1. Introduction
During the building of a structure, concrete is utilized to give strength, applicability, and durability. Due to its exceptional qualities, concrete is a dependable and long-lasting material choice for both commercial and residential structures. However, the usability of concrete is highly dependent on its quality in terms of strength [
1]. Gaining concrete strength is a rather complex process. Different internal and external processes have an impact on the concrete’s increase in strength. All of this is relevant to the long-term functionality of concrete during the course of its life cycle [
2]. Throughout its lifetime of use, a concrete structure should be secure, reliable, usable, and sustainable [
3]. To protect structures from collapsing, stability represents a fundamental challenge in solid mechanics that must be solved [
4,
5,
6]. Generally speaking, concrete structures are anticipated to perform satisfactorily for the duration of their service life with minimal maintenance. However, due to things such as concrete deterioration, overburden, or physical damage, such expectations are typically not met [
7]. The quality of the raw materials, the proportion of water to cement, the coarseness and fineness of the aggregate, the age of the concrete, the degree of compaction, the temperature, the relative humidity, and the curing process all have an impact on the strength of the concrete [
8]. Therefore, it is crucial to keep moisture loss under control throughout the entire hardening process, whereby the process of controlling the rate and amount of moisture loss from concrete during cement hydration is known as curing [
9,
10]. Curing is accomplished by continually misting the exposed surface to stop moisture loss.
In this regard, water is an essential ingredient in the processes of hardening and preparing concrete. Unfortunately, while being an essential component, water quality should always be checked before being used in the process of making concrete. According to the study conducted by Nikhil [
11], the findings show that using potable water improves the strength qualities of concrete and increases compressive strength by 33.34% when compared to using sewage water. That is to say, the pH of the water used to prepare concrete is one of the most crucial water quality parameters to be examined [
12]. It should also be noted that the main causes of damage to concrete in all its forms worldwide are moisture and pH fluctuations [
13]. Therefore, it is significantly important to investigate the potential of pH in the initial mixing water to provide the construction industry with a piece of crucial information for the production of high-quality concrete.
Due to the porous nature of concrete, moisture can rather quickly infiltrate the material, changing the pH and hastening the carbonation process. The pH scale has a range of 0 to 14, with a neutral or “basic” pH of 7. Acidity is indicated by numbers less than 7, and the lower the value, the more acidic. Contrarily, a reading greater than 7 indicates that the substance being measured is alkaline, and the closer readings are to 14, the more alkaline is the substance [
14]. Portland cement, the binding component in concrete, has a pH of 12, and if this pH varies too much, the cement loses its ability to bond as it is broken down [
15]. As a result, the concrete will develop more pores and cracks, significantly speeding up the process and causing an increase in issues and deterioration. There may be problems with pH and moisture levels when it comes to the making of concrete [
16]. The pH level of the concrete mix can change depending on the location’s water, and this issue can be sped up if too much water is utilized during the concrete-making process. Concrete will show visible disintegration and surface damage if the pH falls below 7, as the cement will no longer be able to hold the concrete together. If ignored, it will keep getting more acidic, which will eventually cause the concrete to lose its structural integrity and require replacement [
17].
Temperature is another essential factor, in addition to pH, that governs how concrete gains strength over time [
18,
19]. Unfortunately, little to no effort is made to look at how internal temperature may affect concrete strength increase; the majority of temperature-related studies on concrete are based on external temperature. It should also be noted that Delayed Ettringite Formation is more likely to occur when internal concrete temperatures are high (DEF). DEF is a material-related problem that may result in significant cracking [
20,
21].
The strength and maturity of concrete at any given time are two of the most crucial factors to understand during a construction process [
22,
23]. This information has previously been gathered by contractors through protracted break tests conducted in laboratories, but in recent years, new technology has made it possible for contractors to acquire concrete strength and maturity in place and in realtime [
24,
25]. Many issues that plagued the engineering and construction sectors for a while have been resolved thanks to smart technology, including challenges with data compilation, data sharing, and time-consuming data collection procedures. In that matter, the use of monitoring sensors in concrete has gained more interest in the recent past [
9,
26,
27].
The timing of these technological developments could not be more ideal as the complexity and cost of construction projects expand globally. The early age strength growth of concrete, which is closely correlated with the cementitious paste’s history of hydration temperature, can be predicted using in-situ concrete maturity data [
28]. Contractors may incur costs due to inaccurate data on concrete maturity and strength. Contractors either act prematurely by removing formwork and moving on to the next stage of the project before optimal results have been achieved, wasting valuable time and lengthening the project timeframe, or they wait too long for results.
