Validation of a Sapphire Gas-Pressure Cell for Real-Time In Situ Neutron Diffraction Studies of Hydrogenation Reactions
Abstract
:1. Introduction and Nomenclature
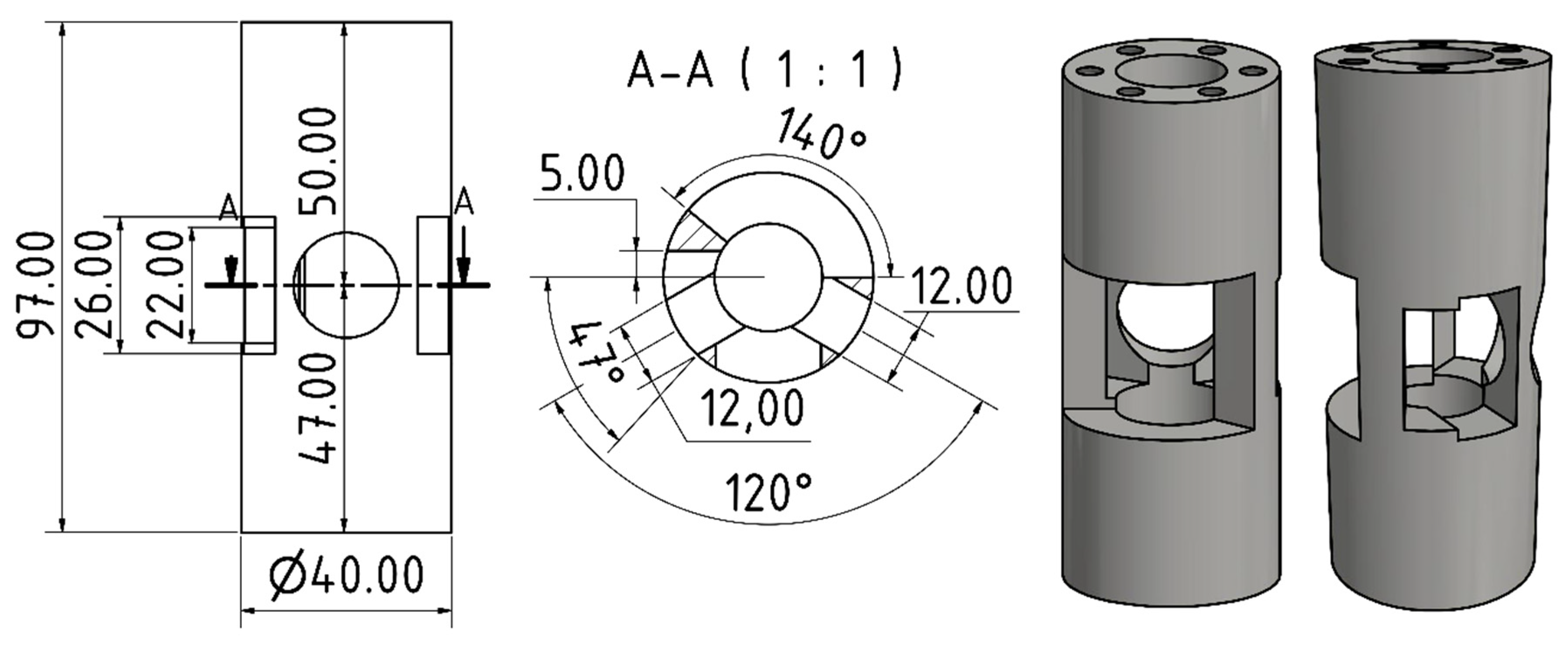
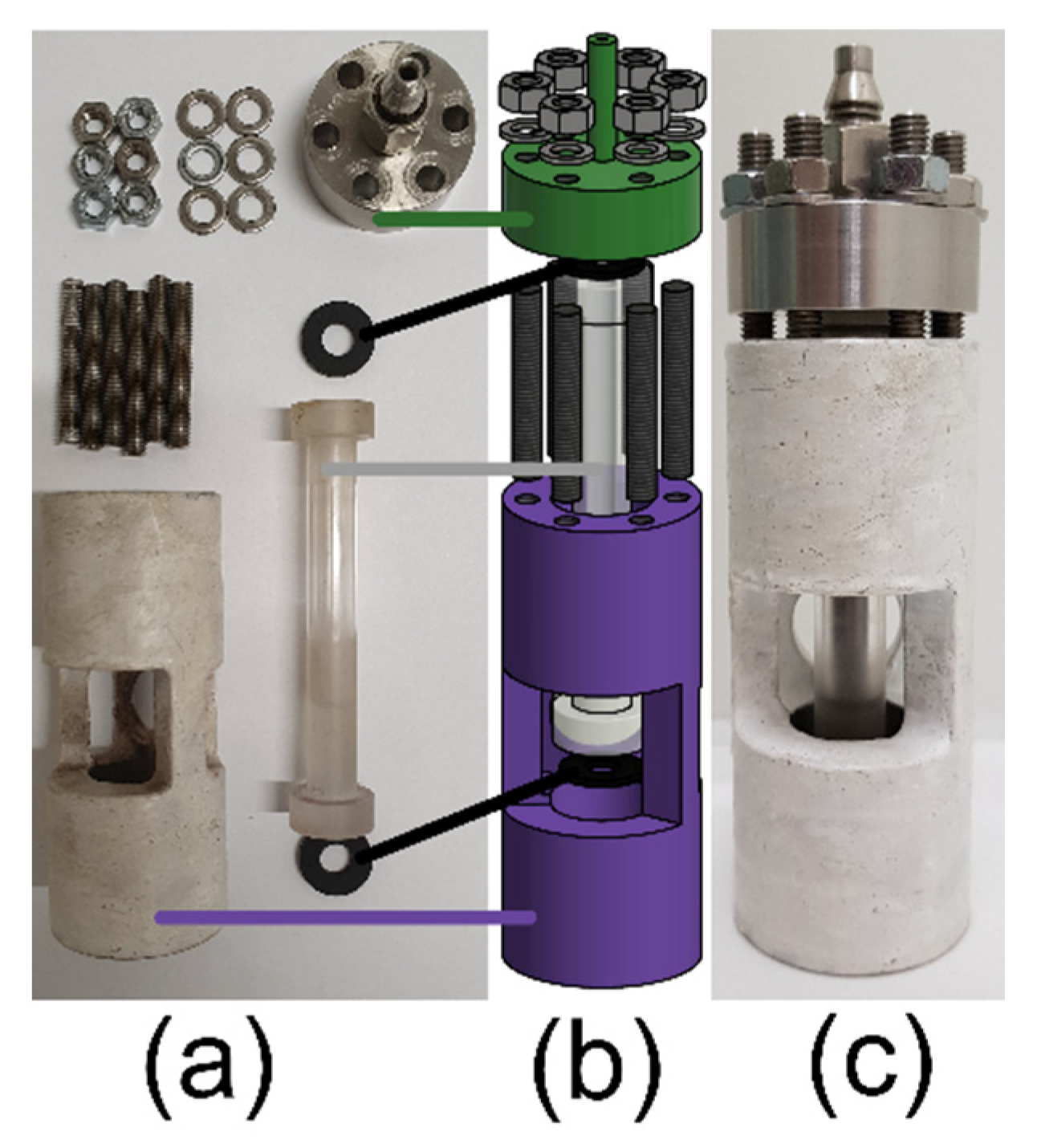
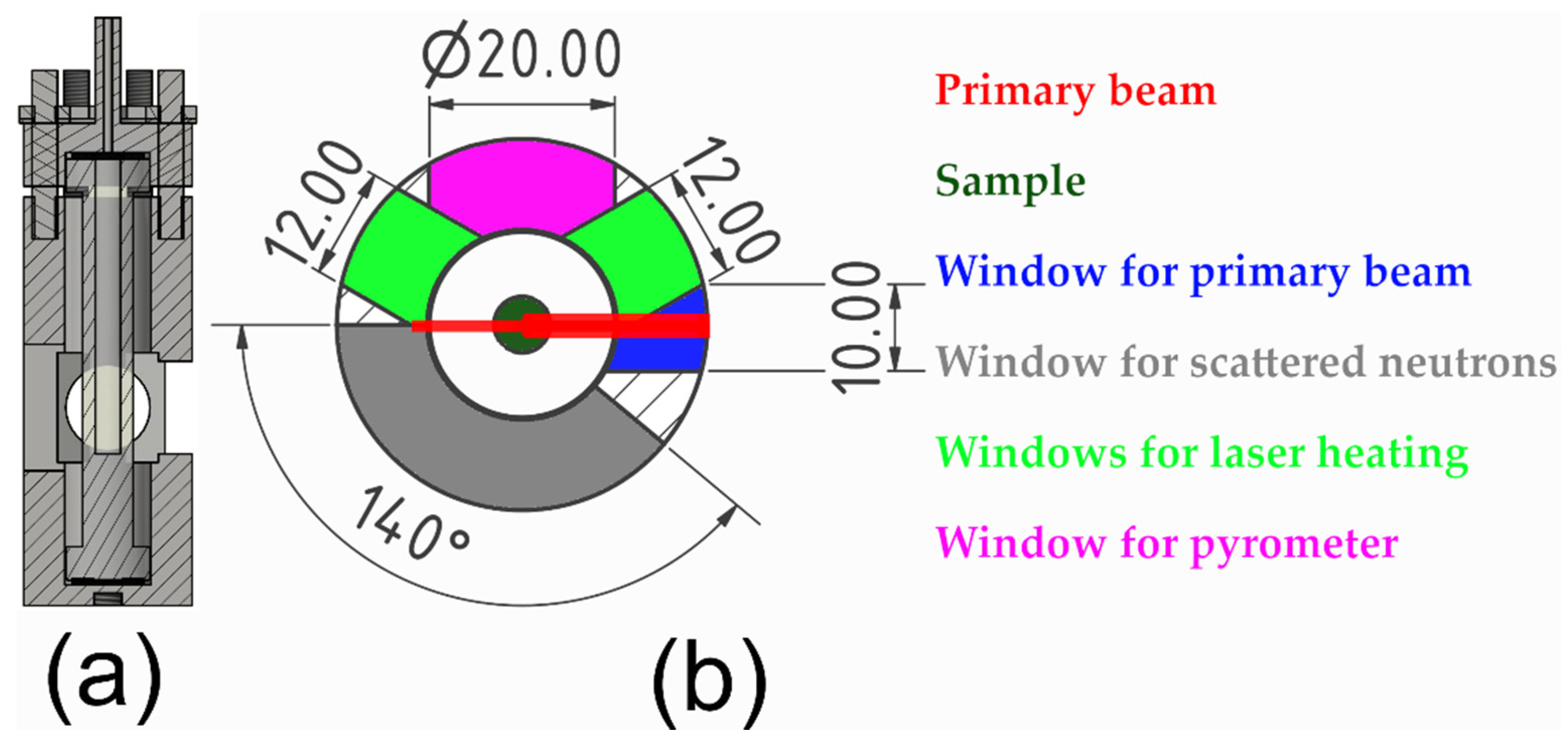
2. Assembly and Use of the Single-Crystal Gas-Pressure Cell (Type II)
