Abstract
The accumulation of steel slag has become a significant obstacle for the steel industry in achieving ultra-low emission targets. Given its composition is similar to that of road construction materials, steel slag holds substantial potential for application in sustainable road construction. This study investigated the current status and future trends of steel slag applications in road construction through a bibliometric analysis. The findings reveal that steel slag applications primarily focus on steel slag concrete, asphalt, steel slag aggregates, and steel slag processing technologies. The activation of its reactivity and stability emerged as a key research direction, with carbonated steel slag demonstrating exceptional performance in road construction. This study provides a scientific foundation for the high-value utilization of steel slag. It suggests optimizing its reactivity, stability, and carbonation, which will be crucial for expanding its use in road construction.
1. Introduction
The steel industry was long regarded as a fundamental pillar of modern society, yet the substantial volumes of steel slag produced as a byproduct during steel manufacturing presented ongoing challenges for effective disposal [1]. Steel slag, primarily generated from iron and steel making processes, consisted of the residue left after the high-temperature reduction and smelting of ores, apart from the extracted iron and steel [2]. According to statistics, China’s steel slag generation in 2022 was about 300 million tons, and only about 80 million tons was used for resource utilization [3]. The accumulation of steel slag not only occupied significant land resources but also posed environmental risks, such as heavy metal leaching and groundwater pollution [4]. Traditional methods of steel slag disposal, such as stockpiling and reuse, often lead to environmental pollution and resource wastage [5]. Thus, finding efficient ways to process and utilize steel slag has become a critical focus of research. In recent years, with growing awareness of resource recovery and environmental protection, researchers increasingly sought more sustainable methods for managing steel slag.
Road construction was a key component of national infrastructure development, and with the accelerated global urbanization, the demand for high-quality and durable road materials grew substantially [6]. However, the extraction and use of conventional road construction materials, such as natural stone and gravel, faced challenges of resource depletion and significant environmental impact [7]. Steel slag, as a potential alternative material, exhibited physical and chemical properties that made it particularly suitable for road construction [1]. First, its high density and abrasion resistance allowed it to withstand heavy loads in road construction, positioning it as a premium substitute for traditional aggregates [8]. Compared to conventional natural aggregates, steel slag aggregates provided enhanced load-bearing capacity and superior fatigue resistance. Its rough surface texture also improved the bonding strength of road materials, thereby increasing the overall strength and stability of road surfaces [5]. Research by Kedar indicated that roads constructed with steel slag aggregates demonstrated an approximately 10% improvement in seismic resistance compared to traditional roads [9]. Additionally, the active oxide components in steel slag engaged in hydration reactions with cement, producing denser hydration products, which enhanced the durability and impermeability of concrete. Fan’s research showed that incorporating steel slag into concrete increased compressive strength by more than 15% [10]. The effective use of steel slag in road construction depended critically on its processing technology. Steel slag underwent several processing steps, including crushing, screening, and magnetic separation, to produce aggregates of appropriate size and purity [11]. Crushing aimed to reduce large steel slag pieces into suitable aggregate sizes, while screening separated aggregates into different size ranges to meet various engineering requirements.
Magnetic separation removed metallic impurities from steel slag, ensuring the purity and uniformity of the aggregate. In recent years, advances in technology have significantly improved the efficiency of steel slag processing [12]. According to Moura’s research, high-efficiency crushing equipment and precise screening technologies not only enhanced the quality of steel slag aggregates but also significantly reduced processing costs [13]. These technological advancements provided strong support for the widespread application of steel slag in road construction. The complex mineral composition of steel slag, rich in silicates and aluminates, contributed to its excellent rutting resistance and high-temperature stability in asphalt mixtures, making it suitable for high-traffic roads and regions with hot climates [14]. Research by Dondi revealed that steel slag roads demonstrated superior durability and fatigue resistance compared to traditional roads [15]. Jonczy’s findings indicated that the rough surface of steel slag enhanced the friction coefficient of road surfaces, improving skid resistance and road safety [16]. In addition, Wu’s research indicated that steel slag had excellent water absorption and drainage properties, which made it effective as a road sub-base material [12]. Its application improved the water resistance and freeze–thaw durability of roads. Thus, the diverse properties of steel slag rendered it an ideal material for road construction. However, excessive chemical activity in steel slag could lead to volume expansion, affecting the stability of road surfaces. Wang’s research showed that when highly active steel slag was incorporated into concrete, roads developed bulging at temperatures as low as −30 °C [17]. Korkmaz’s research highlighted that roads constructed with steel slag aggregates experienced a more than 50% variation in bumpiness between the summer and winter seasons [18]. Therefore, strict control of the chemical composition and particle distribution of steel slag was necessary to improve its stability and optimize its performance in road construction. Furthermore, Liu’s research indicated that appropriate treatment processes, such as aging and the addition of stabilizers, effectively reduced the expansion potential of steel slag, further enhancing its applicability in road construction [19]. With the increasing emphasis on sustainable development and resource recycling, the application of steel slag in road construction is expected to expand further. Nevertheless, the current research has not explicitly addressed the major challenges and bottlenecks in the application of steel slag in road construction, nor has it outlined the future direction of research efforts. It was worth noting that China banned the use of steel slag as a blending material for ordinary silicate cement in building construction on 1 June 2024, and exploring new utilization pathways for steel slag has become increasingly urgent [20,21].
This study employed a bibliometric analysis to quantitatively assess the current status of steel slag applications in road construction and to identify the key challenges and future development trends through bibliometric methods. The aim was to enhance the industrial processes for applying steel slag in road construction, providing a promising solution to the dual challenges of industrial waste disposal and infrastructure development.
2. Research Tendencies of Steel Slag Application in Road Construction
In this study, a search phrase using keywords such as (“roadway” or “road”) and (steel slag or steel scrap) was applied to retrieve all the indexed articles published from 1900 to 2023 from the Web of Science database. All the indexed records were downloaded into Excel 2020 for digital logic analysis [22]. During the data processing, certain keywords with similar meanings, such as “aggregate” and “skeletal material”, were grouped together. After classifying all the relevant data, the publication trends were analyzed in 10-year intervals to minimize annual fluctuations.
Upon analyzing the keywords, it was evident that “aggregate” appeared most frequently, ranking first among all keywords. “Mix Design”, “processing technology”, and “concrete” ranked second, third, and fourth, respectively, consistently holding the top positions since 1968. Moreover, “stability” and “reactivity” have shown the fastest growth as keywords in recent years. The specific data can be found in Table 1. Through an analysis of keyword trends, several conclusions were drawn. The primary application of steel slag in road construction has been as aggregate, mixtures, and concrete. Processing technology is the key factor in promoting the broader application of steel slag in road construction. The stabilization of steel slag ensures its safe application in road construction, while the activation of steel slag’s reactivity represents a focal challenge in its use. These foundational studies provide a solid basis for the application and development of steel slag in road construction and offer recommendations for future research.

Table 1.
Author keywords on steel slag in road construction from 1968 to 2023.
3. Application of Steel Slag in Road Construction
According to the theory of road construction, it was known that the layering of a road is from top to bottom: surface, base layer, sub-base, and road base. Each layer had different requirements for construction materials, and the road construction materials were adapted according to the nature of steel slag and modified steel slag, as shown in Figure 1.
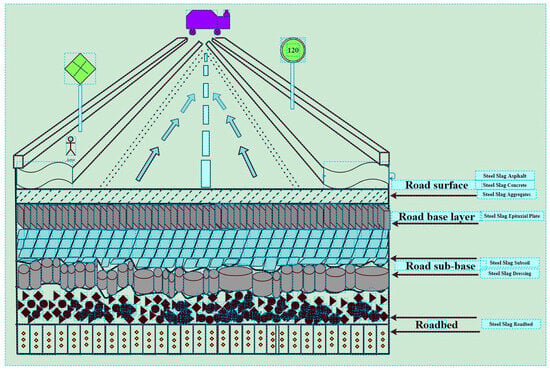
Figure 1.
Steel slag application scenarios in road construction.
3.1. Steel Slag Aggregate
In road construction, aggregates are fundamental materials constituting the road structure, categorized based on their position and function within the road structure as surface aggregates and base and sub-base aggregates. Surface aggregates were employed in the top layer of the road, which is directly exposed to vehicular traffic, providing the necessary mechanical strength and stability to withstand the load of passing vehicles and daily traffic wear [23]. Consequently, surface aggregates needed to exhibit excellent abrasion resistance, skid resistance, and adhesion, which enhanced road durability and extended its service life, thereby reducing maintenance and repair frequency and ensuring driving safety while lowering long-term maintenance costs [24]. In contrast, base and sub-base aggregates were used in the lower layers of the road structure, including the foundation and sub-foundation layers [25]. These aggregates typically consisted of fine particles, designed to create a porous structure that allowed for water infiltration and drainage, reducing surface water and preventing damage from prolonged water exposure [26]. Additionally, fine aggregates could regulate the expansion and contraction of road materials under varying temperatures, minimizing temperature-induced cracking. Figure 2 presents the Energy Dispersive Spectroscopy (EDS) analysis of metals in typical road construction aggregates, including crushed stone [27], bulk stone [28], and typical steel slag [29]. Based on the metal content, it was concluded that the metal content of steel slag falls between that of crushed stone and bulk stone, indicating its potential as a road construction aggregate.
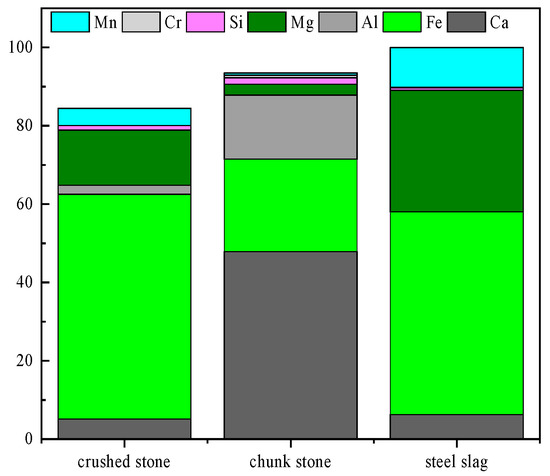
Figure 2.
Energy Dispersive Spectroscopy analysis of crushed stone, bulk stone, and steel slag.
The high strength and hardness of steel slag made it an ideal aggregate for road construction to withstand heavy traffic loads [29]. These properties were crucial for enhancing road load-bearing capacity and significantly improving road durability [26]. The high abrasion resistance of steel slag contributed to extending the road’s service life and reducing maintenance and repair needs due to frequent traffic wear, thereby lowering long-term maintenance costs [30]. The chemical stability of steel slag allowed it to maintain performance under varying environmental conditions, aiding in the long-term stability of the road structure [2]. The thermal expansion characteristics of steel slag matched those of commonly used materials in road construction, helping to reduce pavement cracking caused by temperature fluctuations and improving deformation resistance [5]. Furthermore, the low water absorption of steel slag improved road frost resistance and reduced damage from freeze–thaw cycles, which was particularly significant for pavement construction in cold regions [9]. Using steel slag as an aggregate promoted the recycling of industrial waste, reducing the exploitation of natural resources and environmental emissions, reflecting the principles of environmental friendliness and sustainability [31]. As an industrial byproduct, steel slag typically had lower costs compared to conventional aggregates, offering significant economic advantages. The sharp edges of steel slag particles increased adhesion with asphalt, enhancing skid resistance and improving driving safety [21]. Although steel slag had some beneficial effects on concrete strength as an aggregate, the issue of stability required attention. The variability in the stability of steel slag aggregates could lead to uneven expansion stresses within the concrete, damaging its microstructure [32]. Therefore, steel slag needed to undergo pretreatment to address stability issues. Common pretreatment methods included aging, dissolution, carbonation, weathering, and autoclaving [21].

