 2017, 8, 65957. [Google Scholar] [CrossRef]
- Li, G.; Su, Q.; Liu, H.; Wang, D.; Zhang, W.; Lu, Z.; Chen, Y.; Huang, X.; Li, W.; Zhang, C.; et al. Frizzled7 Promotes Epithelial-to-mesenchymal Transition and Stemness Via Activating Canonical Wnt/β-catenin Pathway in Gastric Cancer. Int. J. Biol. Sci. 2018, 14, 280–293. [Google Scholar] [CrossRef]
- Xi, L.; Liu, Q.; Zhang, W.; Luo, L.; Song, J.; Liu, R.; Wei, S.; Wang, Y. Circular RNA circCSPP1 knockdown attenuates doxorubicin resistance and suppresses tumor progression of colorectal cancer via miR-944/FZD7 axis. Cancer Cell Int. 2021, 21, 153. [Google Scholar] [CrossRef]
- Yang, L.; Wu, X.; Wang, Y.; Zhang, K.; Wu, J.; Yuan, Y.C.; Deng, X.; Chen, L.; Kim, C.C.H.; Lau, S.; et al. FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene 2011, 30, 4437–4446. [Google Scholar] [CrossRef]
- Simmons, G.E., Jr.; Pandey, S.; Nedeljkovic-Kurepa, A.; Saxena, M.; Wang, A.; Pruitt, K. Frizzled 7 Expression Is Positively Regulated by SIRT1 and β-Catenin in Breast Cancer Cells. PLoS ONE 2014, 9, e98861. [Google Scholar] [CrossRef]
- Yin, P.; Bai, Y.; Wang, Z.; Sun, Y.; Gao, J.; Na, L.; Zhang, Z.; Wang, W.; Zhao, C. Non-canonical Fzd7 signaling contributes to breast cancer mesenchymal-like stemness involving Col6a1. Cell Commun. Signal. 2020, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Dang, S.; Feng, Q.; Liang, J.; Wang, Y.; Fan, N. MicroRNA-542-3p inhibits the growth of hepatocellular carcinoma cells by targeting FZD7/Wnt signaling pathway. Biochem. Biophys. Res. Commun. 2017, 482, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Li, B.; Yang, J. MicroRNA-504 functions as a tumor suppressor in hepatocellular carcinoma through inhibiting Frizzled-7-mediated-Wnt/β-catenin signaling. Biomed. Pharmacother. 2018, 107, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Javanmard, D.; Karbalaie Niya, M.H.; Kalafkhany, D.; Najafi, M.; Ziaee, M.; Babaei, M.R.; Kiani, S.J.; Esghaei, M.; Jazayeri, S.M.; Panahi, M.; et al. Downregulation of gsk3β and upregulation of urg7 in hepatitis b-related hepatocellular carcinoma. Hepat. Mon. 2020, 20, e100899. [Google Scholar] [CrossRef]
- Dang, M.N.; Suri, S.; Li, K.; Gomez Casas, C.; Stigliano, G.; Riley, R.S.; Scully, M.A.; Hoover, E.C.; Aboeleneen, S.B.; Kramarenko, G.C.; et al. Antibody and siRNA Nanocarriers to Suppress Wnt Signaling, Tumor Growth, and Lung Metastasis in Triple-Negative Breast Cancer. Adv. Ther. 2024, 7, 2300426. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, T.; Cao, Y.W.; Ding, X.Q. Antitumor effect of miR-27b-3p on lung cancer cells via targeting Fzd7. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4113–4123. [Google Scholar]
- Condello, S.; Sima, L.; Ivan, C.; Cardenas, H.; Schiltz, G.; Mishra, R.K.; Matei, D. Tissue tranglutaminase regulates interactions between ovarian cancer stem cells and the tumor niche. Cancer Res. 2018, 78, 2990–3001. [Google Scholar] [CrossRef]
- Sima, L.E.; Yakubov, B.; Zhang, S.; Condello, S.; Grigorescu, A.A.; Nwani, N.G.; Chen, L.; Schiltz, G.E.; Arvanitis, C.; Zhang, Z.Y.; et al. Small molecules target the interaction between tissue transglutaminase and fibronectin. Mol. Cancer Ther. 2019, 18, 1057–1068. [Google Scholar] [CrossRef]