3. Validation of the Type II Sapphire Gas-Pressure Cell
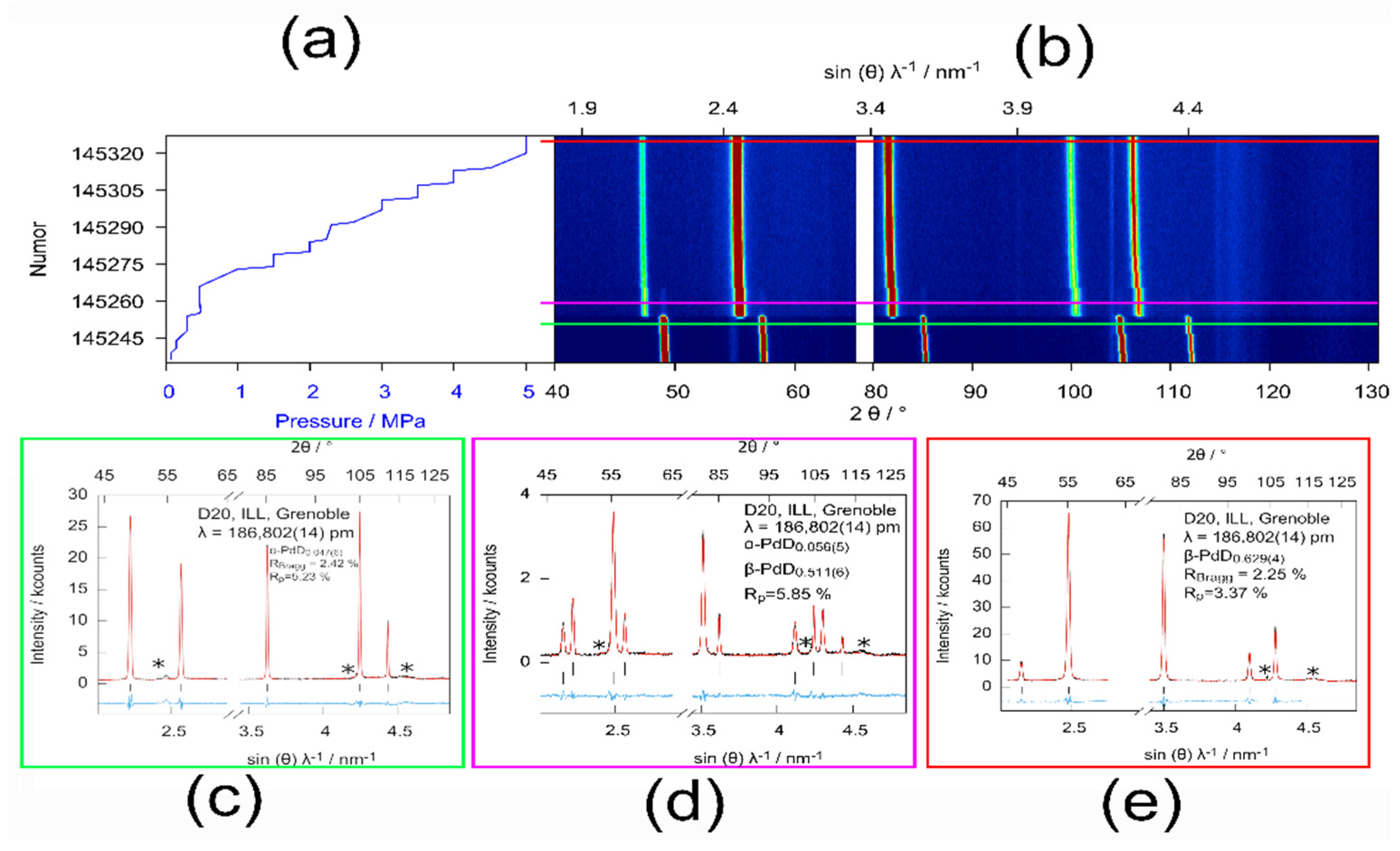
3.1. Experimental Details
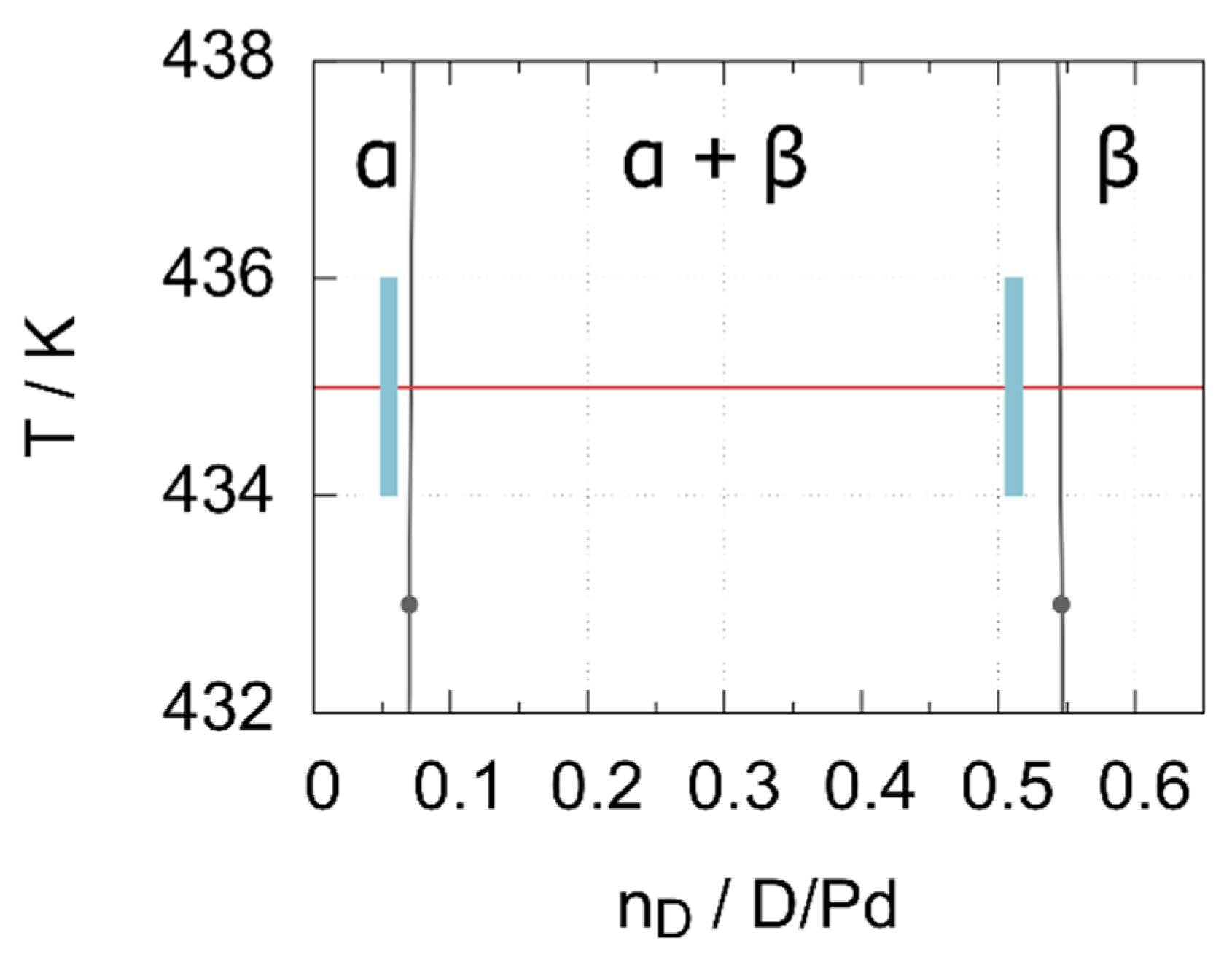
3.2. Results and Discussion