Table 2.
Performance characterization of steel slag with different incorporation amount as aggregate in road construction.
Table 2.
Performance characterization of steel slag with different incorporation amount as aggregate in road construction.
| Steel Slag Admixtured | Aggregate Size/mm | The Sand Rate/% | Slump/mm | Stick Degree | Water Retention |
|---|---|---|---|---|---|
| 0% | 5~10 | 36 | 70 | Middle | No |
| 5% | 5~10 | 38 | 65 | Hight | No |
| 10% | 5~16 | 36 | 70 | Middle | No |
| 7.5% | 5~15 | 34 | 85 | Hight | Low |
| 12.5% | 5~15 | 36 | 75 | Middle | Low |
| 15% | 5~10 | 38 | 65 | Hight | Low |
| 20% | 5~25 | 36 | 85 | Hight | middle |
The data in Table 2 were obtained from the references [4,11,33,34,35,36,37].
As shown in Table 2, the inclusion of steel slag in aggregates led to improvements in consistency, sand content, and water retention. Furthermore, higher incorporation levels generally resulted in better performance of the aggregates. Feng investigated the impact of steel slag aggregates on concrete performance, noting that fine steel slag aggregates reduced the workability of fresh concrete but could enhance the compressive strength, splitting tensile strength of ordinary concrete, and splitting tensile strength of high-strength concrete under high-temperature curing conditions [38]. Huang employed high-speed stirring and chelating agents to pretreat steel slag to remove free CaO, finding that cement mortar made with treated steel slag as the fine aggregate achieved a compressive strength of up to 45.4 MPa [39]. Deng directly used steel slag as the fine aggregate in concrete, replacing natural sand on an equal volume basis, and observed that replacing 20% of natural sand with steel slag improved the impact resistance of steel slag concrete [40]. Zhang studied the effects of replacing sand and cement with converter steel slag and silica on concrete properties, finding that a 50% replacement of converter steel slag increased compressive strength by 18%, reduced water absorption by 50%, and decreased slump by 40% [41]. Lopes compared the effects of steam curing and autoclaving on the stability of steel slag, using a high-pressure vessel to pretreat steel slag (t = 3 h, P = 2.0 MPa, and T = 215 °C), which significantly improved the volumetric stability of fine steel slag aggregates [42]. Rodrigues used untreated steel slag with low CaO content as the fine aggregate, finding that when the replacement rate was 30–50%, the 28-day tensile strength of concrete increased, and when the replacement rate was 15–30%, the tensile strength also improved [43]. These studies indicated that while direct application of steel slag in concrete aggregates could positively affect concrete strength at appropriate replacement rates, stability issues remained a concern. Pretreating steel slag to address stability problems made its use as a fine aggregate in concrete feasible. Steel slag particles were uniformly sized, with diameters less than 5 mm, and did not require further crushing to meet fine aggregate requirements [44]. Additionally, steel slag microspheres had low f-CaO content and good sphericity, suggesting that steel slag microspheres could potentially replace natural river sand as a fine aggregate [45]. Zhao applied the rapidly quenched slag from steel, as a fine aggregate in dry mortar, asphalt mixtures, and concrete, showing that its mechanical properties were similar to those before replacement, thereby demonstrating the feasibility of using rapidly quenched slag as a substitute for fine aggregate [46].
3.2. Steel Slag Asphalt Mixtures
The replacement of natural mixtures with steel slag in concrete was identified as one of the primary pathways for large-scale resource utilization of steel slag [47]. Therefore, a comprehensive investigation of the mixture properties of steel slag was of great significance for its effective recycling in concrete. Table 3 presents a comparative analysis of the properties of steel slag and natural mixtures.

Table 3.
Comparison of steel slag pad and traditional pad material properties.
It was observed that steel slag exhibited superior physical properties compared to natural mixtures. Indicators such as density, abrasion resistance, adhesion to asphalt, and strength of steel slag were found to be equivalent to or even better than natural mixtures such as basalt, limestone, and granite. Additionally, these technical parameters met the regulatory requirements for mixtures used in construction materials. Notably, the polishing value of steel slag reached 70 Gu, which was significantly higher than that of natural steel slag asphalt mixtures like basalt, limestone, and granite. The residual lime from the slag-forming process rendered the surface of the steel slag slightly alkaline, resulting in the highest adhesion rating (level 5) between steel slag and acidic asphalt [52]. Furthermore, the crushing value and Los Angeles abrasion rate of steel slag were approximately 15% and 30% lower than those of natural mixtures, respectively, indicating excellent strength and durability [53]. The metallic iron content in steel slag further enhanced its wear and impact resistance. From a mineralogical perspective, steel slag contained significant amounts of minerals such as rhodonite and olivine, both with a Mohs hardness above 6.5 [54]. In comparison, basalt, primarily composed of feldspar and pyroxene, had a Mohs hardness of 6 to 6.5, while the primary mineral in limestone, calcite, had a hardness of only 3.0 [55]. Hence, steel slag demonstrated greater hardness and strength than typical natural mixtures.
The superior mixture properties of steel slag laid a solid foundation for its use in pavement maintenance materials [56]. Figure 3 summarizes the test results of base layers incorporating different proportions of steel slag asphalt mixtures. The data in Figure 3 were obtained from references [7,33,34,35,57,58,59,60,61,62,63]. The data indicated that when the steel slag content did not exceed 85%, the mixtures met the design standards for road construction, making steel slag a highly suitable mixture material for road infrastructure [64]. However, the presence of unreacted free calcium oxide (f-CaO) in steel slag, which could hydrate into calcium hydroxide upon contact with water, potentially led to volume expansion. Therefore, in addition to conventional engineering mixture performance, the volume stability of steel slag in construction materials could not be overlooked [65]. Zepper utilized a hot-stuffing method to pretreat the steel slag, where the saturated steam in the hot-stuffing tank reacted with the free calcium oxide, effectively reducing the f-CaO content and mitigating the risk of volume expansion [66]. Jiang employed a rotary kiln method to pretreat the steel slag, reducing the f-CaO content to undetectable levels [67]. Wan used air quenching combined with hydrothermal and steam aging post-treatment processes to completely resolve the volume stability issue of steel slag [68]. Li and Liu, through the hot-stuffing process, were able to reduce the f-CaO content from 8% to below 3%, further validating the effectiveness of this method [61,69]. In conclusion, compared to natural mixtures, steel slag exhibited superior physical properties, and its risk of volume expansion could be effectively controlled through pretreatment techniques. These characteristics made steel slag meet the performance requirements for steel slag asphalt mixtures in road engineering, demonstrating its feasibility as a substitute for natural mixtures in road construction. Similar conclusions were reached by Schneider, who found that when steel slag was used as the coarse mixture in asphalt mixtures, the coating effect of the asphalt partially inhibited the leaching of heavy metal ions from the steel slag [70]. Kamata’s research further indicated that compared to steel slag exposed to air and rainwater, the concentration of leached heavy metal ions in steel slag asphalt concrete was generally reduced by 20–50% [71]. Furthermore, replacing natural mixtures with steel slag was shown to reduce the environmental impact throughout the life cycle of roads.
Figure 3.
Results of steel slag with different mixing ratios in cushion detection.
Neves conducted a comparative environmental impact assessment of asphalt concrete with 75% steel slag replacing natural mixtures and conventional asphalt concrete. The results demonstrated significant environmental benefits from recycling steel slag for road engineering, with only minor increases in ozone depletion and photochemical oxidation impacts compared to natural mixtures [72]. Ma’s life cycle assessment of steel slag as a replacement steel slag asphalt mixture revealed a 10–20% reduction in global warming potential, marine eco-toxicity, and human toxicity, highlighting its positive environmental impact [73]. However, Sukak’s research indicated that the initial construction cost of steel slag asphalt concrete was over 30% higher than that of conventional asphalt concrete due to additional processing, transportation costs, and higher asphalt usage [74]. Alves’ findings showed that the routine maintenance costs of steel slag asphalt concrete decreased from 550,000 CNY/km to 520,000 CNY/km, and major repair costs dropped from 1.17 million CNY/km to 570,000 CNY/km [75]. Overall, steel slag asphalt concrete still exhibited significant environmental and economic benefits.
3.3. Steel Slag Concrete
Researchers both domestically and internationally have conducted extensive studies on the pavement performance of steel slag asphalt concrete, including high-temperature rutting resistance, low-temperature cracking resistance, water stability, fatigue resistance, volume stability, and skid resistance. Steel slag, as a recycled material in concrete production, has been widely applied in Western countries. Approximately 50% of steel slag in European countries is used in concrete production, with the figure rising to about 70% in the United States, and exceeding 98% in the UK and Germany [76]. In contrast, research on steel slag concrete in China began earlier but its utilization remains relatively low due to limitations in technology and processes. It is mainly applied in road subgrades, and the progress in resource utilization in road engineering is slow, lagging behind that of developed countries [77]. Since the 2nd International Conference on Mineral Waste Utilization in 1970 proposed the application of steel slag in road engineering, countries have begun to explore its use in road construction [78]. In 1974, a steel slag aggregate pavement test section in Toronto, Canada, showed a slower decline in skid resistance compared to regular asphalt pavement after four years of use [79]. In the 1980s, Japan began using steel slag as a replacement for natural aggregates in several regions to enhance the rutting resistance of pavements. In 1994, a steel slag asphalt concrete test section in Oregon, USA, despite having higher initial costs, exhibited excellent long-term performance, with good skid and wear resistance [80]. Subsequently, other European countries began to standardize the use of steel slag in road engineering. In 1997, China constructed its first steel slag asphalt concrete pavement in Shanghai, which, despite heavy traffic loads, maintained good performance over the years [28]. In 2003, the Baoxie section of the Wuhuang Expressway used steel slag as coarse concrete in its asphalt concrete ramps, and after six years of service, the pavement performance remained excellent. In 2009, 1275 tons of steel slag asphalt mix were used in the renovation of Beijing’s Chang’an Avenue, and the road has remained in good condition. In 2011, the first steel slag asphalt concrete pavement in Xinjiang was laid at the S114 interchange in Urumqi, demonstrating good smoothness, impermeability, and skid resistance [81].
Steel slag possesses good cementitious properties and, after volume stabilization treatments such as melting and conditioning, aging, acidification, or surface modification, it can be used to produce asphalt concrete [68]. From Table 4, it can be observed that when the steel slag content in the concrete does not exceed 50%, the steel slag concrete exhibits good slump, compressive strength, and frost resistance. Additionally, the higher the proportion of steel slag, the better the overall performance of the concrete.

Table 4.
Road performance of steel slag concrete.