- Tan, M.; Asad, M.; Heong, V.; Wong, M.K.; Tan, T.Z.; Ye, J.; Kuay, K.T.; Thiery, J.P.; Scott, C.; Huang, R.Y.J. The FZD7-TWIST1 axis is responsible for anoikis resistance and tumorigenesis in ovarian carcinoma. Mol. Oncol. 2019, 13, 757–780. [Google Scholar] [CrossRef]
- Liu, N.; Zang, S.; Liu, Y.; Wang, Y.; Li, W.; Liu, Q.; Ji, M.; Ma, D.; Ji, C. FZD7 regulates BMSCs-mediated protection of CML cells. Oncotarget 2015, 7, 6175. [Google Scholar] [CrossRef]
- Ren, W.; Li, C.; Duan, W.; Du, S.; Yang, F.; Zhou, J.; Xing, J. MicroRNA-613 represses prostate cancer cell proliferation and invasion through targeting Frizzled7. Biochem. Biophys. Res. Commun. 2016, 469, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, S.; Xu, Y.; Zhao, C. Regulation of ABCG2 expression by Wnt5a through FZD7 in human pancreatic cancer cells. Mol. Med. Rep. 2021, 23, 52. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, J.; Ren, J.; Zhang, D. MicroRNA-485-5p represses melanoma cell invasion and proliferation by suppressing Frizzled7. Biomed. Pharmacother. 2017, 90, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Hernandez, I.; Maiques, O.; Kohlhammer, L.; Cantelli, G.; Perdrix-Rosell, A.; Monger, J.; Fanshawe, B.; Bridgeman, V.L.; Karagiannis, S.N.; Penin, R.M.; et al. WNT11-FZD7-DAAM1 signalling supports tumour initiating abilities and melanoma amoeboid invasion. Nat. Commun. 2020, 11, 5315. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, L.; Pan, S.; Jiang, H.; Jin, H. Targeting Frizzled-7 decreases stemness and chemotherapeutic resistance in gastric cancer cells by suppressing MYC expression. Med. Sci. Monit. 2019, 25, 8637–8644. [Google Scholar] [CrossRef]
- Fazeli, M.; Zarei, N.; Moazen, B.; Nejatollahi, F. Anti-proliferative effects of human anti-FZD7 single chain antibodies on colorectal cancer cells. Shiraz E Med. J. 2017, 18, e45219. [Google Scholar] [CrossRef]
- Hafezi, N.; Valadan, R.; Asgarian, O.H.; Ajami, A. Anti-tumor activity of a recombinant soluble Fzd7 decoy receptor in human gastric and colon cancer cells. Iran. J. Basic Med. Sci. 2022, 25, 187–192. [Google Scholar] [CrossRef]
- Li, J.; Lin, N.; Zhang, S.; Weng, L.; Chen, C.; Ou, W.; Cao, Y. Characterization of the tumor microenvironment in breast cancer brain metastasis. Heliyon 2024, 10, e34876. [Google Scholar] [CrossRef]
- Li, K.; Mao, S.; Li, X.; Zhao, H.; Wang, J.; Wang, C.; Wu, L.; Zhang, K.; Yang, H.; Jin, M.; et al. Frizzled-7-targeting antibody (SHH002-hu1) potently suppresses non–small-cell lung cancer via Wnt/β-catenin signaling. Cancer Sci. 2023, 114, 2109–2122. [Google Scholar] [CrossRef]
- Do, M.; Wu, C.C.N.; Sonavane, P.R.; Juarez, E.F.; Adams, S.R.; Ross, J.; Baena, A.R.y.; Patel, C.; Mesirov, J.P.; Carson, D.A.; et al. A FZD7-specific Antibody–Drug Conjugate Induces Ovarian Tumor Regression in Preclinical Models. Mol. Cancer Ther. 2022, 21, 113–124. [Google Scholar] [CrossRef]