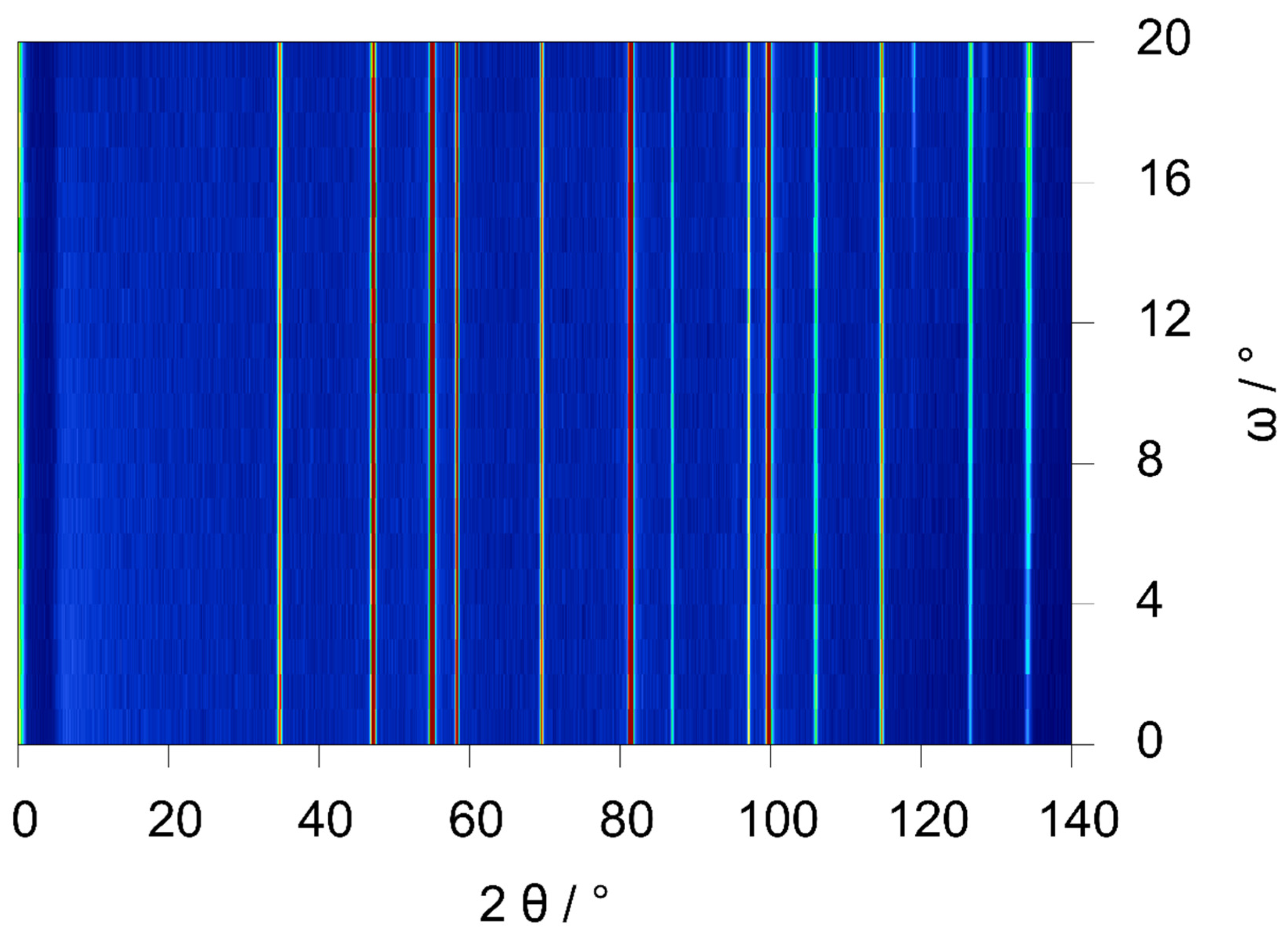
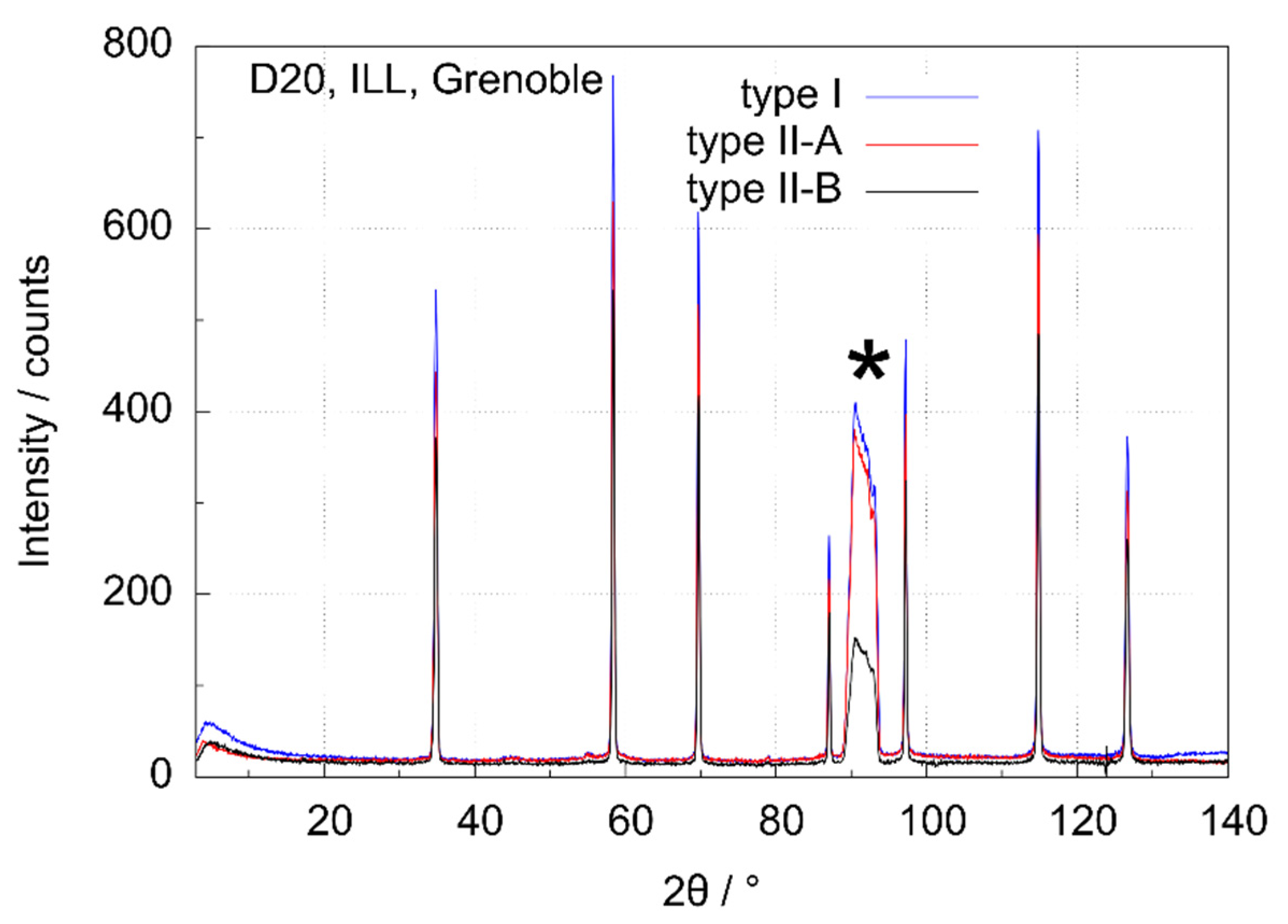
3.3. Data Quality of the Type I and Type II Cell
- The same sample (417.4(1) mg of silicon powder, (Johnson and Matthey, 325 mesh, Alfa Products, Karlsruhe, Germany), filling height 20 mm) was used for all measurements.
- The same sample holder in the same orientation has been used for all measurements, i.e., contributions from the sample holder were identical.
- All measurements were performed using the same diffractometer setup and were performed within 24 h.