Xu prepared dense-graded steel slag asphalt concrete with different limestone replacement ratios (25%, 50%, 75%, and 100%) using limestone as a reference [84]. The results indicated that with the increase in steel slag content, the optimal asphalt binder content showed a rising trend. However, when steel slag completely replaced limestone coarse concrete (100%), the deformation resistance of asphalt concrete was negatively impacted, though other properties improved compared to limestone asphalt concrete. At the other three replacement levels, the performance of steel slag asphalt concrete improved across all properties, with the most significant improvement observed at a 25% steel slag content [84,85]. Castillo also prepared dense-graded asphalt concrete with coarse steel slag and fine limestone concretes, studying its water damage resistance and mechanical properties [85]. The results showed that steel slag asphalt concrete had better water sensitivity, rutting resistance, dynamic creep characteristics, resilient modulus, and resistance to permanent deformation compared to ordinary asphalt concrete. Segui produced stone mastic asphalt (SMA) mixtures with steel slag, using basalt and limestone mixtures as controls. The findings demonstrated that at 60 °C, the dynamic stability of the steel slag concrete was close to 7000 cycles, an increase of 15% and 112% compared to the basalt and limestone control groups, respectively [86]. Lu’s study revealed that the low-temperature critical compressive strain energy density of steel slag concrete was 89 kJ/m3, representing increases of 20.3% and 53.4% compared to basalt and limestone mixtures, respectively, indicating that steel slag mixtures had superior low-temperature cracking resistance [87]. However, Zhang observed that when steel slag replaced 40% and 80% of the coarse basalt concrete in asphalt concrete, the fracture strain decreased by 14.9% and 17.3%, respectively, compared to ordinary asphalt concrete, suggesting that increased steel slag content significantly reduced low-temperature cracking resistance [88]. Ma [89] and Luo [90] used partial steel slag replacement in asphalt concrete, finding that the skid resistance of steel slag asphalt concrete was 10–15% higher than that of natural asphalt concrete. Pazzini compared the water-induced volume expansion rates of coarse steel slag-fine stone asphalt concrete and pure steel slag asphalt concrete [91]. The results showed that the water expansion rate of the composite mixture was 36.8% lower than that of pure steel slag, indicating that the use of coarse steel slag reduced the expansion risk, which was significantly influenced by the cementitious properties of steel slag. It is important to note that steel slag contains small amounts of toxic heavy metals (Cr, Cu, Zn, As, Pb, etc.), which pose a potential risk of heavy metal ion release into the environment when used as concrete or filler in road construction, especially under long-term exposure to rainwater [91]. Various countries have set limits on the heavy metal content and leaching concentrations of steel slag used in road construction. For instance, Austria prohibits the use of steel slag asphalt concrete in groundwater protection areas, and Germany mandates biannual leaching tests for steel slag used in hydraulic and road engineering [92]. Researchers have conducted extensive studies on the leaching behavior of heavy metals from steel slag concretes and steel slag asphalt concrete. Su investigated the leaching behavior of heavy metals from steel slag asphalt concrete and the surrounding soil after 15 years of service. The study revealed a close correlation between the types and concentrations of heavy metal ions leached from steel slag concretes and the heavy metal content in the surrounding soil [93]. Despite this, the concentrations of metal ions remained below the national Grade II surface water environmental quality standards. Yang designed SMA-13 steel slag asphalt concrete and used the Toxicity Characteristic Leaching Procedure (TCLP) to explore the short-term and long-term leaching behaviors of steel slag and steel slag asphalt concrete. The results showed that after short-term leaching, the concentrations of heavy metal ions in the leachate met regulatory standards [94]. However, after long-term leaching, the concentrations of cadmium and nickel ions exceeded the regulatory limits, indicating a long-term leaching risk for steel slag concrete. In contrast, the leaching concentrations of heavy metals from steel slag concrete were significantly lower than those from steel slag concretes and remained below the critical values specified in regulations. This suggests that the encapsulation effect of asphalt can effectively reduce the leaching concentrations of heavy metals from steel slag.
4. Steel Slag Process Technology
Figure 4 summarizes the processing techniques for steel slag application in road construction. The processing technologies for steel slag primarily include coarse and fine processing [35]. Coarse processing involves steps such as cooling, crushing, screening, and chemical stabilization. Fine processing includes the preparation of steel slag micro-powder and steel slag cementitious materials. The goal of coarse processing is to reduce the volume and render steel slag harmless, while simultaneously achieving resource utilization to a certain extent, balancing both economic and ecological benefits. Therefore, the steel slag treatment process should aim to be safe, reliable, simple, and environmentally friendly, providing the optimal treatment conditions for the carbonization of steel slag.
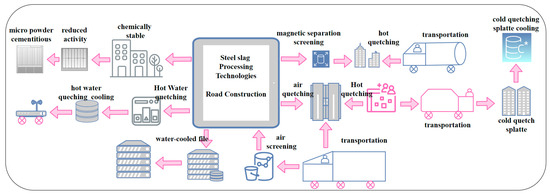
Figure 4.
Processing techniques for steel slag application in road construction.
4.1. Coarse Processing of Steel Slag
The current coarse processing technologies for steel slag mainly include methods such as the cold quenching method, Splat Cooling, hot water quenching method, water quenching method, air quenching method, hot steaming method, rotary drum method, and pressurized steam aging method. Among these, the cold quenching method requires a long aging time, and the treated steel slag has a large particle size, which is not conducive to its utilization and processing. The Splat Cooling is costly and causes significant pollution. The water quenching and air quenching methods are prone to explosions and are hazardous for operation, and none of these methods have been widely adopted on a large scale internationally [58]. Currently, the commonly used methods for steel slag treatment worldwide are the hot water quenching method, rotary drum method, hot steaming method, and pressurized steam aging method. Table 5 summarizes the technical operations, advantages, and disadvantages of the mainstream coarse processing methods.

Table 5.
Types of steel slag roughing and their advantages and disadvantages.
The hot-stuffing technique for steel slag is divided into two main categories: the traditional atmospheric-pressure pool-type hot-stuffing method and the improved roller-crushing and residual-heat-pressurized hot-stuffing process. The atmospheric-pressure pool-type method involved pouring steel slag, at temperatures ranging from 200 to 1650 °C, into a hot-stuffing pool in batches, during which water was sprayed onto the slag. After the slag was fully loaded, the pool was sealed, and water was intermittently added to facilitate the hot-stuffing process. During this period, the slag expanded and disintegrated. After 8 to 12 h, the slag temperature decreased to around 60 °C, allowing for safe removal from the pool. Reactions occurred between free calcium oxide (f-CaO), free magnesium oxide (f-MgO), and high-temperature steam during the hot-stuffing process, as indicated by reactions 1-1 and 1-2. These reactions led to the digestion of f-CaO and f-MgO [96].
f-CaO + H2O → Ca(OH)2 Volume expansion 98% Equations (1)
f-MgO + H2O → Mg(OH)2 Volume expansion 148% Equations (2)
Razzaq’s research demonstrated that the traditional hot-stuffing process did not require specific slag fluidity and could be applied to a wide range of conditions. Furthermore, more than 60% of the processed slag had a particle size of less than 20 mm, and the process generated minimal pollution [97]. However, Nguyen’s findings highlighted some limitations, such as the long process duration (around 10 h), slow processing speed, and significant water consumption (about 500 kg per ton of slag) [98]. The roller-crushing and residual-heat-pressurized hot-stuffing method was an advanced technique developed based on the traditional approach. This newer method involved three main steps: slag pouring, roller-crushing, and pressurized hot-stuffing using residual heat [99]. Slag was transported by slag pot vehicles to a crushing bed, where it underwent roller-crushing and water-cooling simultaneously, reducing its temperature to around 800 °C and breaking it down into particles smaller than 300 mm. The cooled slag was then transferred to a pressurized chamber for sealed water spraying. As liquid water contacted the high-temperature slag, it turned into steam, creating a reaction pressure of about 0.2 to 0.4 MPa, which accelerated the digestion of f-CaO and f-MgO, stabilizing the slag through pulverization [84]. Ma’s research found that, compared to the traditional hot-stuffing process, the pressurized method significantly reduced the reaction time (to approximately 2 h), lowered water consumption (300 to 400 kg per ton of slag), produced over 70% of slag particles smaller than 20 mm, and had a minimal environmental impact [89]. The Splat Cooling involved setting up an elevated slag pan in the steel slag workshop. A crane was used to pour molten slag from the slag pot into the pan, where the slag layer typically had a thickness of 30 to 120 mm. Water was then sprayed onto the slag to induce rapid cooling and fragmentation. The crane subsequently flipped the pan, allowing the fragmented slag to fall onto a slag transport vehicle. The slag was then transported to a nearby pool, where further water spraying cooled it down, reducing the particle size to between 5 and 100 mm. Finally, a grab bucket was used to collect the slag for transportation to the slag processing workshop, where it underwent magnetic separation, crushing, screening, and fine processing. This method was considered safe and reliable, with minimal environmental pollution and a good working environment [100]. However, it had a lower slag processing capacity, and the process was relatively complex with many stages, resulting in higher production costs. Dang applied the Splat Cooling to process steel slag from a site in southern China, obtaining steel slag powder with a particle size of 30 nm, which was suitable for modifying acidic farmland soil [101]. Building on Zhang’s work, Liu colleagues achieved steel slag powder with a particle size of just 5 nm, eliminating the need for further processing into micro-powder and saving over 60% of the energy typically required [61].
The steel slag drum granulation technique involves slowly pouring molten steel slag from a specialized reaction container into an inclined rotating drum, accompanied by water cooling. Within the drum, steel balls collide and rub against the slag, causing it to break into smaller solid particles under the combined effects of thermal stress and mechanical force. Ahirwar’s research indicated that the drum granulation technique requires high slag fluidity, with more than 80% of the processed slag particles meeting the size standard of less than 10 mm [102]. However, Schumacher’s findings highlighted that the steel slag processed by drum granulation tends to have a relatively high f-CaO content (3–5%). Additionally, this technique involves complex procedures and equipment, making maintenance and repair challenging and increasing operational costs [103].
The hot splashing method involves pouring molten steel slag into slag pots, which are then transported to the steel slag hot splashing workshop. Using a crane, the molten slag is layered onto a slag bed and allowed to cool by air. Once the temperature drops to 350–400 °C, water is sprayed on the slag to rapidly cool and fracture it. Excavators and loaders are used to dig and load the slag, which is then transported to a disposal site. For slag intended for further use, it is taken to the processing plant for crushing, screening, and magnetic separation. This method requires large-scale excavation machinery, which leads to significant equipment wear, large land use, and high dust levels during crushing. However, it is a well-established and reliable process with fast slag discharge rates, making it a widely adopted method for basic oxygen furnace slag processing worldwide [104]. Gan’s study showed that, after switching to the hot splashing method in a steel plant in Hebei, China, daily slag processing capacity increased by more than 200%, and energy consumption was reduced by 40% [105]. An’s research indicated that using this method reduced dust emissions by about 30% and significantly decreased labor requirements [83].
Air granulation of steel slag is a technology that atomizes molten steel slag through high-velocity air streams, rapidly solidifying it into fine particles. This method significantly enhances the utilization rate of steel slag, typically reaching around 70%. Compared to water quenching, air granulation is safer and avoids wastewater production since it uses air as the cooling medium. The resulting granulated slag particles possess a stable vitreous structure with minimal free calcium oxide (f-CaO), addressing the issue of poor slag stability. This makes the slag suitable for use in building materials, offering promising economic benefits [90]. Liu’s research demonstrated that after rough air granulation processing, the f-CaO content in steel slag decreased to below 10%, improving its overall stability [95]. Luan’s findings suggested that molten steel slag is more suitable for air granulation, while treating solid slag in this manner leads to significant dust pollution and dispersion of the slag particles [106].