- Nile, A.H.; de Sousa, E.M.F.; Mukund, S.; Piskol, R.; Hansen, S.; Zhou, L.; Zhang, Y.; Fu, Y.; Gogol, E.B.; Kömüves, L.G.; et al. A selective peptide inhibitor of Frizzled 7 receptors disrupts intestinal stem cells. Nat. Chem. Biol. 2018, 14, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, N.; Alizadeh-Navaei, R.; Golpour, M.; Zafari, P.; Ajami, A. Role of Frizzled receptor expression on patients’ survival with gastrointestinal cancers: A systematic review with meta-analysis. Casp. J. Intern. Med. 2022, 13, 1. [Google Scholar] [CrossRef]
- Astudillo, P. A Non-canonical Wnt Signature Correlates with Lower Survival in Gastric Cancer. Front. Cell Dev. Biol. 2021, 9, 633675. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Deng, J.; Wang, P.; Kou, F.; Wu, Z.; Zhang, N.; Zhao, Z.; Nie, Y.; Yang, L. The malignancy suppression and ferroptosis facilitation of BCL6 in gastric cancer mediated by FZD7 repression are strengthened by RNF180/RhoC pathway. Cell Biosci. 2023, 13, 73. [Google Scholar] [CrossRef]
- Chidiac, R.; Yang, A.; Kubarakos, E.; Mikolajewicz, N.; Han, H.; Almeida, M.P.; Thibeault, P.E.; Lin, S.; MacLeod, G.; Gratton, J.P.; et al. Selective activation of FZD2 and FZD7 reveals non-redundant function during mesoderm differentiation. Stem Cell Rep. 2025, 20, 102391. [Google Scholar] [CrossRef]
- Yu, H.; Ye, X.; Guo, N.; Nathans, J. Frizzled 2 and frizzled 7 function redundantly in convergent extension and closure of the ventricular septum and palate: Evidence for a network of interacting genes. Development 2012, 139, 4383–4394. [Google Scholar] [CrossRef]
- Michelli, M.; Zougros, A.; Chatziandreou, I.; Michalopoulos, N.V.; Lazaris, A.C.; Saetta, A.A. Concurrent Wnt pathway component expression in breast and colorectal cancer. Pathol. Res. Pract. 2020, 216, 153005. [Google Scholar] [CrossRef]
- Muzny, D.M.; Bainbridge, M.N.; Chang, K.; Dinh, H.H.; Drummond, J.A.; Fowler, G.; Kovar, C.L.; Lewis, L.R.; Morgan, M.B.; Newsham, I.F.; et al. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef]
- Ferrell, M.; Guven, D.C.; Gomez, C.G.; Nasrollahi, E.; Giza, R.; Cheng, S.; Syed, M.P.; Magge, T.; Singhi, A.; Saeed, A.; et al. Investigating the WNT and TGF-beta pathways alterations and tumor mutation burden in young-onset colorectal cancer. Sci. Rep. 2024, 14, 17884. [Google Scholar] [CrossRef]
- DeAlmeida, V.I.; Miao, L.; Ernst, J.A.; Koeppen, H.; Polakis, P.; Rubinfeld, B. The Soluble Wnt Receptor Frizzled8CRD-hFc Inhibits the Growth of Teratocarcinomas In vivo. Cancer Res. 2007, 67, 5371–5379. [Google Scholar] [CrossRef]
- Sung, V.Y.C.; Knight, J.F.; Johnson, R.M.; Stern, Y.E.; Saleh, S.M.; Savage, P.; Monast, A.; Zuo, D.; Duhamel, S.; Park, M. Co-dependency for MET and FGFR1 in basal triple-negative breast cancers. NPJ Breast Cancer 2021, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, M.; Dong, Z.X.; Jiang, D.; Li, X.; Lin, S.; Chen, D.; Zou, X.; Zhang, X.D.; Luker, G.D. Heterogeneous microenvironmental stiffness regulates pro-metastatic functions of breast cancer cells. Acta Biomater. 2021, 131, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Trayes, K.P.; Cokenakes, S.E.H. Breast Cancer Treatment. Am. Fam. Physician 2021, 104, 171–178. [Google Scholar] [PubMed]
- Hoover, E.C.; Ruggiero, O.M.; Swingler, R.N.; Day, E.S. FZD7-Targeted Nanoparticles to Enhance Doxorubicin Treatment of Triple-Negative Breast Cancer. ACS Omega 2024, 9, 14323–14335. [Google Scholar] [CrossRef]