4. Further Developments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Hassan, Z.; Amir, A.; Selvaraj, J.; Rahim, N.A. A review on current injection techniques for low-voltage ride-through and grid fault conditions in grid-connected photovoltaic system. Sol. Energy 2020, 207, 851–873. [Google Scholar] [CrossRef]
- Kougias, I.; Aggidis, G.; Avellan, F.; Deniz, S.; Lundin, U.; Moro, A.; Muntean, S.; Novara, D.; Pérez-Díaz, J.I.; Quaranta, E.; et al. Analysis of emerging technologies in the hydropower sector. Renew. Sust. Energ. Rev. 2019, 113, 109257. [Google Scholar] [CrossRef]
- International Energy Agency. World Energy Outlook; International Energy Agency: Paris, France, 2017. [Google Scholar]
- Bundesministerium für Wirtschaft und Energie. Die Nationale Wasserstoffstrategie; Bundesministerium für Wirtschaft und Energie: Berlin, Germany, 2020. [Google Scholar]
- Elberry, A.M.; Thakur, J.; Santasalo-Aarnio, A.; Larmi, M. Large-scale compressed hydrogen storage as part of renewable electricity storage systems. Int. J. Hydrog. Energy 2021, 46, 15671–15690. [Google Scholar] [CrossRef]
- Singh, R.; Altaee, A.; Gautam, S. Nanomaterials in the advancement of hydrogen energy storage. Helyion 2020, 6, e04487. [Google Scholar] [CrossRef]
- Reilly, J.J.; Wiswall, R.H. Formation and properties of iron titanium hydride. Inorg. Chem. 1974, 13, 218–222. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, S.; Lu, H.; Wu, J.; Yan, K.; Cheng, H.; Liu, J. Stability of LaNi5-Co alloys cycled in hydrogen—Part 1 evolution in gaseous hydrogen storage performance. Int. J. Hydrog. Energy 2019, 44, 15159–15172. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrog. Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Boateng, E.; Chen, A. Recent advances in nanomaterial-based solid-state hydrogen storage. Mater. Today Adv. 2020, 6, 100022. [Google Scholar] [CrossRef]
- Jain, I.P.; Jain, P.; Jain, A. Novel hydrogen storage materials: A review of lightweight complex hydrides. J. Alloys Compd. 2010, 503, 303–339. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, P.; Niu, M.; Maddy, J. The survey of key technologies in hydrogen energy storage. Int. J. Hydrog. Energy 2016, 41, 14535–14552. [Google Scholar] [CrossRef]
- Hansen, T.C.; Kohlmann, H. Chemical Reactions followed by in situ Neutron Powder Diffraction. Z. Anorg. Allg. Chem. 2014, 640, 3044–3063. [Google Scholar] [CrossRef]
- Cordova, D.L.M.; Johnson, D.C. Synthesis of Metastable Inorganic Solids with Extended Structures. Chem. Phys. Chem. 2020, 21, 1345–1368. [Google Scholar] [CrossRef]
- Peterson, V.K.; Auckett, J.E.; Pang, W.-K. Real-time powder diffraction studies of energy materials under non-equilibrium conditions. IUCrJ 2017, 4, 540–554. [Google Scholar] [CrossRef]
- Kohlmann, H. Looking into the Black Box of Solid-State Synthesis. Eur. J. Inorg. Chem. 2019, 4174–4180. [Google Scholar] [CrossRef] [Green Version]
- Kohlmann, H. Solid-Gas Reactions in Synthetic Chemistry: What can we learn from reaction pathways? Russ. Chem. Rev. 2020, 89, 275–280. [Google Scholar] [CrossRef]
- Finger, R.; Kurtzemann, N.; Hansen, T.C. Design and use of a sapphire single-crystal gas-pressure cell for in situ neutron diffraction. J. Appl. Crystallogr. 2021, 54, 839–846. [Google Scholar] [CrossRef]
- Shull, C.G.; Wollan, E.O.; Morton, G.A.; Davidson, W.L. Neutron Diffraction Studies of NaH and NaD. Phys. Rev. 1948, 73, 842–847. [Google Scholar] [CrossRef]
- Yvon, K. Structural aspects of ternary metal hydrides: A critical review. J. Less. Common. Met. 1984, 103, 53–70. [Google Scholar] [CrossRef]