4.2. Fine Processing of Steel Slag
The fine processing technology of steel slag builds upon the basic processing steps, aiming to further refine steel slag to produce materials with significant industrial value tailored to specific application requirements. These materials primarily include steel slag micro-powder and steel slag-based cementitious materials. According to the YB/T 022 standard, steel slag—whether from a basic oxygen furnace (BOF) or electric arc furnace (EAF)—is subjected to iron removal via magnetic separation, followed by grinding to a specified fineness, resulting in what is defined as steel slag powder [107]. The production process of steel slag micro-powder encompasses drying, fine grinding, grading, and mixing with an appropriate amount of water. The mixture is then compressed and cured in a carbonation chamber. This process not only significantly improves the early strength of the steel slag but also addresses stability issues arising from volumetric expansion, thereby enhancing the overall utilization rate of steel slag. Despite these advancements, the current market sales of steel slag micro-powder remain limited, largely due to the presence of free calcium oxide (CaO) and magnesium oxide (MgO) in steel slag, which negatively affect its volumetric stability [98]. Additionally, the relatively high iron oxide content in steel slag increases the complexity of the grinding process. Several grinding technologies are currently employed in the production of steel slag micro-powder, as summarized in Table 6. These technologies not only ensure the quality and yield of steel slag micro-powder but also provide robust support for the efficient use and environmentally friendly processing of steel slag resources.

Table 6.
Fine processing of steel slag.
Ball mills, widely used in the building materials and mining sectors, are favored for their ease of operation and low failure rate. The equipment’s key advantages include excellent adaptability, a high reduction ratio, the ability to simultaneously grind and dry materials, a simple structural design, and easy maintenance. Ball mills offer superior sealing performance, smooth operation, and reliable functionality. In grinding systems, two configurations are typically employed: open-circuit mills and closed-circuit mills. In an open-circuit mill, material is discharged directly as the final product, while in a closed-circuit mill, the discharged material is classified, with fine particles sent to the storage silo and coarse particles recirculated for further grinding. The closed-circuit system not only increases grinding capacity and reduces over-grinding but also incorporates iron removal equipment to reduce iron content in recirculated material, thus minimizing equipment wear [112]. According to Liu’s research, the specific energy consumption for grinding steel slag powder to a fineness of 400 m2/kg is approximately 100 kWh/t [113]. However, Goli’s study revealed that when ball mill systems are used to produce products with a fineness greater than 450 m2/kg, the specific energy consumption can exceed 75 kWh/t, significantly increasing energy use. Additionally, the consumption of grinding media can surpass 200 g/t, raising operational costs [114].
Roller presses, based on the principle of high-pressure bed grinding, use two rollers to exert high pressure on the material layer, causing it to break apart. The predominant grinding mechanisms in this process are inter-particle compression and shearing, where the energy generated by friction is converted into deformation energy, leading to material breakage [97]. Zhu’s research demonstrated that while roller presses have high energy transmission efficiency, they usually need to be combined with ball mills, complicating the process flow and reducing the utilization rate of steel slag [115]. De’s findings further indicated that roller presses alone are insufficient to grind steel slag into micro-powder. Vertical roller mills (VRMs), which integrate crushing, grinding, drying, and classifying, are highly regarded for their low energy consumption, excellent sealing performance, low noise levels, suitability for outdoor installation, compact footprint, and simple process flow. The product’s fineness and particle size distribution can be effectively controlled by adjusting the separator speed, mill airflow, grinding pressure, and dam ring height [116]. Ma’s study indicated that in a steel plant in Shandong Province, China, the specific energy consumption of VRMs was less than 28 kWh/t, with even greater energy savings observed when grinding hot-stirred steel slag after appropriate crushing and iron removal [73]. Fei’s research showed that in a steel plant in Jiangsu Province, China, the proportion of iron in steel slag micro-powder produced using VRM technology was 2.6%, with the final product iron content reduced to 0.3%. When the specific surface area of the product ranged from 450 to 480 m2/kg, the energy consumption of the VRM was between 25 and 28 kWh/t. Horizontal roller mills, with their larger nip angles and smaller channel contraction rates, perform well in grinding steel slag to a specific surface area of 400 m2/kg, with a main motor energy consumption of approximately 45 kWh/t [117]. Wu’s study in a Shandong steel plant successfully utilized a horizontal roller mill to produce steel slag micro-powder that met the latest national standard [12].
5. The Activity and Stability of Steel Slag
The statistical analysis of keywords revealed a rapidly increasing trend in research focused on “reactivity”, “stability”, and “carbonation”, highlighting these as emerging hot topics. This suggests that in future road construction, effectively utilizing the reactivity of steel slag and ensuring its stability will become key research directions.
5.1. Activity of Steel Slag
Reactivity, in this context, refers to the ability of steel slag to participate in chemical reactions under specific environmental conditions, mainly in hydration and alkaline activation reactions. Free calcium oxide (f-CaO) and magnesium oxide (f-MgO) in steel slag tend to react with moisture in humid environments, potentially causing volumetric expansion and cracks, posing a threat to the long-term stability of road projects. Therefore, controlling the reactivity of steel slag is crucial for its successful application in road construction. The reactivity of steel slag can be categorized into pozzolanic reactivity and hydraulic reactivity. The chemical composition of steel slag primarily includes calcium oxide (CaO), silicon dioxide (SiO2), aluminum oxide (Al2O3), ferric oxide (Fe2O3), and small amounts of magnesium, manganese, and other metal oxides [1]. The pozzolanic reactivity of steel slag is closely related to the content of CaO, SiO2, and Al2O3. Under suitable conditions, these components can form calcium silicate hydrate (C-S-H) through secondary hydration reactions, exhibiting pozzolanic activity. However, compared to materials like slag and fly ash, the pozzolanic reactivity of steel slag is generally lower. This is mainly due to its higher CaO content and specific crystalline structure, which limit its hydration reactions [118]. The presence of f-CaO and f-MgO in steel slag can react with water, reducing the durability of concrete. Additionally, the presence of Fe2O3 in steel slag may negatively impact its hydration reactivity. Thus, strategies for activating the pozzolanic reactivity of steel slag focus on modifying its chemical composition and phase structure through physical or chemical methods [13]. To enhance the pozzolanic reactivity of steel slag, researchers have developed various activation techniques, mainly including mechanical activation, thermal treatment, and chemical activation, as summarized in Table 7. The application of these activation techniques can effectively improve the reactivity of steel slag, thereby enhancing its performance and application value in road construction. As research on the reactivity and stability of steel slag continues to progress, its potential as an eco-friendly and cost-effective building material in road construction will be further developed and utilized.

Table 7.
Activation of steel slag.
In research on the use of steel slag in road construction, enhancing its reactivity and stability has been a key focus. Mechanical activation, thermal treatment, and chemical activation are the three primary techniques employed to improve the pozzolanic activity of steel slag, thereby increasing its potential applications in road engineering [72].
Mechanical activation mainly involved using superfine grinding technology to reduce the particle size of steel slag and increase its specific surface area, which enhanced its reactivity. Studies indicated that particle refinement accelerated the hydration reaction and increased the production of C-S-H gel, thus improving the pozzolanic activity of steel slag [75]. Jiao’s research showed that mechanical activation could disrupt the crystalline structure of steel slag, increasing the glass-phase content and further enhancing its pozzolanic activity [120]. However, Cao’s research pointed out that mechanical activation was energy-intensive, and the activation effect tended to saturate as particle refinement progressed, limiting the extent of activity improvement. Thermal treatment involved preheating steel slag at high temperatures to alter its crystalline structure. Under high temperatures, phase transitions occurred in the steel slag, with some crystalline phases transforming into glass phases, which improved its pozzolanic activity [77]. Nicula’s research found that thermal treatment of steel slag at temperatures between 800 °C and 1000 °C significantly enhanced its pozzolanic activity [121]. Abji’s study also noted that high-temperature treatment could break down Fe2O3 crystalline phases, promoting reactions with SiO2 and CaO to form more reactive mineral phases [122]. However, Xu warned that thermal treatment was energy-intensive and required careful consideration of environmental and economic costs during operation, necessitating optimization of the treatment process for practical applications [84]. Chemical activation enhanced steel slag activity by adding external additives or alkaline activators, such as sodium hydroxide (NaOH), sodium sulfate (Na2SO4), and sodium silicate (Na2SiO3). Alkaline activators disrupted stable crystalline phases in steel slag, stimulating its latent activity [123]. Yang’s research demonstrated that the combined use of steel slag and other industrial waste materials like fly ash, under alkaline activation, significantly improved the early strength and durability of cementitious materials [25]. Moreover, the type and dosage of chemical activators greatly influenced the activation effect, requiring careful selection and optimization based on specific application scenarios. In conclusion, mechanical activation, thermal treatment, and chemical activation techniques significantly enhanced the pozzolanic activity of steel slag, increasing its application value in road construction [38]. However, these methods presented challenges such as high energy consumption and costs, necessitating careful consideration of environmental, economic, and engineering factors in practical applications. Future research could explore optimized combinations of these activation techniques and their application conditions to maximize the utilization of steel slag in road construction.
5.2. Stability of Steel Slag
The chemical composition of steel slag determines its hydraulic properties. Steel slag primarily consists of compounds such as calcium oxide (CaO), silicon dioxide (SiO2), aluminum oxide (Al2O3), and ferric oxide (Fe2O3), among which CaO plays a crucial role in the slag’s hydraulic properties. During hydration, CaO reacts with water to form calcium hydroxide (Ca(OH)2), which then reacts with SiO2 to produce calcium silicate hydrate (C-S-H). These hydrates act as binding agents in cementitious materials, providing strength and stability [31]. However, not all the CaO in steel slag is present in a reactive form, with some existing as free CaO. This may limit its hydration reactions and can potentially lead to the expansion and cracking of materials during use. Additionally, impurities such as Fe2O3 and MgO in steel slag can affect both the rate of hydration reactions and the final mechanical properties [46]. Due to these factors, the hydraulic properties of steel slag are generally lower than those of cement clinker, which restricts its direct application in cement and concrete. Alkaline activation technology offers an effective way to enhance the hydraulic properties of steel slag. As can be seen from Table 8, by adding strong alkaline activators, such as sodium hydroxide (NaOH), potassium hydroxide (KOH), or sodium silicate (Na2SiO3), the crystalline structure of steel slag can be disrupted, activating its latent reactivity and promoting rapid hydration reactions that form large quantities of C-S-H gel and other binding products. This process simulates the hydration of traditional cement but without the need for clinker or high-temperature calcination, offering significant energy savings and environmental benefits [58]. The alkaline activation mechanism of steel slag involves two main aspects: firstly, alkaline activators promote the reaction between CaO and SiO2 in steel slag to form C-S-H gel; secondly, the activators break down the stable crystalline phases in steel slag, releasing more reactive components [124]. Compared to traditional cement, the hydration reactions of alkali-activated steel slag occur at a faster rate, and the resulting binding products are denser, leading to improved early strength and durability. The type and concentration of alkaline activators significantly influence the activation effect of steel slag. Common alkaline activators include sodium hydroxide (NaOH), potassium hydroxide (KOH), sodium silicate (Na2SiO3), and sodium sulfate (Na2SO4). These activators produce varying effects on steel slag through different chemical reaction mechanisms. For instance, NaOH and KOH enhance hydration by increasing the pH of the solution, while Na2SiO3 strengthens the binding properties by forming a silicate framework structure [49]. Each activator has its unique advantages and limitations, so selecting and optimizing the appropriate activator depends on specific engineering requirements and environmental conditions.