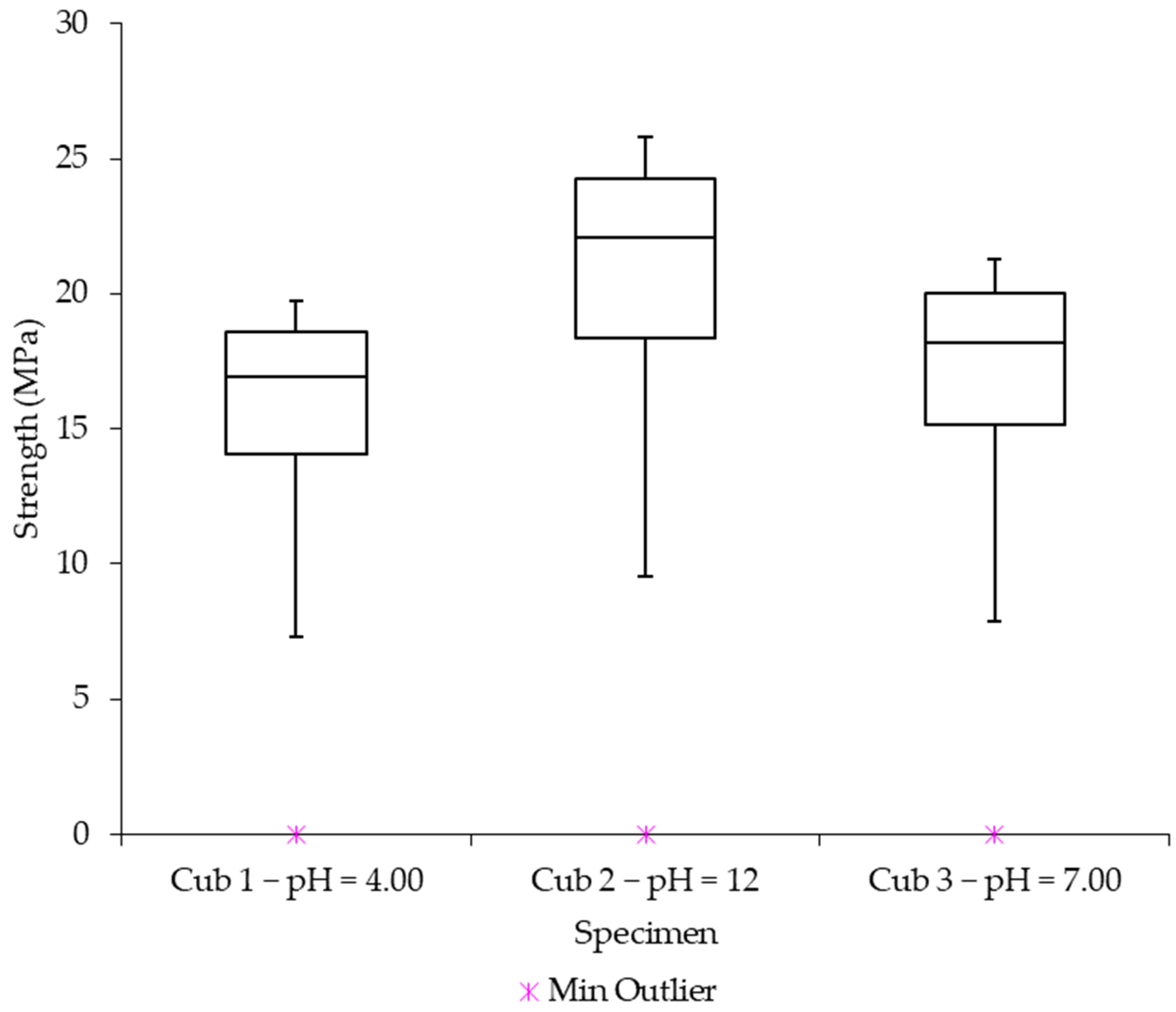
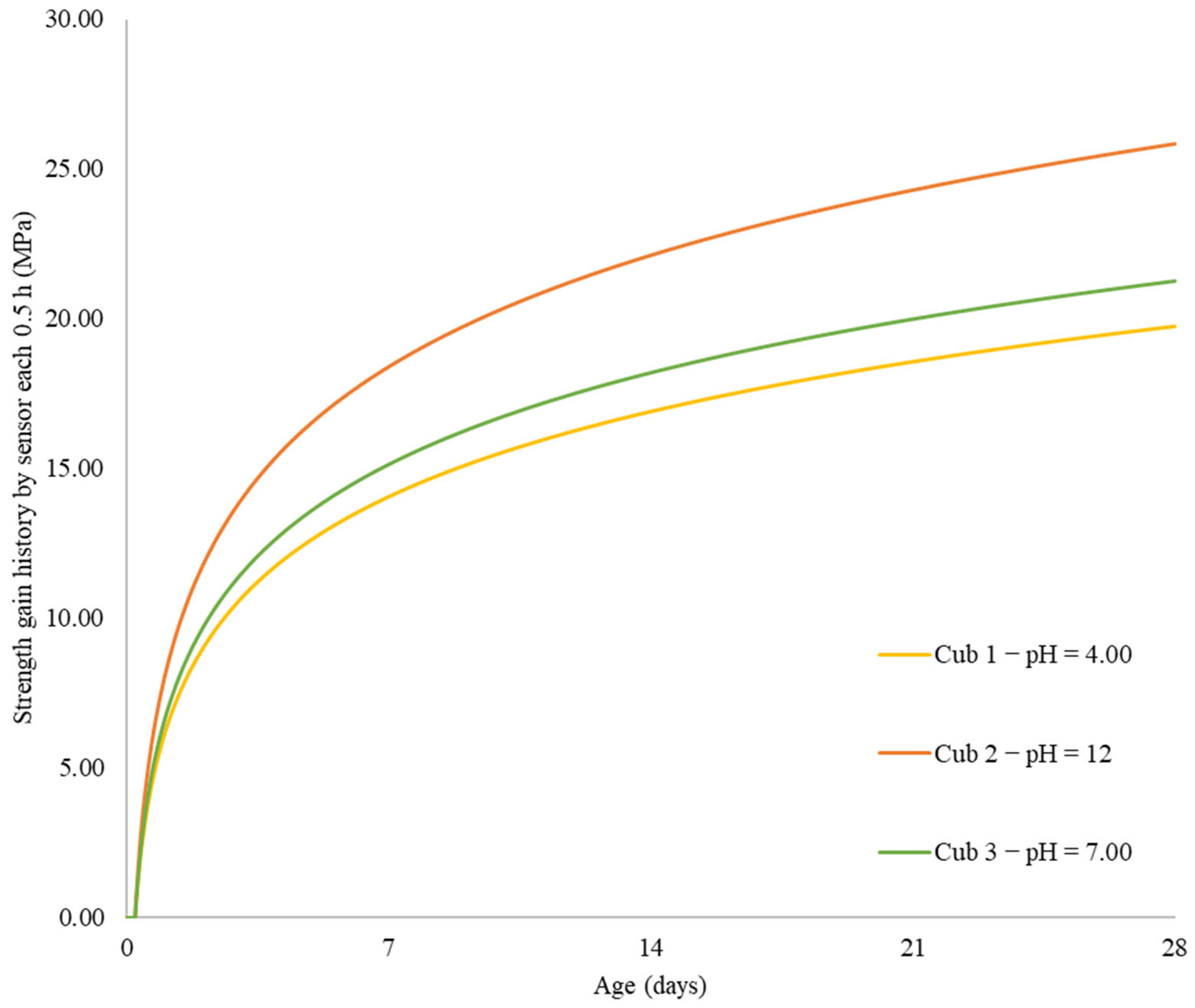
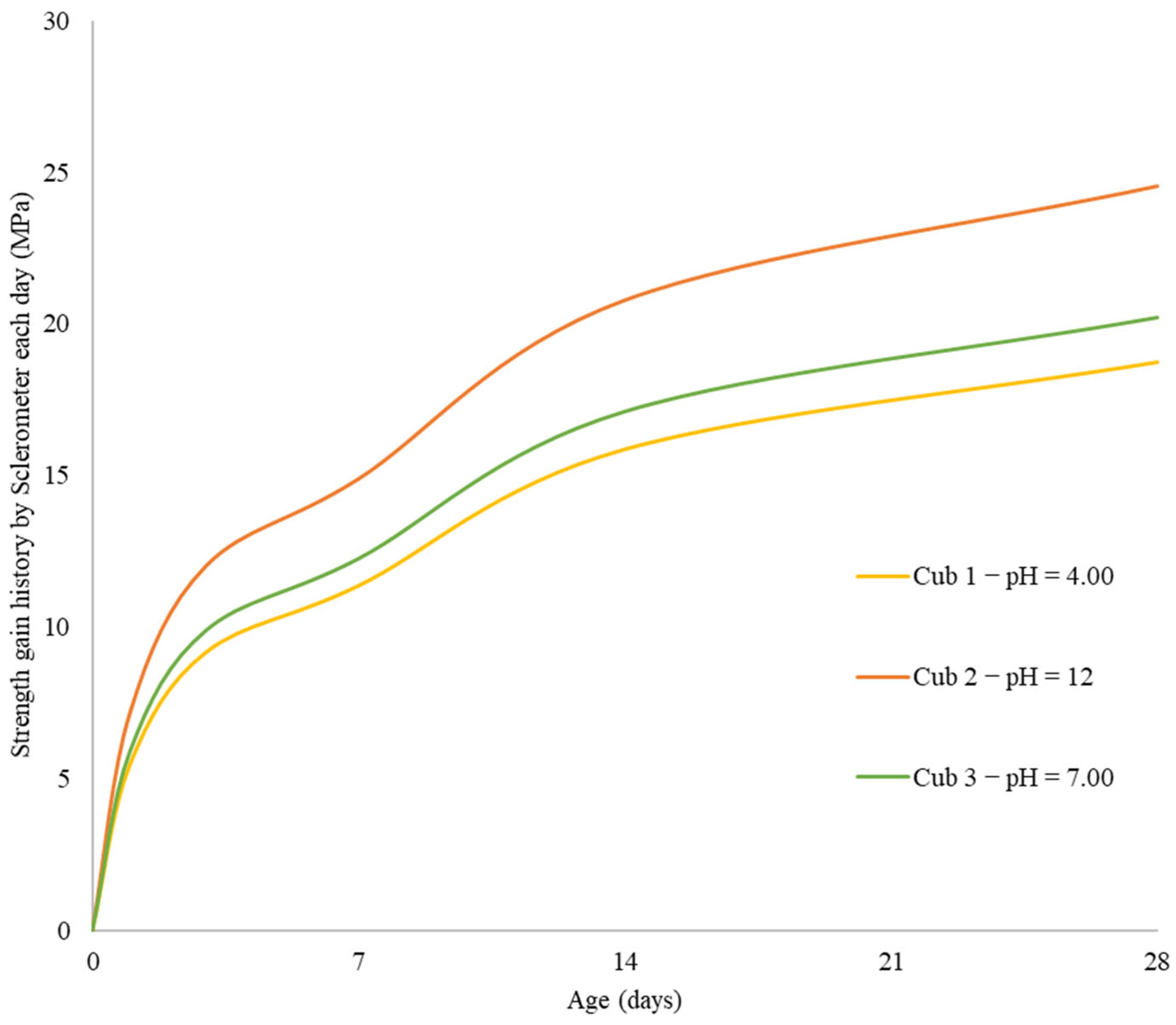
In this work, mechanical-electrical sensors are used to examine any potential impacts of the initial water pH on the concrete strength gain. Utilizing three different pH values, the effect of initial pH on the strength-gain process was examined (4.0, 7.0, and 12). The interior temperature, strength increase, and pH changes over time are the main factors evaluated. A number of statistical methods, such as Single-way Analysis of Variance, Scheffé’s method, and Correlation Matrixes, are used to further analyze the problem.