- Götze, A.; Auer, H.; Finger, R.; Hansen, T.C.; Kohlmann, H. A sapphire single-crystal cell for in situ neutron powder diffraction of solid-gas reactions. Phys. B Amst. Neth. 2018, 551, 395–400. [Google Scholar] [CrossRef]
- Kohlmann, H.; Kurtzemann, N.; Hansen, T.C. Metal hydride formation in palladium and palladium rich intermetallic compounds studied by in situ neutron diffraction. Powder Diffr. 2013, 28, S242–S255. [Google Scholar] [CrossRef]
- Widenmeyer, M.; Niewa, R.; Hansen, T.C.; Kohlmann, H. In situ Neutron Diffraction as a Probe on Formation and Decomposition of Nitrides and Hydrides: A Case Study. Z. Anorg. Allg. Chem. 2013, 639, 285–295. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Amst. Neth. 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Pitt, M.P.; Gray, E.M. Tetrahedral occupancy in the Pd-D system observed by in situ neutron powder diffraction. Europhys. Lett. 2003, 64, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Wicke, E.; Blaurock, J. New experiments on and interpretations of hysteresis effects of Pd-D2 and Pd-H2. J. Less. Common. Met. 1987, 130, 351–363. [Google Scholar] [CrossRef]
- Többens, D.M.; Stüßer, N.; Knorr, K.; Mayer, H.M.; Lampert, G. E9: The New High-Resolution Neutron Powder Diffractometer at the Berlin Neutron Scattering Center. Mater. Sci. Forum. 2001, 378, 288–293. [Google Scholar] [CrossRef]

| Wall Thickness/mm | Gas Pressure/MPa | T/K |
|---|---|---|
| 3 | 0.1 air | 571 |
| 3 | 2.5 H2 | 524 |
| 3 | 5.0 H2 | 435 |
| 3 | 15.0 H2 | 298 |
| 2 | 0.1 air | 524 |
| 2 | 15.0 H2 | 298 |
| 1 | 0.1 air | 718 |
| 1 | 9.5 H2 | 298 |
| 1 | 9.5 H2 | 584 |
| Phase | p(D2)/MPa | Lattice Parameter/Å | Deuterium Content x | Biso(Pd)/Å2 | Biso(D)/Å2 |
|---|---|---|---|---|---|
| α-PdDx | 0.3 | 3.91016(19) | 0.047(6) | 1.17(5) | 4(1) |
| α-PdDx | 0.5 | 3.91285(13) | 0.056(5) | 0.97(8) | 4 * |
| β-PdDx | 0.5 | 4.03114(15) | 0.512(6) | 1.24(6) | 3.9(1) |
| β-PdDx | 5.0 | 4.05067(22) | 0.629(4) | 1.44(4) | 4.54(6) |
| hkl | Position 2θ/° | RI Type I | RI Type II-A | RI Type II-B |
|---|---|---|---|---|
| 111 | 34.80 | 27.3(2) | 24.5(2) | 23.8(3) |
| 220 | 58.35 | 35.0(3) | 29.7(2) | 32.1(3) |
| 311 | 69.70 | 32.3(3) | 26.8(3) | 29.7(4) |
| 400 | 87.10 | 10.5(1) | 8.7(1) | 10.3(2) |
| 331 | 97.25 | 18.9(2) | 16.0(2) | 18.6(2) |
| 422 | 114.85 | 30.5(2) | 25.2(0) | 29.8(3) |
| 511/333 | 126.65 | 15.6(3) | 15.5(2) | 15.2(2) |
| Type | Average Low Angle Background/Counts (5° ≤ 2θ ≤ 25°) | Average High Angle Background/Counts (130° ≤ 2θ ≤ 140°) |
|---|---|---|
| I | 29.8 | 24.5 |
| II-A | 21.4 | 17.4 |
| II-B | 21.0 | 16.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finger, R.; Hansen, T.C.; Kohlmann, H. Validation of a Sapphire Gas-Pressure Cell for Real-Time In Situ Neutron Diffraction Studies of Hydrogenation Reactions. Quantum Beam Sci. 2021, 5, 22. https://doi.org/10.3390/qubs5030022
Finger R, Hansen TC, Kohlmann H. Validation of a Sapphire Gas-Pressure Cell for Real-Time In Situ Neutron Diffraction Studies of Hydrogenation Reactions. Quantum Beam Science. 2021; 5(3):22. https://doi.org/10.3390/qubs5030022
Chicago/Turabian StyleFinger, Raphael, Thomas C. Hansen, and Holger Kohlmann. 2021. "Validation of a Sapphire Gas-Pressure Cell for Real-Time In Situ Neutron Diffraction Studies of Hydrogenation Reactions" Quantum Beam Science 5, no. 3: 22. https://doi.org/10.3390/qubs5030022
APA StyleFinger, R., Hansen, T. C., & Kohlmann, H. (2021). Validation of a Sapphire Gas-Pressure Cell for Real-Time In Situ Neutron Diffraction Studies of Hydrogenation Reactions. Quantum Beam Science, 5(3), 22. https://doi.org/10.3390/qubs5030022







