Differentiation of Heterogeneous Mouse Liver from HCC by Hyperpolarized 13C Magnetic Resonance
Abstract
1. Introduction
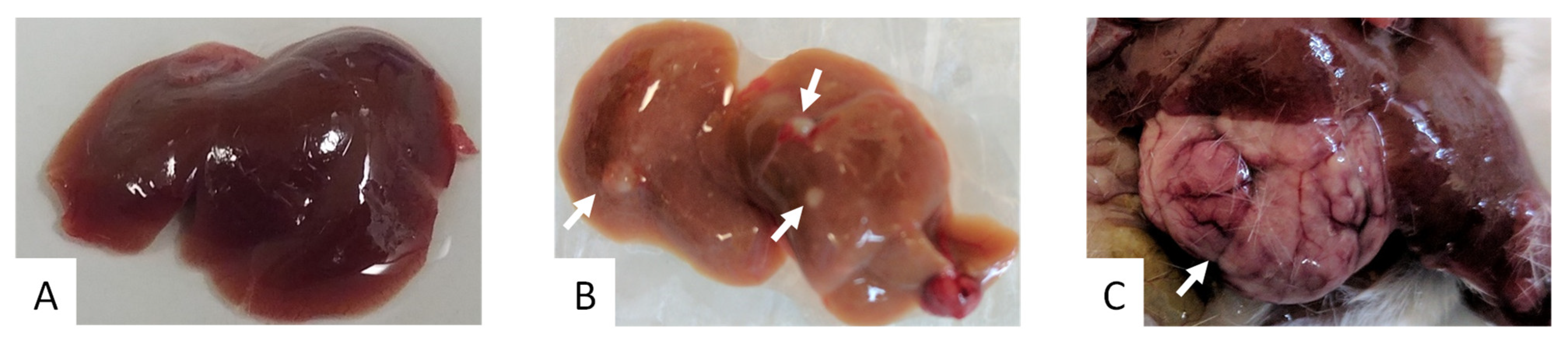
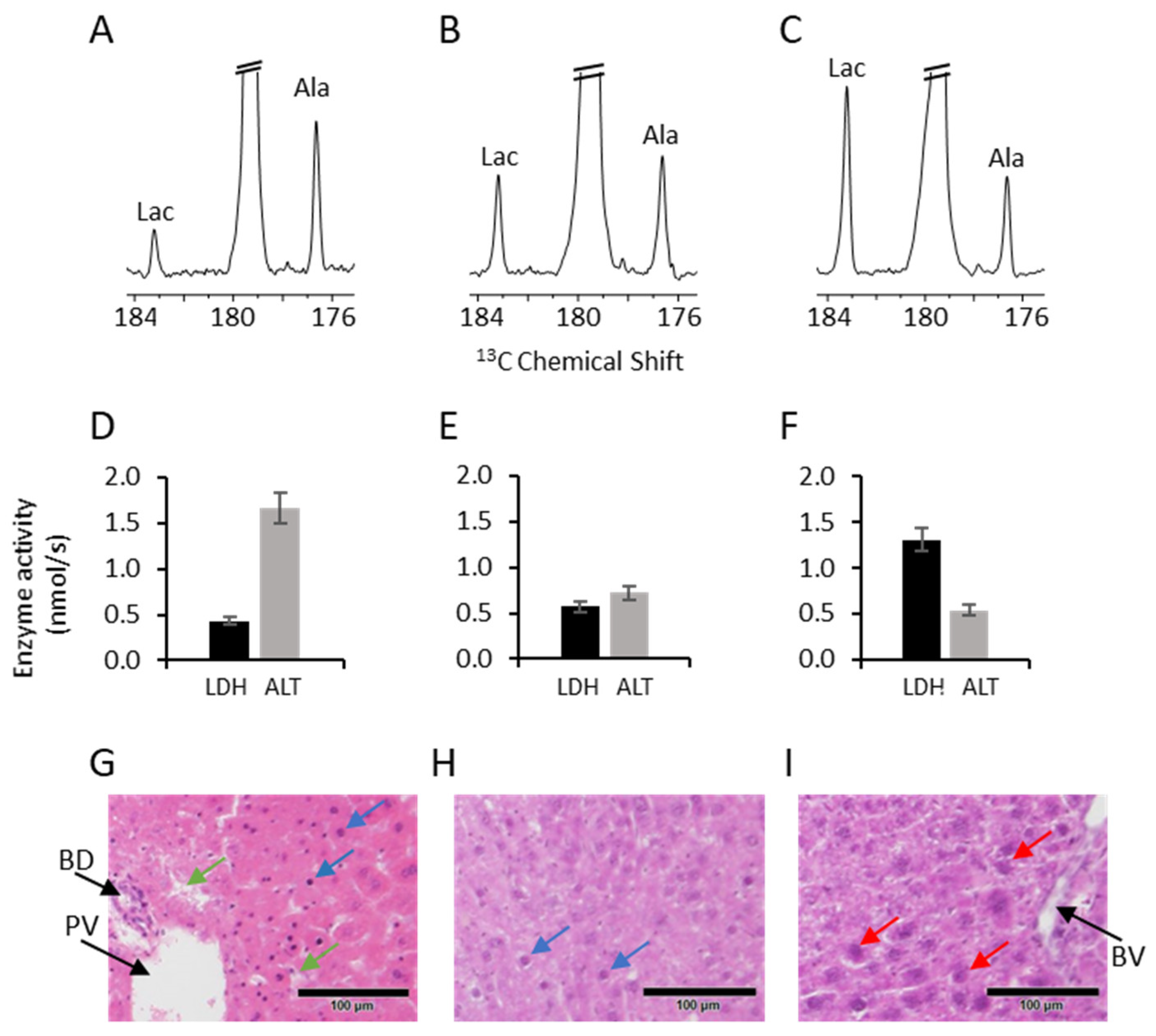
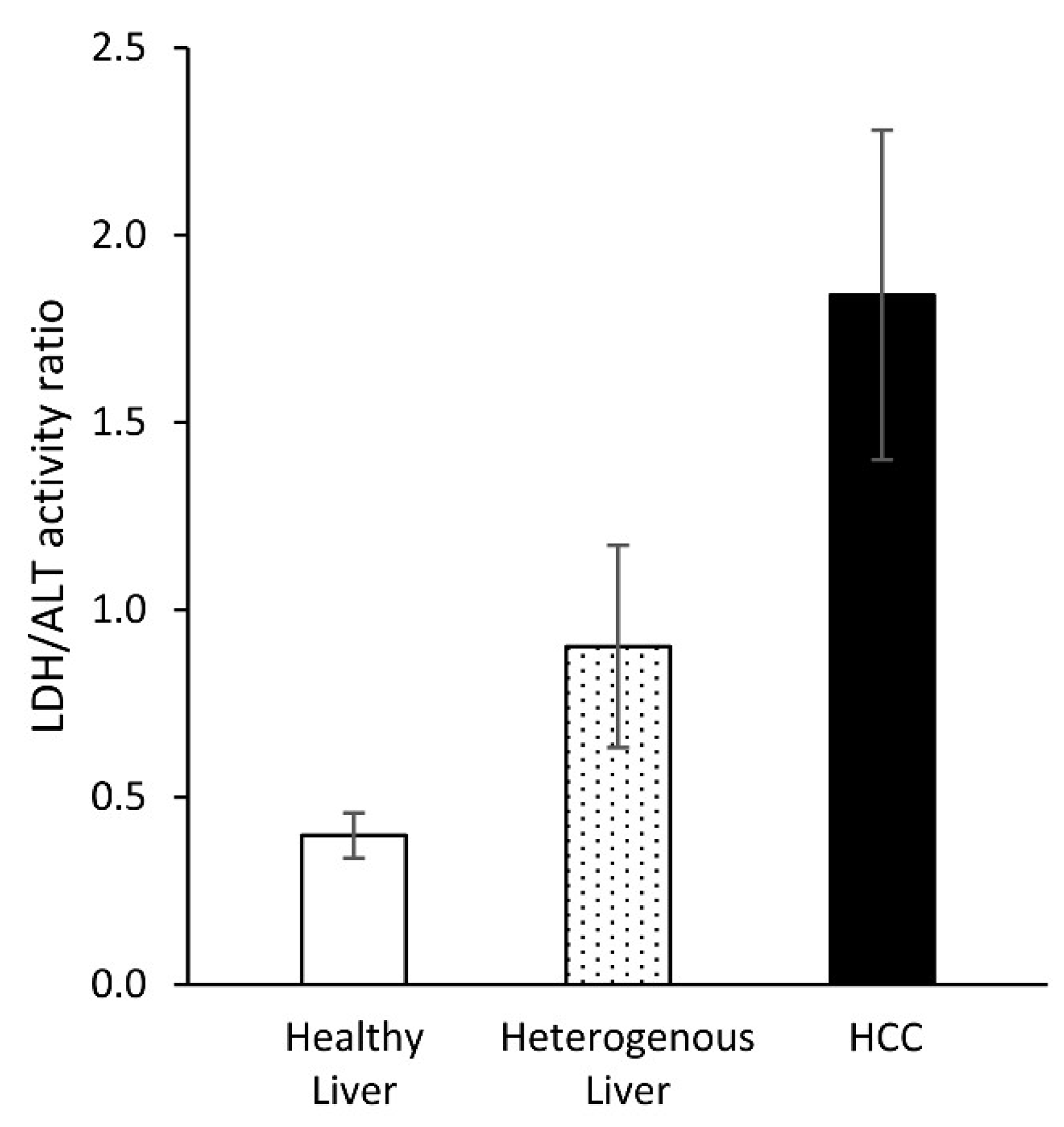
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Animals, Surgery, Tissue Processing and Categorization
5.3. PCLS Preparation
5.4. PCLS Perfusion in the Spectrometer
5.5. Hyperpolarization and Dissolution
5.6. Histological Study of the Same PCLS Batch Used for the Metabolic Study
5.7. Determination of Enzyme Activities
5.8. Parameters Used for Enzymatic Rate Calculations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| dDNP | dissolution dynamic nuclear polarization |
| LDH | lactate dehydrogenase |
| ALT | alanine transaminase |
| TR | repetition time |
References
- Forner, A.; Bruix, J. Biomarkers for early diagnosis of hepatocellular carcinoma. Lancet Oncol. 2012, 13, 750–751. [Google Scholar] [CrossRef]
- Llovet, J.M.; Bru, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Ardenkjær-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef] [PubMed]
- Duwel, S.; Durst, M.; Gringeri, C.V.; Kosanke, Y.; Gross, C.; Janich, M.A.; Haase, A.; Glaser, S.J.; Schwaiger, M.; Schulte, R.F.; et al. Multiparametric human hepatocellular carcinoma characterization and therapy response evaluation by hyperpolarized C-13 MRSI. NMR Biomed. 2016, 29, 952–960. [Google Scholar] [CrossRef]
- Darpolor, M.M.; Kaplan, D.E.; Pedersen, P.L.; Glickson, J.D. Human hepatocellular carcinoma metabolism: Imaging by hyperpolarized 13C magnetic resonance spectroscopy. J. Liver Dis. Transpl. 2012, 1, 1. [Google Scholar] [CrossRef]
- Cunningham, C.H.; Lau, J.Y.C.; Chen, A.P.; Geraghty, B.J.; Perks, W.J.; Roifman, I.; Wright, G.A.; Connelly, K.A. Hyperpolarized 13C metabolic MRI of the human heart initial experience. Circ. Res. 2016, 119, 1177–1182. [Google Scholar] [CrossRef]