- Ma, K.; Wu, H.; Ji, L. Construction of HBV gene-related prognostic and diagnostic models for hepatocellular carcinoma. Front. Genet. 2023, 13, 1065644. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, C.; Ma, T.; Jiang, L.; Tang, L.; Shi, T.; Zhang, S.; Zhang, L.; Zhu, P.; Li, J.; et al. Reversal effect of quercetin on multidrug resistance via FZD7/β-catenin pathway in hepatocellular carcinoma cells. Phytomedicine 2018, 43, 37–45. [Google Scholar] [CrossRef]
- Huang, J.; Chan, W.C.; Ngai, C.H.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.J.; Elcarte, E.; Withers, M.; et al. Worldwide Burden, Risk Factors, and Temporal Trends of Ovarian Cancer: A Global Study. Cancers 2022, 14, 2230. [Google Scholar] [CrossRef]
- Asad, M.; Wong, M.K.; Tan, T.Z.; Choolani, M.; Low, J.; Mori, S.; Virshup, D.; Thiery, J.P.; Huang, R.Y.J. FZD7 drives in vitro aggressiveness in stem-A subtype of ovarian cancer via regulation of non-canonical wnt/PCP pathway. Cell Death Dis. 2014, 5, e1346. [Google Scholar] [CrossRef]
- Atwani, R.; Nagare, R.; Rogers, A.; Prasad, M.; Lazar, V.; Sandusky, G.; Tong, Y.; Pin, F.; Condello, S. Integrin-linked kinase-frizzled 7 interaction maintains cancer stem cells to drive platinum resistance in ovarian cancer. J. Exp. Clin. Cancer Res. 2024, 43, 156. [Google Scholar] [CrossRef]
- Diamond, J.R.; Becerra, C.; Richards, D.; Mita, A.; Osborne, C.; O’Shaughnessy, J.; Zhang, C.; Henner, R.; Kapoun, A.M.; Xu, L.; et al. Phase Ib clinical trial of the anti-frizzled antibody vantictumab (OMP-18R5) plus paclitaxel in patients with locally advanced or metastatic HER2-negative breast cancer. Breast Cancer Res. Treat. 2020, 184, 53–62. [Google Scholar] [CrossRef]
- Jabbour, E.; Kantarjian, H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am. J. Hematol. 2020, 95, 691–709. [Google Scholar] [CrossRef] [PubMed]
- Senapati, J.; Sasaki, K.; Issa, G.C.; Lipton, J.H.; Radich, J.P.; Jabbour, E.; Kantarjian, H.M. Management of chronic myeloid leukemia in 2023—Common ground and common sense. Blood Cancer J. 2023, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- de Cássia Viu Carrara, R.; Fontes, A.M.; Abraham, K.J.; Orellana, M.D.; Haddad, S.K.; Palma, P.V.B.; Panepucci, R.A.; Zago, M.A.; Covas, D.T. Expression differences of genes in the PI3K/AKT, WNT/b-catenin, SHH, NOTCH and MAPK signaling pathways in CD34+ hematopoietic cells obtained from chronic phase patients with chronic myeloid leukemia and from healthy controls. Clin. Transl. Oncol. 2018, 20, 542–549. [Google Scholar] [CrossRef]
- Wang, W.; Lyu, C.; Wang, F.; Wang, C.; Wu, F.; Li, X.; Gan, S. Identification of Potential Signatures and Their Functions for Acute Lymphoblastic Leukemia: A Study Based on the Cancer Genome Atlas. Front. Genet. 2021, 12, 656042. [Google Scholar] [CrossRef]
- Lv, L.; Chen, G.; Zhou, J.; Li, J.; Gong, J. WT1-AS promotes cell apoptosis in hepatocellular carcinoma through down-regulating of WT1. J. Exp. Clin. Cancer Res. 2015, 34, 119. [Google Scholar] [CrossRef]