Table 8.
Steel slag stability technology and advantages and disadvantages.
NaOH, as a strong alkaline activator, played a key role in the chemical activation of steel slag. It rapidly disrupted the crystalline structure of the slag, releasing significant amounts of Ca2⁺ and SiO44− ions, thereby promoting the formation of calcium silicate hydrate (C-S-H) gel [68]. Zhang’s research indicated that when the NaOH concentration was high, the hydration reaction rate of the slag accelerated markedly, leading to a significant increase in early strength [127]. However, Cui’s study found that excessive NaOH concentrations could result in overly high alkalinity, potentially compromising the material’s long-term durability [119]. Therefore, precise control of the NaOH dosage was necessary in practical applications. Sodium silicate (Na2SiO3), a silicate-based activator, effectively increased the formation of calcium silicate hydrate in steel slag, enhancing its cementitious properties. Jiang’s research demonstrated that compared to NaOH, sodium silicate provided a more sustained activation effect, improving the long-term mechanical performance of the material. This made sodium silicate a promising activator for enhancing the long-term performance of slag-based materials [67]. Sodium sulfate (Na2SO4) was often used as a supplementary activator in combination with other alkaline activators. Pooni’s research revealed that sodium sulfate could enhance early strength by generating calcium sulfate crystals (gypsum) and also improve the chemical resistance of slag. The use of such auxiliary activators offered additional performance improvements in the chemical activation of slag. The choice and dosage of activators in the chemical activation of steel slag had a decisive impact on the material’s performance [122]. Therefore, future research needed to further explore the optimal combinations and dosages of different activators, as well as their specific effects on slag-based materials. These studies could provide a scientific basis for the efficient utilization of steel slag in applications such as road construction and promote its further development in the field of sustainable building materials.
6. Perspectives—Carbonation of Steel Slag
As shown in Table 1, the keyword “carbonation” received little attention in research before 2001. However, after 2002, especially following 2013, it exhibited an exponential increase. This indicates that carbonation of steel slag has become a research hotspot in the field of steel slag-based road construction.
6.1. Reaction Mechanism of Steel Slag Carbonation
Steel slag carbonation is a green process that utilizes mineral carbonation technology to absorb and sequester CO2. Through the reaction between steel slag and CO2, CO2 is fixed as carbonates, achieving two goals: reducing CO2 emissions and recycling steel slag. Carbonated steel slag, due to its enhanced structural stability and improved mechanical properties, has shown wide applicability in building materials and road engineering, particularly in the application of subgrade materials, where it demonstrates significant potential [128]. Chen’s research results suggest that the carbonation process consumes low energy and is an exothermic reaction. From an energy perspective, the energy state of CO2 is 400 kJ/mol lower than that of carbon, and the energy state of carbonates is 60–180 kJ/mol lower than that of CO2. This indicates that the conversion of CO2 into carbonates is a spontaneous reaction [129].
The composition analysis of steel slag revealed that the primary substances responsible for CO2 adsorption in steel slag are Ca, Mg, and Si compounds, with the carbonation reaction pathways outlined roughly in Figure 5. Under conditions with sufficient CO2, a small portion of CaO can directly react with CO2 to form CaCO3. The remaining CaO reacts with H2O to form Ca(OH)2, which then further reacts with CO2 to produce CaCO3, thereby completing the adsorption and sequestration of CO2. A small amount of MgO can directly combine with CO2 to form MgCO3. The majority of MgO reacts with H2O to form Mg(OH)2, which then fixes CO2. Under CO2-rich conditions, Ca3SiO5 and Ca2SiO4 can react to form SiO2 and CaCO3, thereby completing the CO2 absorption reaction [130].
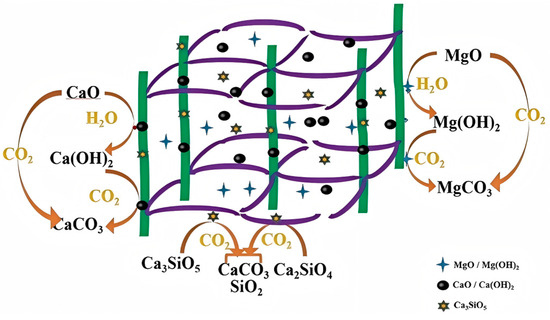
Figure 5.
Mechanism of carbonation of steel slag.
6.2. Classification and Research Progress of Steel Slag Carbonation Process
Steel slag carbonation can be classified into direct carbonation and indirect carbonation according to the mainstream processes. Direct carbonation involves the direct reaction between CO2 and mineral particles. Natural reactions are slow and require the material to be crushed into small particles, along with the addition of a certain amount of solvent (e.g., water, sodium chloride, etc.). The direct carbonation of steel slag is generally divided into direct dry carbonation and direct wet carbonation reactions [131]. Table 9 summarizes the more widely used direct carbonation method.

Table 9.
Direct carbonation of steel slag.
Dry carbonation primarily refers to the reaction between steel slag and CO2 under gas–solid conditions. For example, Japan’s JFE Steel used the dry carbonation of steel slag to produce “marine blocks” [138]. However, this process requires a high CO2 concentration and pressure, and the carbonation efficiency is limited, typically ranging from 4% to 20%, mainly due to the formation of a dense CaCO3 layer that hinders further carbonation. Due to the relatively low efficiency of dry carbonation, many researchers have focused on wet carbonation. Wet carbonation primarily occurs through gas–water or gas–alkaline liquid reactions, enabling steel slag to react with CO2 in the solution. Liu’s research indicated that adding a small amount of water (liquid-to-solid ratio of approximately 0.2 L/kg) increased the reaction rate, with carbonation efficiency improving to 30% [139]. Yao’s study found that under optimal conditions (high temperature, high pressure, and sufficient stirring), the degree of carbonation could reach 74% [140]. However, wet carbonation requires a significant amount of water, and the solid calcium carbonate formed on the surface can still hinder deep carbonation. Indirect carbonation involves the use of acids, bases, or salts to dissolve steel slag and extract calcium and magnesium ions, followed by a carbonation reaction. The disadvantage of this method is that it requires additional energy or chemical reagents. Based on the differences in solution acidity and alkalinity, Table 10 summarizes the common indirect carbonation methods.

Table 10.
Indirect carbonation of steel slag.
In indirect carbonation, calcium ions are extracted from steel slag and then react with CO2 to form CaCO3. The process promotes calcium leaching and carbonation depth by adjusting the pH of the solution or adding ion-enhancing agents. For example, Gao’s study indicated that adding NaCl could increase the carbonation efficiency of steel slag from 59% to 71% [146]. Li conducted carbonation experiments on steel slag using a 5% NaOH solution, and the results showed that carbonation could be achieved in an environment as low as −10 °C [147]. Paul used 85% concentrated sulfuric acid and 15% diluted sulfuric acid for carbonation experiments on steel slag, and the results showed that concentrated sulfuric acid achieved a carbonation efficiency of 68%, much higher than the 32% efficiency of diluted sulfuric acid [148]. However, the indirect carbonation process requires large amounts of acid, base, or salt solutions, which increases the cost in practical applications and complicates the subsequent treatment of waste liquids. Therefore, the indirect carbonation process needs to improve the recovery rate of leaching agents to enhance its economic feasibility.
6.3. Comparison of Properties of Steel Slag Before and After Carbonation
Carbonation improved the microstructure and physical properties of steel slag and enhanced its potential for use in road construction. Table 11 compares the properties of steel slag before and after carbonation.

Table 11.
Properties of steel slag before and after carbonation.
From Table 11, it can be concluded that steel slag that has not undergone carbonation is suitable for temporary or secondary road construction projects, but it requires additional treatment processes to reduce risks. On the other hand, carbonated steel slag is more appropriate for road projects with higher loads and long-term use, such as subgrade materials for highways or urban main roads.
The most significant change resulting from the carbonation treatment of steel slag is the transformation of its chemical composition. The carbonation reaction converts CaO in steel slag into CaCO3, effectively reducing the alkalinity of the slag. Wang’s study indicated that the CaO content of carbonated steel slag significantly decreased, and the formation of carbonates enhanced its stability. During the carbonation process, the reaction between CaO and CO2 not only reduced the concentration of CaO but also possibly altered the interactions of other components in the slag, leading to a reduction in the release of soluble metals [156]. This change significantly lowers the environmental risks of steel slag. Wei’s research showed that after carbonation treatment, the pore structure of the steel slag changed, with reduced porosity and pore size distribution. The carbonated steel slag exhibited a lower specific surface area and higher density [157]. Wang’s study indicated that the overall structure of the carbonated steel slag became denser, mainly due to the change in the crystal structure when CaO was converted into CaCO3 [158]. Furthermore, Lou’s research showed that carbonated steel slag exhibited better water stability and freeze resistance, which broadens its potential for use in construction materials [159]. According to Xie’s findings, the high alkalinity of steel slag is one of the primary sources of its environmental pollution. Carbonation treatment significantly reduced the alkalinity of the slag [160]. Kuo’s research demonstrated that the pH of the carbonated steel slag decreased drastically, approaching neutral, which effectively mitigated its negative environmental impact [161]. Patel utilized high-concentration CO2 from blast furnace off-gases to perform carbonation treatment on steel slag, and the results showed that the pH of the slag dropped from 13.2 to 7.5 after carbonation. This change not only reduced water pollution but also made the steel slag more suitable for soil improvement and building material production [162]. According to Lind’s study, heavy metal leaching tests were conducted on steel slag before and after carbonation, and the results showed that CaCO3 in the slag could encapsulate some harmful metals, leading to the conclusion that carbonation treatment significantly reduced the leaching of harmful metals from the slag [163]. Milad’s research indicated that carbonation treatment reduced the leaching of heavy metals from steel slag by more than 40%, making the use of carbonated steel slag in environmentally friendly construction materials possible [164]. In addition, an important advantage of steel slag carbonation is the sequestration of CO2. The carbonation reaction can convert atmospheric CO2 into stable minerals, such as CaCO3, thus achieving carbon sequestration. This process not only effectively reduces the environmental burden of steel slag but also plays a significant role in addressing global climate change. Bonoli’s study indicated that carbonation treatment not only enhanced the stability of steel slag but also effectively absorbed and sequestered CO2, providing new avenues for the resource utilization of steel slag [165].
7. Conclusions
The application of steel slag in road construction demonstrated significant value and potential. Bibliometric analysis revealed that steel slag, when used as an aggregate or subgrade material, exhibited excellent mechanical properties and durability. Its incorporation into concrete not only enhanced the stability and rutting resistance of roads but also significantly improved the strength and durability of concrete. Furthermore, advancements in steel slag processing technologies, such as the production of steel slag powder and cementitious materials, further enhanced its applicability in practical engineering. However, the stability and reactivity of steel slag in applications required optimization through modification treatments and rigorous testing to ensure long-term performance. Notably, steel slag carbonation technology provided an important approach for CO2 sequestration in road construction, offering notable environmental benefits. Future research should focus on the reactivity, stability, and carbonation mechanisms of steel slag to promote its broader application in road construction. With continuous advancements in technological innovation and standardization research, steel slag was expected to become a crucial sustainable material in the road construction industry, contributing significantly to both industry development and environmental protection.