- Nelson, S.J.; Kurhanewicz, J.; Vigneron, D.B.; Larson, P.E.Z.; Harzstark, A.L.; Ferrone, M.; Criekinge, M.; Chang, J.W.; Bok, R.; Park, I.; et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C] pyruvate. Sci. Transl. Med. 2013, 5, 198ra108. [Google Scholar] [CrossRef]
- Park, I.; Autry, A.; Yang, X.D.; Zhai, Y.Y.; Sriram, R.; Korenchan, D.; Kurhanewicz, J.; Cunha, A.; Hsu, I.C.; Nelson, S.; et al. Noninvasive assessment of treatment response for diffuse intrinsic pontine glioma using hyperpolarized 13C metabolic imaging. Neurooncol. Pract. 2017, 19, 194. [Google Scholar]
- Chung, B.T.; Chen, H.Y.; Gordon, J.; Mammoli, D.; Sriram, R.; Autry, A.W.; Page, L.M.; Chaumeil, M.M.; Shin, P.; Slater, J.; et al. First hyperpolarized 2-13C pyruvate MR studies of human brain metabolism. J. Magn. Reson. 2019, 309, 106617. [Google Scholar] [CrossRef]
- Kurhanewicz, J.; Vigneron, D.B.; Ardenkjaer, L.J.H.; Bankson, J.A.; Brindle, K.; Cunningham, C.H.; Gallagher, F.A.; Keshari, K.R.; Kjaer, A.; Laustsen, C.; et al. Hyperpolarized 13C MRI: Path to Clinical Translation in Oncology. Neoplasia 2019, 21, 1–16. [Google Scholar] [CrossRef]
- Fussenich, L.M.; Desar, I.M.E.; Peters, M.; Teerenstra, S.; Graaf, W.T.A.; Timmer-Bonte, J.N.H.; Herpen, C.M.L. A new, simple and objective prognostic score for phase I cancer patients. Eur. J. Cancer 2011, 47, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Girgis, H.; Masui, O.; White, N.M.A.; Scorilas, A.; Rotondo, F.; Seivwright, A.; Gabril, M.; Filter, E.R.; Girgis, A.H.A.; Bjarnason, G.A.; et al. Lactate dehydrogenase A is a potential prognostic marker in clear cell renal cell carcinoma. Mol. Cancer 2014, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Kim, W.R.; Benson, J.T.; Therneau, T.M.; Melton, L.J. Serum aminotransferase activity and mortality risk in a United States community. Hepatology 2008, 47, 880–887. [Google Scholar] [CrossRef]