- Du, T.; Zhang, B.; Zhang, S.; Jiang, X.; Zheng, P.; Li, J.; Yan, M.; Zhu, Z.; Liu, B. Decreased expression of long non-coding RNA WT1-AS promotes cell proliferation and invasion in gastric cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 12–19. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Fang, L. LncRNA WT1-AS over-expression inhibits non-small cell lung cancer cell stemness by down-regulating TGF-β1. BMC Pulm. Med. 2020, 20, 113. [Google Scholar] [CrossRef]
- Le, F.; Luo, P.; Ouyang, Q.; Zhong, X. LncRNA WT1-AS downregulates survivin by upregulating miR-203 in papillary thyroid carcinoma. Cancer Manag. Res. 2020, 12, 443–449. [Google Scholar] [CrossRef]
- Tong, W.; Zhang, H. Overexpression of long non-coding RNA WT1-AS or silencing of PIK3AP1 are inhibitory to cervical cancer progression. Cell Cycle 2021, 20, 2583–2596. [Google Scholar] [CrossRef]
- Sun, M.; Wu, D.; Zhou, K.; Li, H.; Gong, X.; Wei, Q.; Du, M.; Lei, P.; Zha, J.; Zhu, H.; et al. An eight-lncRNA signature predicts survival of breast cancer patients: A comprehensive study based on weighted gene co-expression network analysis and competing endogenous RNA network. Breast Cancer Res. Treat. 2019, 175, 59–75. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Wu, J.; Ma, R.; Feng, J. Long noncoding RNAs predict the survival of patients with colorectal cancer as revealed by constructing an endogenous RNA network using bioinformation analysis. Cancer Med. 2019, 8, 863–873. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Yin, X.; Guan, X.; Li, Y.; Xin, C.; Liu, J. GIPC2 interacts with Fzd7 to promote prostate cancer metastasis by activating WNT signaling. Oncogene 2022, 41, 2609–2623. [Google Scholar] [CrossRef] [PubMed]
- Ilic, I.; Ilic, M. International patterns in incidence and mortality trends of pancreatic cancer in the last three decades: A joinpoint regression analysis. World J. Gastroenterol. 2022, 28, 4698–4715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, Y. FZD7 accelerates hepatic metastases in pancreatic cancer by strengthening EMT and stemness associated with TGF-β/SMAD3 signaling. Mol. Med. 2022, 28, 82. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, F.; Xue, Q.; Weng, X.; Xu, F. Metastatic pancreatic cancer: Mechanisms and detection (Review). Oncol. Rep. 2021, 46, 231. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Zhang, Y. Expression and prognostic impact of FZDs in pancreatic adenocarcinoma. BMC Gastroenterol. 2021, 21, 79. [Google Scholar] [CrossRef]
- Jiang, H.; Gao, B.; Meng, Z.; Wang, Y.; Jiao, T.; Li, J.; Li, X.; Cao, Y.; Zhang, X.; Li, C.; et al. Integrative multi-omics analysis reveals the role of tumor-associated endothelial cells and their signature in prognosis of intrahepatic cholangiocarcinoma. J. Transl. Med. 2024, 22, 948. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, H.; Li, Z.; Li, F.; Liang, L.; Zou, Y.; Shen, H.; Li, J.; Xia, Y.; Cheng, Z.; et al. Circular RNA ACTN4 promotes intrahepatic cholangiocarcinoma progression by recruiting YBX1 to initiate FZD7 transcription. J. Hepatol. 2022, 76, 135–147. [Google Scholar] [CrossRef]