4. Discussion
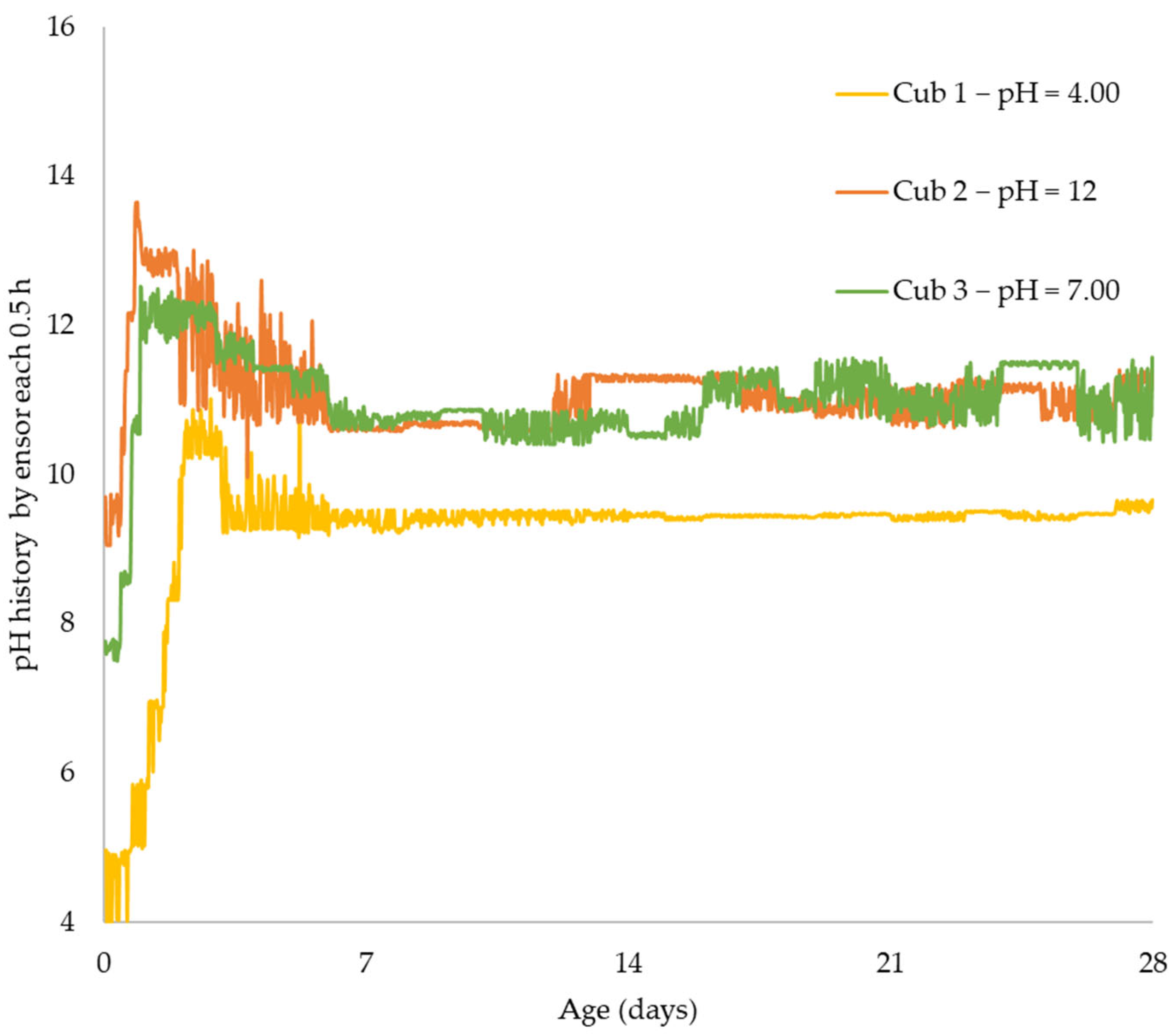
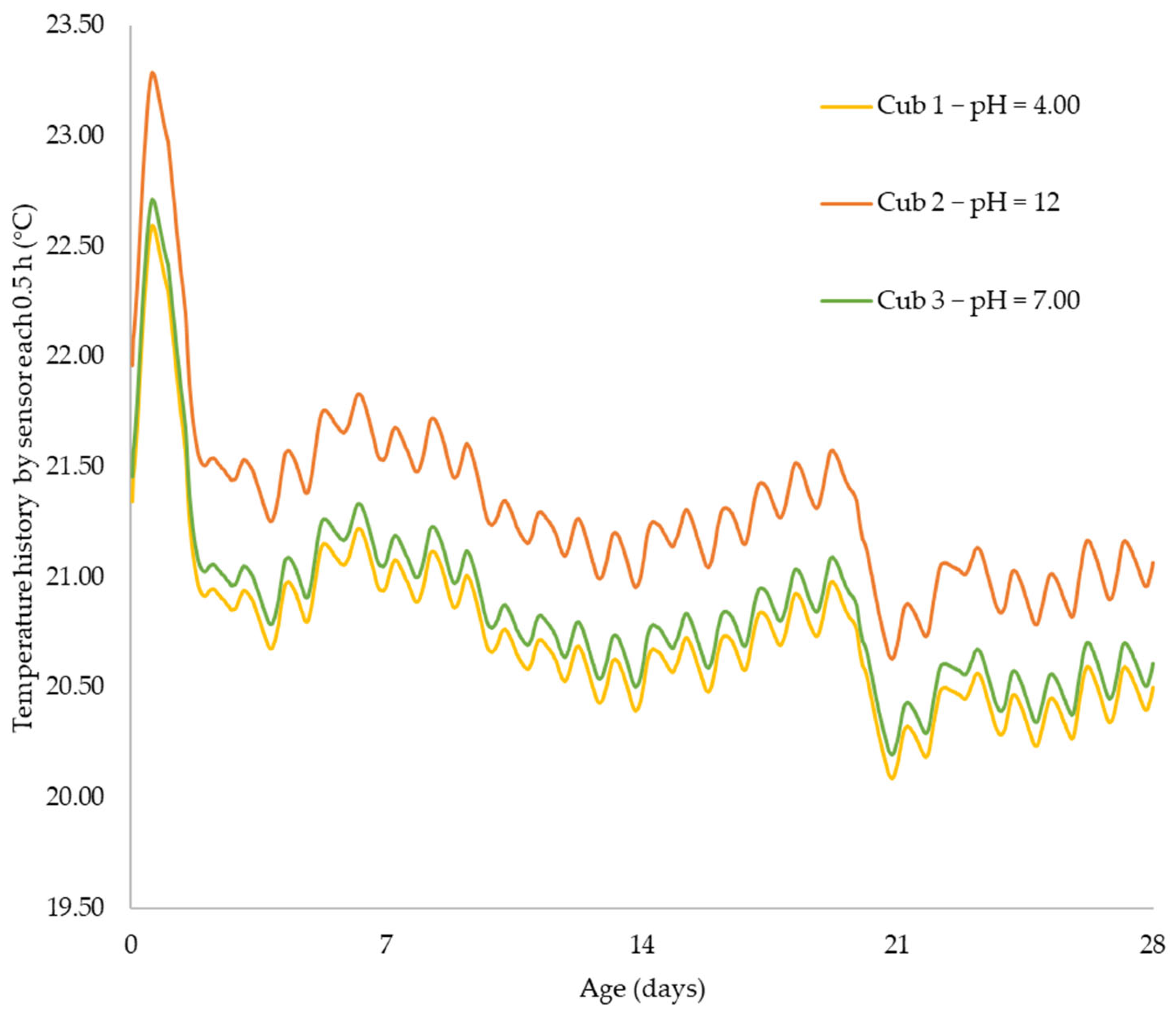
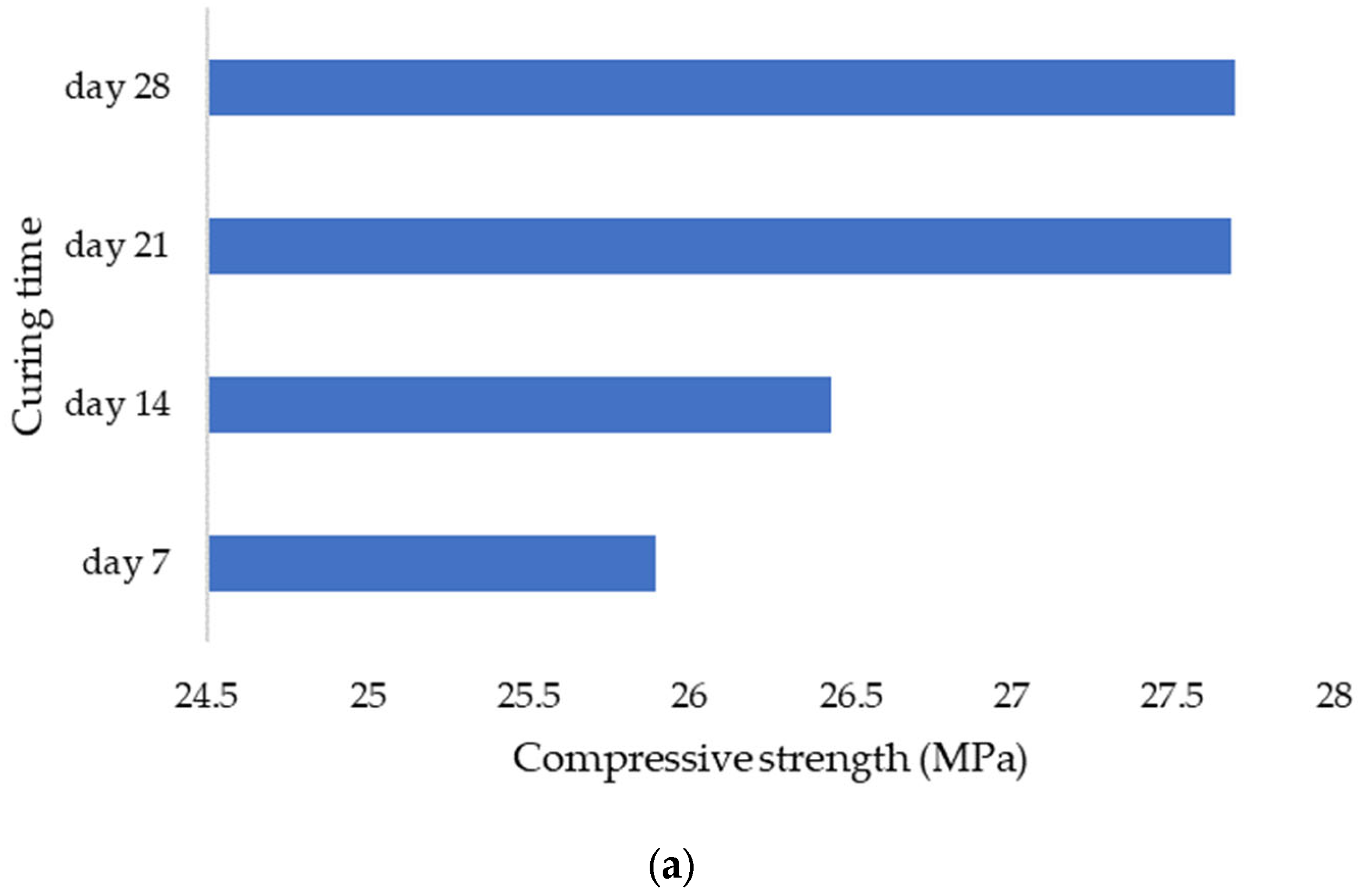
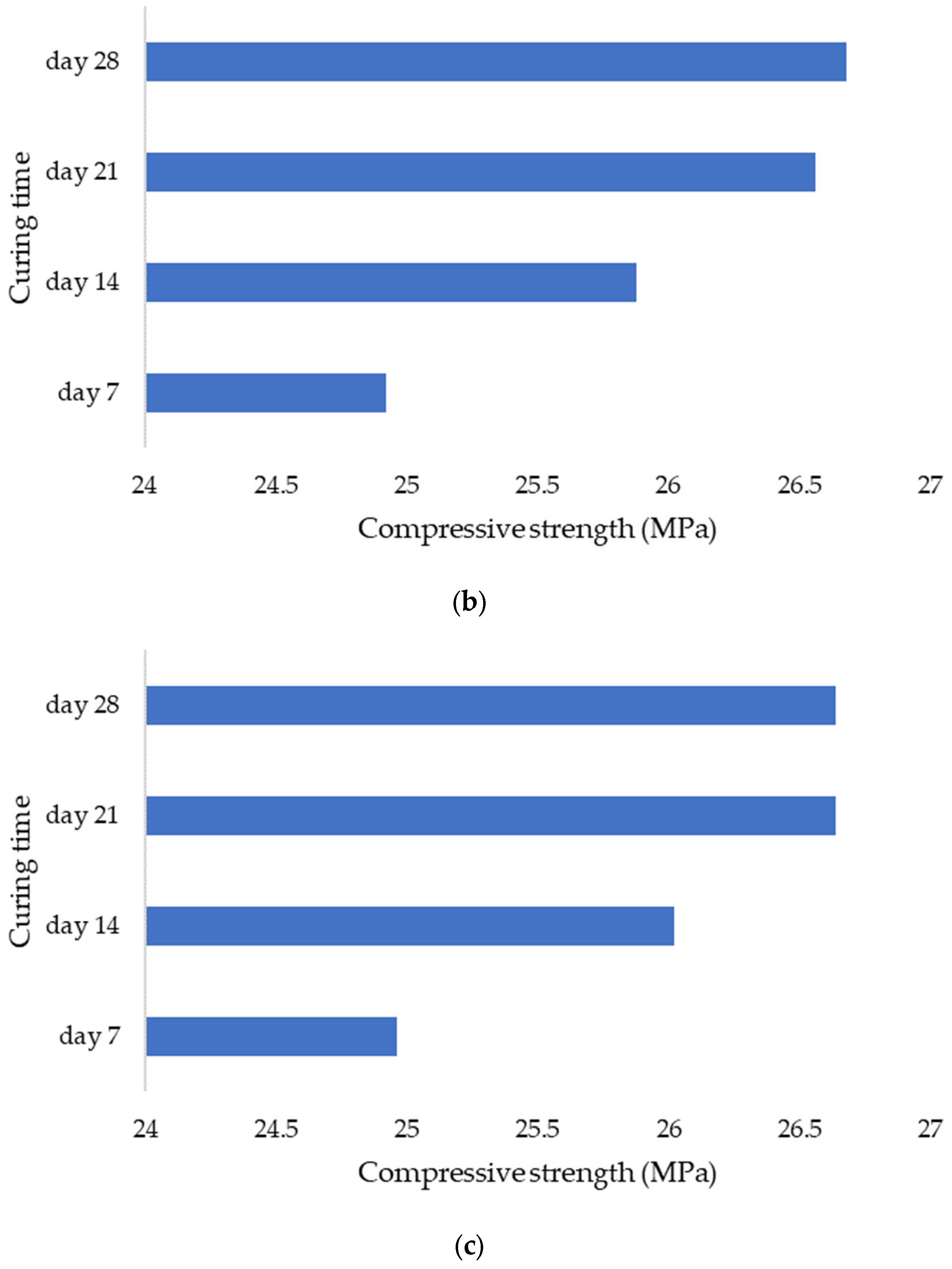
Using sensors and a sclerometric test, the potential impact of the initial water pH on the increase in concrete strength was examined in this study. Utilizing three different pH values, the effect of initial pH on the strength-gain process was examined (4.0, 7.0, and 12). The main factors investigated were changes in pH over time, body temperature, and strength increase. All of the samples’ pH levels were seen to dramatically rise in the early days of the experiment before gradually falling over time. That is to say, the concrete still goes through chemical reactions over time, changing the overall pH in the concrete in addition to the starting pH level given by the pH in the mixing water. The temperature data also revealed a similar phenomenon. As previously highlighted, the main cause of the pH of new concrete being between 12 and 13 is normally the presence of calcium hydroxide, which is typically a byproduct of cement hydration. Through a process known as carbonation, the pH of a concrete surface gradually decreases to approximately 8.5 when it reacts with the carbon dioxide in the air. For the installation of flooring and the performance of adhesives, a dry, typically carbonated concrete surface is desirable. Flooring failures can result from floor coverings being damaged and adhesives being broken down on a surface with a high pH and lots of moisture.