- Sherman, K.E. Alanine aminotransferase in clinical-practice—A review. Arch. Intern. Med. 1991, 151, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Z.; Park, S.; Reagan, W.J.; Goldstein, R.; Zhong, S.; Lawton, M.; Rajamohan, F.; Qian, K.; Liu, L.; Gong, D.W.; et al. Alanine aminotransferase isoenzymes: Molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity. Hepatology 2009, 49, 598–607. [Google Scholar] [CrossRef]
- Bilgic, I.; Gelecek, S.; Akgun, A.E.; Ozmen, M.M. Predictive value of liver transaminases levels in abdominal trauma. Am. J. Emerg. Med. 2014, 32, 705–708. [Google Scholar] [CrossRef]
- Park, J.M.; Khemtong, C.; Liu, S.C.; Hurd, R.E.; Spielman, D.M. In vivo assessment of intracellular redox state in rat liver using hyperpolarized 1-13C Alanine. Magn. Reson. Med. 2017, 77, 1741–1748. [Google Scholar] [CrossRef]
- Hu, S.; Chen, A.P.; Zierhut, M.L.; Bok, R.; Yen, Y.F.; Schroeder, M.A.; Hurd, R.E.; Nelson, S.J.; Kurhanewicz, J.; Vigneron, D.B. In vivo carbon-13 dynamic MRS and MRSI of normal and fasted rat liver with hyperpolarized 13C-pyruvate. Mol. Imaging Biol. 2009, 11, 399–407. [Google Scholar] [CrossRef]
- Jin, E.S.; Moreno, K.X.; Wang, J.X.; Fidelino, L.; Merritt, M.E.; Sherry, A.D.; Malloy, C.R. Metabolism of hyperpolarized [1-13C] pyruvate through alternate pathways in rat liver. NMR Biomed. 2016, 29, 466–474. [Google Scholar] [CrossRef]
- Lee, P.; Leong, W.; Tan, T.; Lim, M.; Han, W.P.; Radda, G.K. In vivo hyperpolarized carbon-13 magnetic resonance spectroscopy reveals increased pyruvate carboxylase flux in an insulin-resistant mouse model. Hepatology 2013, 57, 515–524. [Google Scholar] [CrossRef]
- Lev-Cohain, N.; Sapir, G.; Harris, T.; Azar, A.; Gamliel, A.; Nardi-Schreiber, A.; Uppala, S.; Sosna, J.; Gomori, J.M.; Katz-Brull, R. Real-time ALT and LDH activities determined in viable precision-cut mouse liver slices using hyperpolarized 1-13C pyruvate-Implications for studies on biopsied liver tissues. NMR Biomed. 2019, 32, e4043. [Google Scholar] [CrossRef] [PubMed]