Author Contributions
J.Y.: Conceptualization; Data curation; Formal analysis; Software; Writing. R.M.: Resources; Supervision. B.D.: Validation. H.M.: Funding acquisition. Y.W.: Funding acquisition. M.G.: Investigation. Y.S.: Investigation. Y.J.: Investigation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Jing-Jin-Ji Regional Integrated Environmental Improvement–National Science and Technology Major Project (No. 2024ZD1200404), Key R&D Program of Xinjiang Uygur Autonomous Region (2022B02021), Ordos Science and Technology Major Project (ZD20232319), and Guangdong Foundation for Program of Science and Technology Research (2023B1212060044).
Data Availability Statement
All the relevant data are within this manuscript and its additional files.
Conflicts of Interest
Authors Yujia Sun and Yonglong Jin are employed by HBIS Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zhang, Z.; Zheng, X.G.; Li, J.Y.; Xu, G.; Tan, L.J. Mechanism of reinforced interfacial adhesion between steel slag and highly devulcanized waste rubber modified asphalt and its influence on the volume stability in steel slag asphalt mixture. Constr. Build. Mater. 2024, 447, 138129. [Google Scholar] [CrossRef]
- Yang, C.; Huang, Z.W.; Wu, S.P.; He, X.Y.; Su, Y.; Zhao, Z.G.; Xu, H.Q.; Wang, F.S.; Zhang, L. Recycling steel slag as aggregate in developing an ultra-thin friction course with high comprehensive road performance. Constr. Build. Mater. 2024, 449, 138539. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chen, Z.B.; Wang, S.Y.; Chen, Y.P.; Chen, G.X. Application of steel slag in road Semi-Rigid subgrade and its volume stability modification progress study. JOM 2024, 77, 400–414. [Google Scholar] [CrossRef]
- Zhao, W.X.; Wen, W.; Li, H.R.; Hu, J. Research on the performance of asphalt mixture with acid-treated steel slag based on microscopic properties. Constr. Build. Mater. 2024, 455, 139134. [Google Scholar] [CrossRef]
- Song, Y.; Xu, H.Q.; Wu, S.P.; Xie, J.; Chen, A.Q.; Lv, Y.; Cheng, Y.X.; Li, Y.Y. High-quality utilization of reclaimed asphalt pavement (RAP) in asphalt mixture with the enhancement of steel slag and epoxy asphalt. Constr. Build. Mater. 2024, 445, 137963. [Google Scholar] [CrossRef]
- Chen, C.; Deng, Q.H.; Li, C.M.; Yi, S.B.; Liu, L.B. Research on the preparation and self-healing performance of microwave-induced functional steel slag asphalt mixture. Case Stud. Constr. Mater. 2024, 20, e03038. [Google Scholar] [CrossRef]
- Puma, G.; Salles, A.; Turk, J.; Ungureanu, V.; Bragança, L. Utilisation of reused steel and slag: Analysing the circular economy benefits through three case studies. Buildings 2024, 14, 979. [Google Scholar] [CrossRef]
- Kamalasekar, A.; Murugasan, R.; Makendran, C.; Francis, M.R. Static and cyclic behaviour of fibre-reinforced pavement concrete with copper slag as fine aggregate. Materia 2024, 29, e20230323. [Google Scholar] [CrossRef]
- Kedar, H.N.; Patel, S.; Shirol, S.S. Bulk utilization of steel slag-fly ash composite: A sustainable alternative for use as road construction materials. Innov. Infrastruct. Solut. 2024, 9, 21. [Google Scholar] [CrossRef]
- Fan, M.M.; Lyu, Z.; Liu, L.; Qin, J.X.; Liang, G.R.; Huang, N.J. Preparation of pavement base material by using steel slag powder and steel slag aggregate. Materia 2024, 29, e20240257. [Google Scholar] [CrossRef]
- Yuan, B.; Zhao, D.N.; Lei, J.L.; Song, S.Q. Preparation and performance testing of steel slag concrete from steel solid waste. Buildings 2024, 14, 2437. [Google Scholar] [CrossRef]
- Wu, H.Y.; Xu, F.; Li, B.Y.; Gao, Q.J. Study on Expansion Rate of Steel Slag Cement-Stabilized Macadam Based on BP Neural Network. Materials 2024, 17, 3558. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.; Silva, H.; Oliveira, J.; Loureiro, C. A life cycle assessment of an asphalt mixture with steel slag and reclaimed asphalt. In Proceedings of the 10th International Conference on Maintenance and Rehabilitation of Pavements, MAIREPAV-10, Guimarães, Portugal, 24–26 July 2024; Pereira, P., Pais, J., Eds.; Springer: Cham, Switzerland, 2024; Volume 1, pp. 605–615. [Google Scholar]
- Kumar, H.; Varma, S. A review on utilization of steel slag in hot mix asphalt. Int. J. Pavement Res. Technol. 2021, 14, 232–242. [Google Scholar] [CrossRef]
- Dondi, G.; Mazzotta, F.; Lantieri, C.; Cuppi, F.; Vignali, V.; Sangiovanni, C. Use of steel slag as an alternative to aggregate and filler in road pavements. Materials 2021, 14, 345. [Google Scholar] [CrossRef]
- Jonczy, I.; Grzesik, B. Polymorphic transformations of dicalcium silicates in steel slags used in the production of road aggregates. Gospod. Surowcami Min. 2021, 37, 97–116. [Google Scholar] [CrossRef]
- Wang, X.Y.; Hui, Y.X.; Xu, X.Q.; Xu, T.F.; Zhao, T.; Li, B.W. Multi-scale study on the whole process adhesion behaviour of steel slag and SBS/CR composite modified asphalt. Int. J. Pavement Eng. 2024, 25, 2419937. [Google Scholar] [CrossRef]
- Korkmaz, B.; Dayioglu, A.Y. Utilization of natural soils as a remediation method for electric arc furnace and ladle slags. Sustainability 2024, 16, 5244. [Google Scholar] [CrossRef]
- Liu, L.; Liu, L.X.; Liu, Z.H.; Yang, C.C.; Li, X.; Huang, Y. Effects of the aging treatment process on the properties of steel slag. J. Mater. Civ. Eng. 2024, 36, 04024019. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Zhang, Y.S.; Wu, X.; Zhang, F.Q.; Zhang, J.L.; Li, X.M. The mechanical strength, microstructure, and transport properties of steel slag reinforced loess soil system. Case Stud. Constr. Mater. 2024, 20, e02702. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, D.; Falchetto, A.C.; Cao, Y.S. Microwave deicing properties and carbon emissions assessment of asphalt mixtures containing steel slag towards resource conservation and waste reuse. Sci. Total Environ. 2024, 912, 169189. [Google Scholar] [CrossRef]
- Yang, J.; Hong, C.; Xing, Y.; Zheng, Z.X.; Li, Z.X.; Zhao, X.M.; Qi, C.H. Research progress and hot spots of hydrothermal liquefaction for bio-oil production based on bibliometric analysis. Environ. Sci. Pollut. Res. 2021, 28, 7621–7635. [Google Scholar] [CrossRef] [PubMed]
- Alnadish, A.M.; Ramu, M.B.; Kasim, N.; Alawag, A.M.; Baarimah, A.O. A bibliometric analysis and review on applications of industrial By-Products in asphalt mixtures for sustainable road construction. Buildings 2024, 14, 3240. [Google Scholar] [CrossRef]
- Hassan, H.F.; Al-Shamsi, K.; Al-Jabri, K. Effect of steel slag on the permanent deformation and life cycle cost of asphalt concrete pavements. Int. J. Pavement Res. Technol. 2024, 17, 1513–1530. [Google Scholar] [CrossRef]
- Yang, X.K.; Wu, S.P.; Chen, B.Y.; Ye, G.; Xu, S. Development of a sustainable stabilized macadam road base using steel slag as supplementary cementitious material. Constr. Build. Mater. 2024, 449, 138566. [Google Scholar] [CrossRef]
- Rahaman, O.; Sarker, A.; Das Hasi, A.; Al Mamun, A. A case study on steel slag as an alternative sustainable material for subbase layer. In Proceedings of the International Conference on Transportation and Development 2024: Pavements and Infrastructure Systems, ICTD 2024, Atlanta, GA, USA, 15–18 June 2024; Wei, H., Ed.; ASCE: Reston, VA, USA, 2024; pp. 93–106. [Google Scholar]
- Mymrin, V.A.; Ponte, H.A.; Yamamoto, C.I. Synthesis of new colloidal formations during the strengthening of different activated hydrated metallurgical slags. Colloids Surf. A Physicochem. Eng. Asp. 2003, 220, 211–221. [Google Scholar] [CrossRef]
- Lin, Z.Z.; Yang, H.; Chen, H.M.; Ouyang, X.Y.; Liu, Z.Q. Comparison of the decontamination performance of three permeable bricks: Adsorption and filtration experiments. Pol. J. Environ. Stud. 2020, 29, 3225–3233. [Google Scholar] [CrossRef] [PubMed]
- Mukiza, E.; Zhang, L.L.; Liu, X.M. Durability and microstructure analysis of the road base material prepared from red mud and flue gas desulfurization fly ash. Int. J. Min. Met. Mater. 2020, 27, 555–568. [Google Scholar] [CrossRef]
- Son, P.; Van Nam, N.; Tran, N.P.; Le-Hoai, L.; Ngo, T.D. Steel slag aggregate low-cement concrete: Engineering performance, microstructure and sustainability. Constr. Build. Mater. 2024, 436, 136827. [Google Scholar] [CrossRef]
- Plati, C.; Tsakoumaki, M.; Loizos, A. Quality assurance of steel slag asphalt mixtures for sustainable pavement surface courses. Recycling 2024, 9, 91. [Google Scholar] [CrossRef]
- Zepper, J.; van der Laan, S.R.; Schollbach, K.; Brouwers, H. A Bogue approach applied to basic oxygen furnace slag. Cement Concrete Res. 2024, 175, 107344. [Google Scholar] [CrossRef]
- Abed, Z.M.; Khalil, W.I.; Ahmed, H.K. Effect of waste tire products on some characteristics of roller-compacted concrete. Open Eng. 2024, 14, 20220559. [Google Scholar] [CrossRef]
- Ji, K.; Tian, Y.G.; Jiang, J.; Yan, X.H.; Tian, J.; Wang, Z.J.; Zhang, J. The void characteristics of cement emulsified bitumen mixture under microwave heating by X-ray computed tomography. Constr. Build. Mater. 2024, 425, 136023. [Google Scholar] [CrossRef]
- Ziari, H.; Zalnezhad, M.; Ziari, M.A. Performance evaluation of colored slurry seal mixture with steel slag as a substituent of natural aggregates. J. Mater. Civ. Eng. 2024, 36, 04024192. [Google Scholar] [CrossRef]
- Xu, S.; Xu, W.; Chen, Y.X.; Li, J.Q.; Li, Y.G. Enhancement of Microwave Heating Technology for Emulsified Asphalt Mixtures Using SiC-Fe3O4 Composite Material. Materials 2024, 17, 4572. [Google Scholar] [CrossRef] [PubMed]