- Zapata-García, J.A.; Jave-Suárez, L.F.; Aguilar-Lemarroy, A. Delving into the Role of Receptor-like Tyrosine Kinase (RYK) in Cancer: In Silico Insights into Its Diagnostic and Prognostic Utility. J. Mol. Pathol. 2024, 5, 66–80. [Google Scholar] [CrossRef]
- Tokumoto, N.; Ikeda, S.; Ishizaki, Y.; Kurihara, T.; Ozaki, S.; Iseki, M.; Shimizu, Y.; Itamoto, T.; Arihiro, K.; Okajima, M.; et al. Immunohistochemical and mutational analyses of Wnt signaling components and target genes in intrahepatic cholangiocarcinomas. Int. J. Oncol. 2005, 27, 973–980. [Google Scholar] [CrossRef]
- Xu, R.; Zeng, S.; Xie, W.; Sun, C.; Chen, Y.L.; Chen, M.J.; Zhang, L. The expression and function of Frizzled-7 in human renal cell carcinoma. Clin. Transl. Oncol. 2016, 18, 269–276. [Google Scholar] [CrossRef]
- Muncan, V.; Sansom, O.J.; Tertoolen, L.; Phesse, T.J.; Begthel, H.; Sancho, E.; Cole, A.M.; Gregorieff, A.; de Alboran, I.M.; Clevers, H.; et al. Rapid loss of intestinal crypts upon conditional deletion of the Wnt/Tcf-4 target gene c-Myc. Mol. Cell. Biol. 2006, 26, 8418–8426. [Google Scholar] [CrossRef] [PubMed]
- Gu, N.X.; Guo, Y.R.; Lin, S.E.; Wang, Y.H.; Lin, I.H.; Chen, Y.F.; Yen, Y. Frizzled 7 modulates goblet and Paneth cell fate, and maintains homeostasis in mouse intestine. Development 2023, 150, dev200932. [Google Scholar] [CrossRef] [PubMed]
- Bugter, J.M.; van Kerkhof, P.; Jordens, I.; Janssen, E.; Tran Ngoc Minh, T.; Iglesias van Montfort, D.; Jamieson, C.; Maurice, M.M. E3 ligases RNF43 and ZNRF3 display differential specificity for endocytosis of Frizzled receptors. Life Sci. Alliance 2024, 7, e202402575. [Google Scholar] [CrossRef] [PubMed]
- Nabhan, A.N.; Webster, J.D.; Adams, J.J.; Blazer, L.; Everrett, C.; Eidenschenk, C.; Arlantico, A.; Fleming, I.; Brightbill, H.D.; Wolters, P.J.; et al. Targeted alveolar regeneration with Frizzled-specific agonists. Cell 2023, 186, 2995–3012.e2915. [Google Scholar] [CrossRef]
- Ritchie, M.; Tchistiakova, L.; Scott, N. Implications of receptor-mediated endocytosis and intracellular trafficking dynamics in the development of antibody drug conjugates. mAbs 2013, 5, 13–21. [Google Scholar] [CrossRef]
- Diamantis, N.; Banerji, U. Antibody-drug conjugates—An emerging class of cancer treatment. Br. J. Cancer 2016, 114, 362–367. [Google Scholar] [CrossRef]
- Lambert, J.M.; Berkenblit, A. Antibody–Drug Conjugates for Cancer Treatment. Annu. Rev. Med. 2018, 69, 191–207. [Google Scholar] [CrossRef]
- Samaranayake, H.; Wirth, T.; Schenkwein, D.; Räty, J.K.; Ylä-Herttuala, S. Challenges in monoclonal antibody-based therapies. Ann. Med. 2009, 41, 322–331. [Google Scholar] [CrossRef]
- Gezehagn Kussia, G.; Tessema, T.S. The Potential of Single-Chain Variable Fragment Antibody: Role in Future Therapeutic and Diagnostic Biologics. J. Immunol. Res. 2024, 2024, 1804038. [Google Scholar] [CrossRef]
- DeVito, N.C.; Sturdivant, M.; Thievanthiran, B.; Xiao, C.; Plebanek, M.P.; Salama, A.K.S.; Beasley, G.M.; Holtzhausen, A.; Novotny-Diermayr, V.; Strickler, J.H.; et al. Pharmacological Wnt ligand inhibition overcomes key tumor-mediated resistance pathways to anti-PD-1 immunotherapy. Cell Rep. 2021, 35, 109071. [Google Scholar] [CrossRef]
| Cancer Types | Investigating FZD7 Role in Cancer Function | Reference | |