- Merritt, M.E.; Harrison, C.; Sherry, A.D.; Malloy, C.R.; Burgess, S.C. Flux through hepatic pyruvate carboxylase and phosphoenolpyruvate carboxykinase detected by hyperpolarized 13C magnetic resonance. Proc. Natl. Acad. Sci. USA 2011, 108, 19084–19089. [Google Scholar] [CrossRef] [PubMed]
- Moreno, K.X.; Moore, C.L.; Burgess, S.C.; Sherry, A.D.; Malloy, C.R.; Merritt, M.E. Production of hyperpolarized (CO2)-13C from 1-13C pyruvate in perfused liver does reflect total anaplerosis but is not a reliable biomarker of glucose production. Metabolomics 2015, 11, 1144–1156. [Google Scholar] [CrossRef] [PubMed]
- Spielman, D.M.; Mayer, D.; Yen, Y.F.; Tropp, J.; Hurd, R.E.; Pfefferbaum, A. In vivo measurement of ethanol metabolism in the rat liver using magnetic resonance spectroscopy of hyperpolarized 1-13C pyruvate. Magn. Reson. Med. 2009, 62, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Golman, K.; Zandt, R.; Thaning, M. Real-time metabolic imaging. Proc. Natl. Acad. Sci. USA 2006, 103, 11270–11275. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.J.M.; Miller, J.; McCallum, C.; Rider, O.; Neubauer, S.; Heather, L.; Tyler, D.J. Non-invasive assessment of metformin induced changes in cardiac and hepatic redox state using hyperpolarized 1-13C pyruvate. Heart 2016, 102, A14. [Google Scholar] [CrossRef]
- Josan, S.; Billingsley, K.; Orduna, J.; Park, J.M.; Luong, R.; Yu, L.Q.; Hurd, R.; Pfefferbaum, A.; Spielman, D.; Mayer, D.; et al. Assessing inflammatory liver injury in an acute CCl4 model using dynamic 3D metabolic imaging of hyperpolarized 1-13C pyruvate. NMR Biomed. 2015, 28, 1671–1677. [Google Scholar] [CrossRef]