- Sorociak, W.; Grzesik, B.; Szoltysik, J.; Bzówka, J.; Mieczkowski, P.; Klemens, M. Analysis of reclaimed asphalt pavement heating with microwave radiation. Arab. J. Sci. Eng. 2024, 515, 1–14. [Google Scholar] [CrossRef]
- Feng, T.H.; Wang, W.; Li, N.; Luo, J.L.; Li, B.; Jiang, P.; Pu, S.Y. Mechanical properties and microscopic mechanism of steel slag, sodium sulfate and cement stabilized road demolition waste. Results Eng. 2024, 24, 103338. [Google Scholar] [CrossRef]
- Huang, L.Y.; Wei, G.G.; Lan, Z.X.; Chen, Y.L.; Li, T. Preparation and mechanism analysis of stainless steel AOD slag mixture base materials. Materials 2024, 17, 970. [Google Scholar] [CrossRef]
- Deng, B.; Zeng, G.W.; Ge, R. A creep model of steel Slag-Asphalt mixture based on neural networks. Appl. Sci. 2024, 14, 5820. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Lu, Z.Y.; Chen, A.Q.; Wu, S.P.; Feng, J.L.; Xu, H.Q.; Li, Y.Y. Study on the performance of Epoxy-Modified asphalt and steel slag Ultra-Thin friction course. Materials 2024, 17, 4513. [Google Scholar] [CrossRef]
- Lopes, E.C.; Da Silva, T.O.; Pitanga, H.N.; Pedroti, L.G.; de Carvalho, J.; Nalon, G.H.; de Araújo, E.; Rodrigues, K. Chemical, mineralogical, microstructural and engineering properties of tropical soils stabilised with the combined and individual use of different types of steel slag. Road Mater. Pavement 2024, 25, 1507–1527. [Google Scholar] [CrossRef]
- Rodríguez-Alloza, A.M.; Gulisano, F.; Garraín, D. Environmental benefits of microwave-assisted self-healing technology for pavements—A Life Cycle Assessment comparative study. Mater. Constr. 2024, 74, e346. [Google Scholar] [CrossRef]
- Jahanbakhsh, H.; Nejad, F.M.; Khodaii, A.; Karimi, M.M. Induction heating and induced healing evaluation of the asphalt concretes incorporating conductive aggregates exposed to microwave radiation. Constr. Build. Mater. 2024, 416, 135126. [Google Scholar] [CrossRef]
- Moussaoui, R.; El Alami, S.E.; Aouraghe, H. Valorization of the zellidja lead smelter slag (Eastern morocco) in road building. Int. J. Pavement Res. Technol. 2024, 17, 1620–1636. [Google Scholar] [CrossRef]
- Zhao, W.T.; Yang, Q. Life-cycle assessment of sustainable pavement based on the coordinated application of recycled asphalt pavement and solid waste: Environment and economy. J. Clean. Prod. 2024, 434, 140203. [Google Scholar] [CrossRef]
- Sofilic, T.; Mladenovic, A.; Sofilic, U. Defining of eaf steel slag application possibilities in asphalt mixture production. J. Environ. Eng. Landsc. 2011, 19, 148–157. [Google Scholar] [CrossRef]
- Qi, Y.J.; Indraratna, B.; Ngo, T.; Arachchige, C.; Hettiyahandi, S. Sustainable solutions for railway using recycled rubber. Transp. Geotech. 2024, 46, 101256. [Google Scholar] [CrossRef]
- He, Y.Z.; Xiong, K.; Zhang, J.P.; Guo, F.C.; Li, Y.; Hu, Q.S. A state-of-the-art review and prospectives on the self-healing repair technology for asphalt materials. Constr. Build. Mater. 2024, 421, 135660. [Google Scholar] [CrossRef]
- Chien, H.T.; Chang, J.R.; Hsu, H.M. Determining the content of steel furnace slag in asphalt concrete. Case Stud. Constr. Mater. 2023, 19, e02399. [Google Scholar] [CrossRef]
- Guo, X.; Zeng, M.L.; Yu, H.D.; Lin, F.W.; Li, J.W.; Wang, W.L.; Chen, G.Y. Critical review for the potential analysis of material utilization from inorganic industrial solid waste. J. Clean. Prod. 2024, 459, 142457. [Google Scholar] [CrossRef]
- Jakati, S.S.; Kumar, G.S. Novel cold pothole patching mixtures utilizing EAF steel slag and pyro-oil from municipal plastic solid waste. Innov. Infrastruct. Solut. 2024, 9, 55. [Google Scholar] [CrossRef]
- Mittal, L.; Perry, C.S.; Blanchette, A.D.; Proctor, D.M. Probabilistic risk assessment of residential exposure to electric arc furnace steel slag using Bayesian model of relative bioavailability and PBPK modeling of manganese. Risk Anal. 2024, 44, 2169–2186. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.Y.; Xiang, H.; Hu, X.D.; Wu, H.; Gao, B.; Wan, J.M.; Rao, Z.; Fan, Z.W.; Ma, Y. Evaluation of the influence of Phosphogypsum-Based composite filler on performance of the SMA-13 asphalt mixture and its harmless treatment. Sustainability 2024, 16, 6613. [Google Scholar] [CrossRef]
- Coelho, L.M.; Guimaraes, A.; Moreira, C.; Dos Santos, G.; Monteiro, S.N.; Da Silveira, P. Feasibility of using ferronickel slag as a sustainable alternative aggregate in hot mix asphalt. Sustainability 2024, 16, 8642. [Google Scholar] [CrossRef]
- Li, P.F.; Ji, J.; Wen, L.; Chen, H.; Bian, L.B.; Zhou, W.J.; Wu, Y.B. Quantitative characterization and evaluation of key physicochemical characteristics of steel slag. Constr. Build. Mater. 2024, 414, 134959. [Google Scholar] [CrossRef]
- Ou, L.; Zhu, H.Z.; Chen, R.P.; Su, C.L.; Yang, X.S. Effect of industrial solid waste as fillers on the rheology and surface free energy of asphalt mastic. Materials 2024, 17, 1125. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, K.W.; Yun, Y.M.; Le, T. Evaluation of eco-friendly asphalt mixtures incorporating waste plastic aggregates and additives: Magnesium, fly ash, and steel slag. Case Stud. Constr. Mater. 2024, 20, e02756. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, S.K.; Akbar, M.A. A detailed investigation on assessing the introduction of hybrid fibres in Geopolymer composites for pavement application. Int. J. Pavement Eng. 2024, 25, 2403681. [Google Scholar] [CrossRef]
- Chandel, S.S.; Singh, P.K.; Katiyar, P.K.; Randhawa, N.S. A review on environmental concerns and technological innovations for the valorization of steel industry slag. Min. Metall. Explor. 2023, 40, 2059–2086. [Google Scholar] [CrossRef]
- Liu, J.N.; Wang, Z.J.; Jing, H.S.; Zhang, T.H.; Wang, X.F.; Zhou, X.W.; Xu, X. Self-healing properties of steel slag asphalt mixture based on experimental characterization and 3D reconstruction. Mater. Design 2023, 234, 112358. [Google Scholar] [CrossRef]
- Guo, J.; Bao, Y.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef]
- Dang, D.T.; Nguyen, M.T.; Nguyen, T.P.; Isawa, T.; Ta, Y.; Sato, R. Mechanical properties of steel slag replaced mineral aggregate for road base/sub-base application based Vietnam and Japan standard. Environ. Sci. Pollut. Res. 2022, 29, 42067–42073. [Google Scholar] [CrossRef] [PubMed]
- Gowda, K.; Naveen, G.M. Laboratory performance of asphalt mixtures incorporating electric arc furnace slag and basic oxygen furnace slag as partial replacement for conventional aggregates. Energy Ecol. Environ. 2023, 8, 586–595. [Google Scholar] [CrossRef]
- Liu, J.N.; Yuan, L.J.; Wang, Z.J.; Jing, H.S.; Shao, T.Q.; Chen, H. A new method for evaluating the uniformity of steel slag distribution in steel slag asphalt mixture based on deep learning. Constr. Build. Mater. 2023, 400, 132766. [Google Scholar] [CrossRef]
- Zepper, J.; van der Laan, S.R.; Schollbach, K.; Brouwers, H. Reactivity of BOF slag under autoclaving conditions. Constr. Build. Mater. 2023, 364, 129957. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, W.; Wu, S.P. Analysis on factors affecting moisture stability of steel slag asphalt concrete using grey correlation method. J. Clean. Prod. 2023, 397, 136490. [Google Scholar] [CrossRef]
- Wan, B.C.; Chen, X.Q.; Dong, Q.; Jiang, D.Q. Study on Cracking Behavior of Steel Slag Asphalt Mixture at Different Healing Efficiencies Based on Discrete Element Method. In Proceedings of the 13th International Conference on Road and Airfield Pavement Technology 2023, Beijing, China, 6–8 July 2023; Yao, H., Zhang, J., Fang, F.T., Wang, C., Eds.; ASCE: Reston, VA, USA, 2023; pp. 155–167. [Google Scholar]
- Li, X.Q.; Wu, S.P.; Wang, F.S.; You, L.Y.; Yang, C.; Cui, P.D.; Zhang, X.M. Quantitative assessments of GHG and VOCs emissions of asphalt pavement contained steel slag. Constr. Build. Mater. 2023, 369, 130606. [Google Scholar] [CrossRef]
- Schneider, P.; Ahmed, N.; Mihai, F.C.; Belousova, A.; Kucera, R.; Oswald, K.D.; Lange, T.; Le Hung, A. Life cycle assessment for substitutive building materials using the example of the vietnamese road sector. Appl. Sci. 2023, 13, 6264. [Google Scholar] [CrossRef]
- Kamata, A.; Miura, T.; Katoh, M. Suppression of arsenic leaching from excavated soil and the contribution of soluble and insoluble components in steel slag on arsenic immobilization. Environ. Sci. Pollut. Res. 2023, 30, 19946–19957. [Google Scholar] [CrossRef]
- Neves, J.; Crucho, J. Performance evaluation of steel slag asphalt mixtures for sustainable road pavement rehabilitation. Appl. Sci. 2023, 13, 5716. [Google Scholar] [CrossRef]
- Ma, W.; Wang, N.Q.; Yan, X.S.; Dong, C.X.; Duan, Z.; Li, B. Research on fracture damage characteristics of loess solidified with steel slag, calcium carbide slag and metakaolin. Eng. Fract. Mech. 2023, 289, 109389. [Google Scholar] [CrossRef]
- Sukmak, P.; Sukmak, G.; De Silva, P.; Horpibulsuk, S.; Kassawat, S.; Suddeepong, A. The potential of industrial waste: Electric arc furnace slag (EAF) as recycled road construction materials. Constr. Build. Mater. 2023, 368, 130393. [Google Scholar] [CrossRef]
- Alves, R.; Rios, S.; Fortunato, E.; Da Fonseca, A.V.; Delgado, B.G. Mechanical behaviour of steel Slag-Rubber mixtures: Laboratory assessment. Sustainability 2023, 15, 1563. [Google Scholar] [CrossRef]
- Piemonti, A.; Conforti, A.; Cominoli, L.; Luciano, A.; Plizzari, G.; Sorlini, S. Exploring the potential for steel slags valorisation in an industrial symbiosis perspective at meso-scale level. Waste Biomass Valorization 2023, 14, 3355–3375. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.F.; Hu, X.D.; Wan, J.M.; Wu, S.P.; Gan, W.X.; Zhang, Y.L. Enhanced thermal insulation of steel slag based on ultrathin friction course with ceramic fiber. J. Mater. Civ. Eng. 2023, 35, 04023374. [Google Scholar] [CrossRef]