The temperature and strength gain data from the pH 4.0, 7.0, and 12 samples were subjected to the Single-factor Analysis of Variance. The temperature data from the investigated pH levels showed statistically significant differences with a
p-value of 2.4 × 10
−261, which is less than 0.05 (alpha-value). The strength data for the investigated pH levels had a
p-value of 2.9 × 10
−168, which is less than 0.05 (alpha-value), suggesting that the data variances are statistically significant. It is also worth noting that ANOVA is used to evaluate for differences between three or more population mean values. It permits numerous comparisons while maintaining a predetermined degree of risk for a type I error (rejection of the correct null hypothesis). In an ANOVA, the variance estimates owing to chance components alone and chance factors plus the treatment effect are compared (if there is a treatment effect) [
46]. We may therefore conclude from the retrieved
p-values that variations in the pH of the initial mixing water caused noticeably varied strength gain results, whereby we may also draw the conclusion that variations in the pH of the initial mixing water can significantly affect the concrete strength gain during the curing process. Similar observations are made in the literature; according to the study conducted by Wei et al. [
47], which focused on the low-pH self-compacting concrete’s engineering characteristics for concrete plugs; it was observed that the compressive strength of mixture reached up to 61.66 MPa when the pH value was 10.6 after 90 days. The phenomenon highlights the impact of pH on a concrete’s overall strength increase over time.
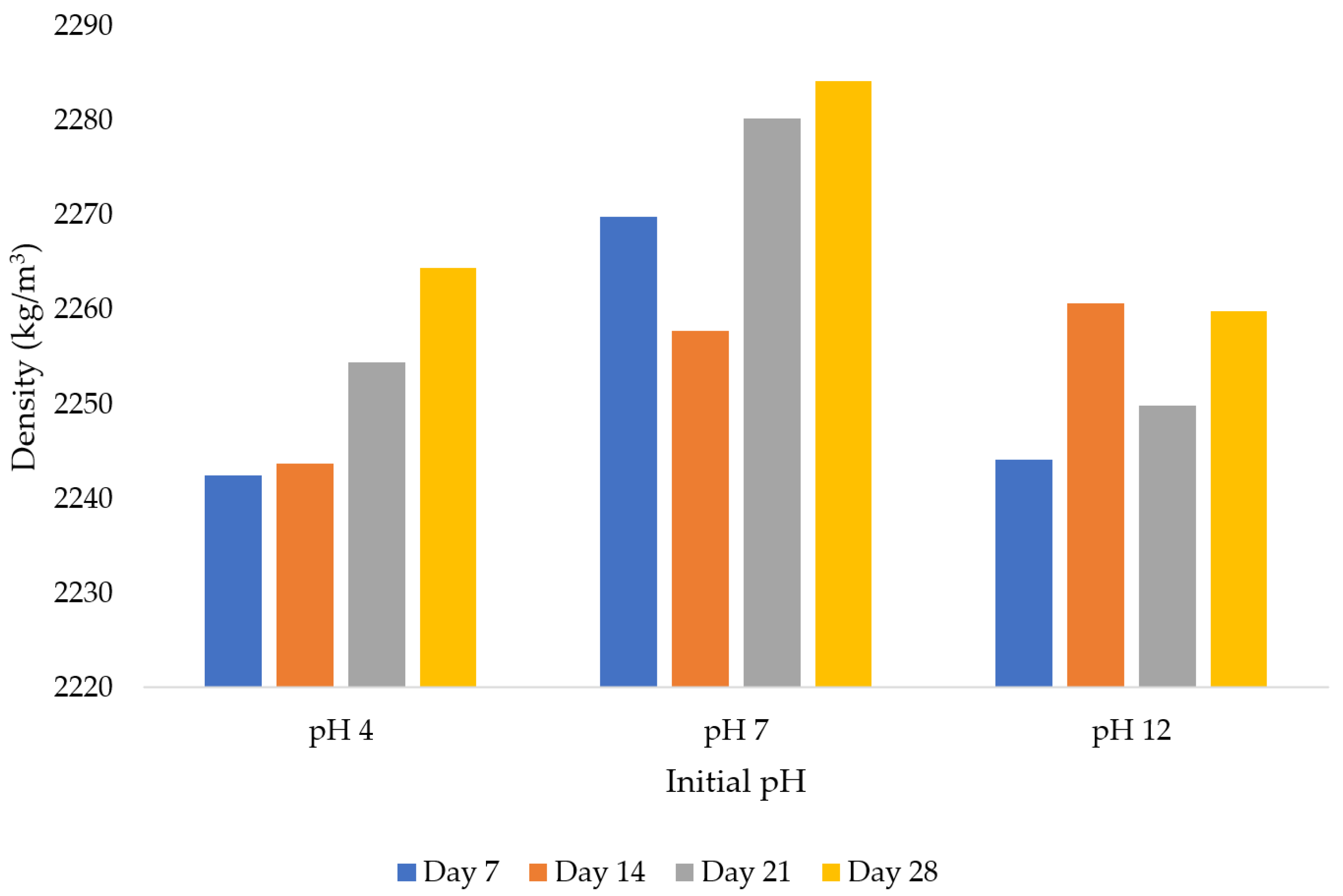
The study also looked into concrete density as a significant element. It is important to note that, concrete’s density significantly influences its mechanical characteristics. Greater strength and fewer voids and porosities are often benefits of dense concrete. Concrete is less vulnerable to water and soluble substances because of the minimal number of voids in the material. It was then observed that the density was 2242.4 kg/m
3 when pH = 4 from day 7. The recorded concrete density in this study at pH = 4 on day 7 is 1.21% smaller than the density attained at pH = 7 on the same day (2269.8 kg/m
3). Moreover, the combination of pH = 12 and day 7 yielded an average density of 2244.1 kg/m
3. According to the findings, the initial mixing water’s pH was near neutral when the highest density was recovered. Similar to the pH = 4 and pH = 12 combinations, pH = 7 and day 21 yielded an average density of 2280.2 kg/m
3, which is roughly 1.13% higher than those of pH = 4 and 1.33% higher than those of pH = 12. However, as previously highlighted there were no discernible variations in the densities obtained from the samples. Moreover, in the literature, it is reported that, typically, barite, magnetite, or hematite aggregates are used to increase concrete density. Concrete has a density of approximately 3400 kg/m
3 when barite aggregate is used, which is 45% denser than regular concrete; 3500 kg/m
3 when magnetite aggregate is used, which is 50% denser than regular concrete; and 3600 kg/m
3 when hematite aggregate is used, which is 53% more dense [
48].