- Katzenellenbogen, M.; Pappo, O.; Barash, H.; Klopstock, N.; Mizrahi, L.; Olam, D.; Jacob-Hirsch, J.; Amariglio, N.; Rechavi, G.; Mitchell, L.A.; et al. Multiple adaptive mechanisms to chronic liver disease revealed at early stages of liver carcinogenesis in the Mdr2-knockout mice. Cancer Res. 2006, 66, 4001–4010. [Google Scholar] [CrossRef]
- Mauad, T.H.; Nieuwkerk, C.M.; Dingemans, K.P.; Smit, J.J.; Schinkel, A.H.; Notenboom, R.G.; Bergh, W.M.A.; Verkruisen, R.P.; Groen, A.K.; Oude, E.R.P. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am. J. Pathol. 1994, 145, 1237–1245. [Google Scholar]
- Katzenellenbogen, M.; Mizrahi, L.; Pappo, O.; Klopstock, N.; Olam, D.; Barash, H.; Domany, E.; Galun, E.; Goldenberg, D. Molecular mechanisms of the chemopreventive effect on hepatocellular carcinoma development in Mdr2 knockout mice. Mol. Cancer. Ther. 2007, 6, 1283–1291. [Google Scholar] [CrossRef]
- Bovenkamp, M.; Groothuis, G.M.M.; Meijer, D.K.F.; Olinga, P. Liver slices as a model to study fibrogenesis and test the effects of anti-fibrotic drugs on fibrogenic cells in human liver. Toxicol. In Vitro 2008, 22, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Bovenkamp, M.; Groothuis, G.M.M.; Meijer, D.K.F.; Olinga, P. Liver fibrosis in vitro: Cell culture models and precision-cut liver slices. Toxicol. In Vitro 2007, 21, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.; Uppala, S.; Lev-Cohain, N.; Adler-Levy, Y.; Shaul, D.; Nardi-Schreiber, A.; Sapir, G.; Azar, A.; Gamliel, A.; Sosna, J.; et al. Hyperpolarized product selective saturating-excitations for determination of changes in metabolic reaction rates in real-time. NMR Biomed. 2020, 33, e4189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P.; Wang, H.B.; Lin, Y.H.; Xu, J.; Wang, J.; Wang, K.; Liu, W.L. Lactate dehydrogenase is an important prognostic indicator for hepatocellular carcinoma after partial hepatectomy. Transl. Oncol. 2015, 8, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.-M.; Shin, S.-S.; Heo, S.-H.; Lim, H.-S.; Moon, M.-J.; Surendran, S.P.; Kim, G.-E.; Park, I.-W.; Jeong, Y.-Y. Metabolic changes in different stages of liver fibrosis: In vivo hyperpolarized 13C MR spectroscopy and metabolic imaging. Mol. Imaging Biol. 2019, 21, 842–851. [Google Scholar] [CrossRef]