|---|---|---|---|
| In Vitro | In Vivo | ||
| Gastric | FZD7 knockdown reduces colony-forming ability, c-MYC and cyclinD1 expression in MKN28 and MKN24. | FZD7 knock out reduces tumour burden, c-MYC, CD44, and cyclinD1 expression in gastric GEMM (gp130F/F). | [17] |
| Colorectal | circCSPP1/miRNA-944-mediated knockdown of FZD7 in CRC cells has been shown to reduce tumorigenic traits and reduces chemoresistance. | shRNA-targeting cricCSPP1 (a positive regulator of FZD7) reduced tumour size in subcutaneous CRC model. | [28] |
| Breast | FZD7 knockdown via shRNA in MDA-MB-231 and BT-20 TNBC BCa cells resulted in reduced migration, invasion, proliferation, and colony formation. | FZD7 shRNA knockdown in MDA-MB-231 reduced xenograft tumour size in subcutaneous model. Additionally, Axin2, Cyclin D1 and MYC expression were all downregulated. | [29,30,31] |
| Hepatocellular Carcinoma | shRNA knockdown of FZD7 in HCC cell lines results in NF-κB-induced apoptosis through downregulation of Bcl-2 and Bcl-XL. siRNA-mediated FZD7 knockdown was able to sensitise HCC cells to several chemo-therapy agents (including 5-FU, MMC, and ADR) significantly reducing their IC50 dosages. Increased FZD7 expression promotes colony formation in HCC cells. | [23,32,33,34,35] | |
| Lung | Downregulation of FZD7 mRNA via miR-27b-3p promotes apoptosis and suppress cancer cell viability. | [36] | |
| Ovarian | shRNA downregulation of FZD7 results in epithelial-like phenotype and reduction in EMT-associated gene expression. | FZD7 knockout in MA-148 cells was unable to prevent tumour formation in a subcutaneous xenograft model of OC. However, direct comparisons of FZD7 proficient and deficient cells were not made. | [37,38,39] |
| Leukaemia | Changes in FZD7 expression in CML cells mirror those in BMSCs. Manipulation of FZD7 expression (knockdown or overexpression) in BMSCs influences FZD7 expression in surrounding CML cells. Direct knockdown of FZD7 in CML cells reduced the expression of Wnt target genes MDR1 and CD44, suggesting that FZD7 acts through the canonical Wnt/β-catenin pathway. | [40] | |
| Prostate | Overexpression of FZD7 led to increased invasion and proliferation in PCa cells, indicating FZD7 plays an oncogenic role in PCa. | [41] | |
| Pancreatic | Knockdown of FZD7 resulted in significantly lower β-catenin levels and ABCG2 in pancreatic cell lines in the presence of Wnt5a, suggesting FZD7 as the bridge between Wnt5a and ABCG2. Additionally, knocking down FZD7 led to increased apoptosis of capan-2 cells when treated with gemcitabine, highlighting the role of FZD7 in gemcitabine resistance in pancreatic cancer. | [24,42] | |
| Melanoma | FZD7 knockdown via miR-486-5p expression resulted in a reversible loss of invasion and proliferation capacity in melanoma cell line. Furthermore, FZD7 is required for melanoma melanosphere formation and invasion via the non-canonical Wnt signalling pathway. | [43,44] | |
| Renal | Inducing overexpression of miR-613 (negative regulator of FZD7) significantly reduced proliferation and invasion of FZD7 dependant RCC cells | [22] | |
| Compound Name | Mechanism of Action | Cancer Types | Reference |