In addition, as previously highlighted, the cumulative intruded pore volume was used to estimate the distribution of pores with respect to pH values. The results of the pore distribution were examined using three different pore diameters: 10, 10–100, and 100–200 μm. Pore diameters less than 10 μm grew when pH levels rose. However, there was a reduction in the distribution of pores between 100 and 200 μm and between 10 and 100 μm. According to a study by Sun et al. [
49], adding alkaline water to concrete can reduce its overall porosity and boost its strength by 21%. On concrete, porosity can have a variety of impacts. Concrete that contains hollow (air-filled) spaces is said to have porosity. Some of these voids might be linked together or not. Generally speaking, the strength of concrete will decrease as porosity increases. This is significant when thinking about permeability. Concrete will often be more permeable if it has more pores. This is crucial in colder locations because water expands up to 9% when it freezes [
50]. If the pores are totally saturated, water may produce cracking or pop-outs as it seeps into the concrete’s pores and freezes. In order to make up for this, the air-entrained admixture is used to create sphere-shaped bubbles that provide a space inside the concrete where the water can expand. Additionally, reinforced concrete may be damaged by water and air penetration due to the linked pores in concrete. The corrosion process can be accelerated by chloride ions’ ability to penetrate [
51]. Additionally, carbonation can happen because concrete is a porous substance. This reaction occurs as water in the pores of the concrete and calcium compounds in the concrete react with air carbon dioxide. This causes the pH of the concrete pore solution to fall, which can damage the reinforcement bars’ passive protective layer. The rebars are subject to corrosion as a result.
Under various hydration environments, notably, the impact of pH value on the hydration reaction, the microscopic properties of cement paste’s hydration products change significantly [
52]. The microstructure of cement hydration products, the development of C-S-H structures during hydration, and even the penetration and diffusion of water and sulfate ions can all be affected by pH value variations [
53], which can also modify the concentration and distribution state of Ca
2+ and SO
42−. The pH value affects the corrosion resistance of cement by altering the diffusion mode of the corrosion medium in cement pores. The sulfate resistance of cement-based materials is related to its composition and the pH value of the sulfate environment. Due to the change in the pH value of the sulfate medium, the porosity of cement-based materials’ hydration products is greatly increased, notably in terms of mass loss and final compressive strength [
54].
At the late stage of hydration, the ettringite (AFt)—aluminum hydroxide (AH3) was gradually transformed into the monosulfate (AFm)—aluminum hydroxide (AH3). As a result, a gradual appearance of the monosulfate (AFm)—aluminum hydroxide (AH3) diffraction peak was detected. It is also important to note that, if a mixture contains enough CaSO
42H
2O, ettringite will develop. When all the CaSO
42H
2O used in the manufacture of ettringite is gone, the compound is changed into monosulfate. In-situ synchrotron X-ray powder diffraction is normally used to study the reactions in the temperature range of 25 to 170 °C [
40].
The pH level of the concrete can be impacted by a variety of natural events in addition to issues that may develop during the mixing and manufacture of the concrete. Depending on the environment, anything that raises the moisture level can cause problems for concrete. For instance, acid rain, which has a pH below 7, significantly lowers the pH level of the concrete mixture overall [
55]. Salt, which is frequently utilized as a remedy to alleviate ice conditions, is still another major problem. In the same manner that acid rain corrodes nearby objects and lowers pH, salt will seep into concrete pores and any cracks it may have.
There is a process that gradually erodes the concrete’s structural integrity even in the absence of the input of acids from saltwater or acid rain. This can happen when any type of water seeps into the porous concrete or even just when carbon dioxide in the air reacts with the cement in the concrete. In addition to lowering the concrete’s pH, carbonation can have a more catastrophic impact on any reinforcements that are there [
56], causing them to expand, breaking the surrounding concrete, and ultimately leading to the collapse of the building [
57].
Additionally, the findings of this study provide some crucial information for the building sector, particularly in relation to controlling concrete strength.