- Chibaudel, B.; Bonnetain, F.; Tournigand, C.; Bengrine-Lefevre, L.; Teixeira, L.; Artru, P.; Desramé, J.; Larsen, A.K.; André, T.; Louvet, C.; et al. Simplified prognostic model in patients with oxaliplatin-based or irinotecan-based first-line chemotherapy for metastatic colorectal cancer: A GERCOR study. Oncologist 2011, 16, 1228–1238. [Google Scholar] [CrossRef]
- Motzer, R.J.; Mazumdar, M.; Bacik, J.; Berg, W.; Amsterdam, A.; Ferrara, J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J. Clin. Oncol. 1999, 17, 2530–2540. [Google Scholar] [CrossRef]
- Faloppi, L.; Scartozzi, M.; Bianconi, M.; Baroni, G.S.; Toniutto, P.; Giampieri, R.; Prete, M.; Minicis, S.; Bitetto, D.; Loretelli, C.; et al. The role of LDH serum levels in predicting global outcome in HCC patients treated with sorafenib: Implications for clinical management. BMC Cancer 2014, 14. [Google Scholar] [CrossRef]
- Kim, W.R.; Flamm, S.L.; Di Bisceglie, A.M.; Bodenheimer, H.C. Public policy committee of the american association for the study of liver disease. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology 2008, 47, 1363–1370. [Google Scholar] [CrossRef]
- Adler-Levy, Y.; Nardi-Schreiber, A.; Harris, T.; Shaul, D.; Uppala, S.; Sapir, G.; Lev-Cohain, N.; Sosna, J.; Goldberg, S.N.; Gomori, J.M.; et al. In-cell determination of lactate dehydrogenase activity in a luminal breast cancer model—Ex vivo investigation of excised xenograft tumor slices using dDNP hyperpolarized [1-13C]pyruvate. Sensors 2019, 19, 2089. [Google Scholar] [CrossRef]
| Mouse Number | Group | Injection Number | Number of Spectral Recordings Used for Rate Calculation | ρ [1-13C]Alanine | ρ [1-13C]Lactate |
|---|---|---|---|---|---|