|---|---|---|---|
| OMP18R5 | Noncompetitive inhibitor of FZD1, 2, 5, 7, and 8. | Gastric | [17,45] |
| FZD7 specific single-chain fragment variable antibodies | Binds and inhibits FZD7 mediated signalling (binding region not mentioned). | CRC | [46] |
| Soluble recombinant FZD7 decoy receptor | Binds to ligands associated with FZD7, limiting the formation of the Wnt ligand/receptor complex. | Gastric and CRC | [47] |
| FZD7 targeting nanoparticles | Deliver doxorubicin or beta-catenin siRNA directly to FZD7 expressing cells. | Breast | [35,48] |
| SHH002-hu1 | Humanised antibody which competitively inhibit FZD7 mediated signalling. | Lung | [49] |
| FZD7-ADC | Humanised antibody which noncompetitively binds to FZD7 and delivers a microtubule inhibitor. | Ovarian | [50] |
| Fz7-21 | Small peptide inhibitor of FZD7 mediated signalling. | N/A | [51] |
| miRNA | Mechanism | Cancer Types | Reference |
|---|---|---|---|
| miR-27b-3p | Directly downregulates FZD7 expression in lung cancer. | Lung | [36] |
| miR-485-5p | Binds to the 3′-untranslated region of FZD7, increased miR-485-5p expression led to reduced FZD7 expression and when FZD7 was restored, the invasive and proliferative capacity of the melanoma cells was restored. | Melanoma | [43] |
| miR-504 | Binds directly to the 3′ untranslated region of FZD7 mRNA, reducing its expression. Downregulated in HCC. | HCC | [33] |
| miR-542-3p | Downregulation in HCC is associated with an increase in clonogenicity and increased FZD7 expression. | HCC | [32] |
| miR-613 | Binds within the 3′-UTR of FZD7, reduces FZD7 expression. Overexpression of miR-613 significantly reduced proliferation and invasion in ACHN and 786-O RCC cells, whereas artificially inducing miR-613 overexpression appears to counteract FZD7-dependant tumour cells. | Prostate and RCC | [22,41] |
| miR-944 | FZD7 is upregulated via circCSPP1-mediated downregulation of miR-944 in doxorubicin resistant patient samples. | CRC | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodson, K.; Arredondo, H.M.; Humphrey, W.E.; Flanagan, D.J.; Vincan, E.; Willert, K.; Pearson, H.B.; Phesse, T.J. Targeting Cancer with Paris’ Arrow: An Updated Perspective on Targeting Wnt Receptor Frizzled 7. Sci 2025, 7, 61. https://doi.org/10.3390/sci7020061
Hodson K, Arredondo HM, Humphrey WE, Flanagan DJ, Vincan E, Willert K, Pearson HB, Phesse TJ. Targeting Cancer with Paris’ Arrow: An Updated Perspective on Targeting Wnt Receptor Frizzled 7. Sci. 2025; 7(2):61. https://doi.org/10.3390/sci7020061
Chicago/Turabian StyleHodson, Kieran, Hector M. Arredondo, William E. Humphrey, Dustin J. Flanagan, Elizabeth Vincan, Karl Willert, Helen B. Pearson, and Toby J. Phesse. 2025. "Targeting Cancer with Paris’ Arrow: An Updated Perspective on Targeting Wnt Receptor Frizzled 7" Sci 7, no. 2: 61. https://doi.org/10.3390/sci7020061
APA StyleHodson, K., Arredondo, H. M., Humphrey, W. E., Flanagan, D. J., Vincan, E., Willert, K., Pearson, H. B., & Phesse, T. J. (2025). Targeting Cancer with Paris’ Arrow: An Updated Perspective on Targeting Wnt Receptor Frizzled 7. Sci, 7(2), 61. https://doi.org/10.3390/sci7020061