| 1 | Healthy Liver | 1 a | 1 | 0.559 | 0.559 |
| 2 | 1 a,c | 2 | 0.276 | 0.276 | |
| 2 a | 2 | 0.292 | 0.292 | ||
| 3 a | 2 | 0.292 | 0.292 | ||
| 3 | 2 a | 1 d | 0.375 | 0.375 | |
| 3 a | 1 e | 0.375 | 0.375 | ||
| 4 | Heterogeneous Liver | 1 b | 5 | 0.062 | 0.058 |
| 2 b | 6 | 0.062 | 0.058 | ||
| 3 b | 2 | 0.062 | 0.058 | ||
| 5 | 1 b,c | 4 | 0.062 | 0.058 | |
| 2 b | 3 | 0.062 | 0.058 | ||
| 6 | 1 b | 2 | 0.062 | 0.058 | |
| 2 b | 2 | 0.062 | 0.058 | ||
| 7 | HCC | 1 b | 6 | 0.244 f | 0.217 f |
| 2 b | 8 | 0.244 f | 0.217 f | ||
| 8 | 1 b | 5 | 0.062 | 0.058 | |
| 2 b,c | 4 | 0.062 | 0.058 | ||
| 9 | 1 b | 1 | 0.062 | 0.058 | |
| 2 b | 1 | 0.062 | 0.058 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lev-Cohain, N.; Sapir, G.; Uppala, S.; Nardi-Schreiber, A.; Goldberg, S.N.; Adler-Levy, Y.; Sosna, J.; Gomori, J.M.; Katz-Brull, R. Differentiation of Heterogeneous Mouse Liver from HCC by Hyperpolarized 13C Magnetic Resonance. Sci 2021, 3, 8. https://doi.org/10.3390/sci3010008
Lev-Cohain N, Sapir G, Uppala S, Nardi-Schreiber A, Goldberg SN, Adler-Levy Y, Sosna J, Gomori JM, Katz-Brull R. Differentiation of Heterogeneous Mouse Liver from HCC by Hyperpolarized 13C Magnetic Resonance. Sci. 2021; 3(1):8. https://doi.org/10.3390/sci3010008
Chicago/Turabian StyleLev-Cohain, Naama, Gal Sapir, Sivaranjan Uppala, Atara Nardi-Schreiber, Shraga Nahum Goldberg, Yael Adler-Levy, Jacob Sosna, J. Moshe Gomori, and Rachel Katz-Brull. 2021. "Differentiation of Heterogeneous Mouse Liver from HCC by Hyperpolarized 13C Magnetic Resonance" Sci 3, no. 1: 8. https://doi.org/10.3390/sci3010008
APA StyleLev-Cohain, N., Sapir, G., Uppala, S., Nardi-Schreiber, A., Goldberg, S. N., Adler-Levy, Y., Sosna, J., Gomori, J. M., & Katz-Brull, R. (2021). Differentiation of Heterogeneous Mouse Liver from HCC by Hyperpolarized 13C Magnetic Resonance. Sci, 3(1), 8. https://doi.org/10.3390/sci3010008