- Lopes, E.C.; Da Silva, T.O.; Pitanga, H.N.; Pedroti, L.G.; de Carvalho, J.; Nalon, G.H.; de Lima, G.; de Araújo, E. Stabilisation of clayey and sandy soils with ladle furnace slag fines for road construction. Road Mater. Pavement 2023, 24, 247–266. [Google Scholar] [CrossRef]
- Jayaneththi, Y.H.; Robert, D.; Giustozzi, F. A critical review on leaching of contaminants from asphalt pavements. Sci. Total Environ. 2024, 950, 174967. [Google Scholar] [CrossRef] [PubMed]
- Kuttah, D. Recycling of industrial and construction waste materials in roads construction. Int. J. Geotech. Eng. 2024, 18, 346–359. [Google Scholar] [CrossRef]
- Yang, C.; Xie, J.; Wu, S.P.; Amirkhanian, S.; Zhou, X.J.; Kong, D.Z.; Ye, Q.S.; Hu, J. Revelation and characterization of selective absorption behavior of bitumen to basic oxygen furnace slag. Constr. Build. Mater. 2020, 253, 119210. [Google Scholar] [CrossRef]
- Indraratna, B.; Arachchige, C.; Rujikiatkamjorn, C.; Heitor, A.; Qi, Y.J. Utilization of granular wastes in transportation infrastructure. Geotech. Test. J. 2024, 47, 409–424. [Google Scholar] [CrossRef]
- An, R.; Zhang, X.W.; Wang, Y.X.; Chen, C.; Chen, X. Dynamic evolution of cracks in Slag-Modified soil under uniaxial loading using Real-Time X-Ray computed tomography. J. Mater. Civ. Eng. 2023, 35, 04023159. [Google Scholar] [CrossRef]
- Xu, H.; Sun, M.Z.; Luo, G.B. Enhanced induction heating and Self-Healing properties of steel slag powder based asphalt and asphalt mixture under microwave irradiation. Materials 2023, 16, 3312. [Google Scholar] [CrossRef] [PubMed]
- Castillo, D.; Falchetto, A.C.; Korkiala-Tanttu, L. Results of surface hot-in place recycling (remix) of modified and alternative asphalt mixtures in Finland. Part I: Mixture scale. Int. J. Pavement Eng. 2023, 24, 2134568. [Google Scholar] [CrossRef]
- Segui, P.; Safhi, A.E.; Amrani, M.; Benzaazoua, M. Mining wastes as road construction material: A review. Minerals 2023, 13, 90. [Google Scholar] [CrossRef]
- Lu, X.L.; Yu, Q.; Xu, J.Y.; Yue, B.; Sheng, M.Q. Comparative experimental study on strength properties of red clay modified by cement and industrial solid waste powder. Adv. Civ. Eng. 2023, 2023, 6645563. [Google Scholar] [CrossRef]
- Zhang, L.T.; Zhang, Z.H.; Yu, W.X.; Miao, Y.H. Review of the application of microwave heating technology in asphalt pavement Self-Healing and de-icing. Polymers 2023, 15, 1696. [Google Scholar] [CrossRef]
- Ma, J.; Wang, X.D.; Zhang, Z.; Dai, G.J.; Huo, Y.F.; Zhao, Y.F. Performance Analysis of Industrial-Waste-Based Artificial Aggregates: CO2 Uptake and Applications in Bituminous Pavement. Buildings 2023, 13, 2823. [Google Scholar] [CrossRef]
- Luo, H.; Yang, J.; Zhang, W.H.; Yang, M.Y.; Chen, L.M.; He, B.J. Cooling and purification effect of permeable pavements. J. Water Clim. Change 2023, 14, 2626–2641. [Google Scholar] [CrossRef]
- Pazzini, M.; Tarsi, G.; Tataranni, P.; Lantieri, C.; Dondi, G. Mechanical characterization of thin asphalt overlay mixtures with 100% recycled aggregates. Materials 2023, 16, 188. [Google Scholar] [CrossRef]
- Espinosa, A.B.; Revilla-Cuesta, V.; Skaf, M.; Serrano-López, R.; Ortega-López, V. Strength performance of low-bearing-capacity clayey soils stabilized with ladle furnace slag. Environ. Sci. Pollut. Res. 2023, 30, 101317–101342. [Google Scholar] [CrossRef]
- Su, X.T.; Yu, L.C.; Chen, J.; Li, H.F.; Liu, Q.; Zhang, H.G.; Liu, T. Mechanical properties and microstructure of SWGA-BF improved rock muck discharged from slurry shield tunnels. Constr. Build. Mater. 2023, 409, 133969. [Google Scholar] [CrossRef]
- Yang, C.; Wu, S.P.; Cui, P.D.; Amirkhanian, S.; Zhao, Z.G.; Wang, F.S.; Zhang, L.; Wei, M.H.; Zhou, X.X.; Xie, J. Performance characterization and enhancement mechanism of recycled asphalt mixtures involving high RAP content and steel slag. J. Clean. Prod. 2022, 336, 130484. [Google Scholar] [CrossRef]
- Liu, J.N.; Wang, Z.J.; Li, M.; Wang, X.F.; Wang, Z.H.; Zhang, T.H. Microwave heating uniformity, road performance and internal void characteristics of steel slag asphalt mixtures. Constr. Build. Mater. 2022, 353, 129155. [Google Scholar] [CrossRef]
- Cao, Y.S.; Sha, A.M.; Liu, Z.Z.; Zhang, F.; Li, J.R.; Liu, H. Thermal conductivity evaluation and road performance test of steel slag asphalt mixture. Sustainability 2022, 14, 7288. [Google Scholar] [CrossRef]
- Razzaq, W.; Gowida, A.; Samsuri, A.; Elkatatny, S. Application of silicomanganese fume as a novel bridging material for Water-Based drilling fluids. ACS Omega 2023, 8, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Nguyen, T.D.; Tran, T.; Nguyen, D.L.; Tran, H.S.; Nguyen, T.L.; Nguyen, T.H.; Nguyen, H.G.; Nguyen, T.P.; Nguyen, N.T.; et al. Steel slag quality control for road construction aggregates and its environmental impact: Case study of Vietnamese steel industry-leaching of heavy metals from steel-making slag. Environ. Sci. Pollut. Res. 2022, 29, 41983–41991. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.M.; Yan, F.; Guo, R.X.; He, H. Study on the performance of steel slag and its asphalt mixture with oxalic acid and water erosion. Materials 2022, 15, 6642. [Google Scholar] [CrossRef] [PubMed]
- Bethary, R.T.; Subagio, B.S. Resilient modulus model of asphalt mixture using steel slag. Mag. Civ. Eng. 2022, 115, 11505. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Zhang, Y.; Adhikari, S. Life cycle assessment of asphalt and cement pavements: Comparative cases in Shanxi Province. Constr. Build. Mater. 2022, 315, 125738. [Google Scholar] [CrossRef]
- Ahirwar, A.D.; Chore, H.S. Strength characterization of steel slag-cement-stabilized marine clay mixes for highway construction. Innov. Infrastruct. Solut. 2022, 7, 254. [Google Scholar] [CrossRef]
- Schumacher, A.G.; Gomes, G.; Schneider, D.; Pires, P.; Gomes, R. Blending Linz-Donawitz and Blast Furnace slags with the Kambara reactor byproduct to improve their reuse in roadworks. J. Mater. Cycles Waste 2022, 24, 2555–2568. [Google Scholar] [CrossRef]
- Mohamad, A.Y.; Jamil, M.; Yusoff, N.; Fadzil, S.M.; Taha, M.R. Physical, microstructure and leaching assessments for pavement road base containing mixed steel slag and cathode ray tube glass. Clean. Technol. Environ. 2022, 24, 919–930. [Google Scholar] [CrossRef]
- Gan, Y.W.; Li, C.M.; Ke, W.; Deng, Q.H.; Yu, T. Study on pavement performance of steel slag asphalt mixture based on surface treatment. Case Stud. Constr. Mater. 2022, 16, e01131. [Google Scholar] [CrossRef]
- Luan, Y.C.; Zhang, W.G.; Zhao, Y.L.; Pan, Z.J.; Niu, Z.; Zeng, K.; Chen, X.B.; Mohammad, L.N. Mechanical property evaluation for steel slag in asphalt mixture with different skeleton structures using modified marshall mix design methodology. J. Mater. Civ. Eng. 2022, 34, 04021382. [Google Scholar] [CrossRef]
- Shu, K.Q.; Sasaki, K. Occurrence of steel converter slag and its high value-added conversion for environmental restoration in China: A review. J. Clean. Prod. 2022, 373, 133876. [Google Scholar] [CrossRef]
- Li, Y.Y.; Li, J.; Li, C.; Chen, A.Q.; Bai, T.; Tang, S.M.; Wu, S.P.; Gao, Y.M.; Zhu, H.B.; Feng, J.L. Strength formation mechanism and performance of steel slag self-compacting epoxy resin concrete. Constr. Build. Mater. 2022, 359, 129525. [Google Scholar] [CrossRef]
- Magalhaes, A.J.; Gomes, G.; Pires, P. Toward improved performance of unpaved roads: Laboratory tests and field investigation of a soil-byproduct base layer. Road Mater. Pavement 2022, 23, 184–198. [Google Scholar] [CrossRef]
- Karadag, H.; Firat, S.; Isik, N.S.; Yilmaz, G. Determination of permanent deformation of flexible pavements using finite element model. Gradevinar 2022, 74, 471–480. [Google Scholar] [CrossRef]
- Goli, A. The study of the feasibility of using recycled steel slag aggregate in hot mix asphalt. Case Stud. Constr. Mater. 2022, 16, e00861. [Google Scholar] [CrossRef]
- Imashuku, S.; Wagatsuma, K. Influence of free lime precipitated in a grain boundary of wustite on volume fraction of free lime in steelmaking slag determined via cathodoluminescence imaging. ISIJ Int. 2022, 62, 941–947. [Google Scholar] [CrossRef]
- Liu, J.Z.; Xu, J.; Liu, Q.; Wang, S.Y.; Yu, B. Steel slag for roadway construction: A review of material characteristics and application mechanisms. J. Mater. Civ. Eng. 2022, 34, 03122001. [Google Scholar] [CrossRef]
- Goli, A.; Emadi, H.; Sadeghi, P. Investigating the effect of using steel slag on abrasion resistance of roller-compacted concrete pavement. Innov. Infrastruct. Solut. 2022, 7, 297. [Google Scholar] [CrossRef]
- Zhu, J.G.; Yue, H.Z.; Ma, L.J.; Li, Z.C.; Bai, R. The synergistic hydration mechanism and environmental safety of multiple solid wastes in red mud-based cementitious materials. Environ. Sci. Pollut. Res. 2023, 30, 79241–79257. [Google Scholar] [CrossRef]
- De Pascale, B.; Tataranni, P.; Lantieri, C.; Bonoli, A.; Vignali, V. Mechanical performance and environmental assessment of porous asphalt mixtures produced with EAF steel slags and RAP aggregates. Constr. Build. Mater. 2023, 400, 132889. [Google Scholar] [CrossRef]
- Sun, Y.H.; Zheng, L.J.; Cheng, Y.D.; Chi, F.X.; Liu, K.; Zhu, T.B. Research on maintenance equipment and maintenance technology of steel fiber modified asphalt pavement with microwave heating. Case Stud. Constr. Mater. 2023, 18, e01965. [Google Scholar] [CrossRef]
- Wang, H.L.; Qian, J.S.; Zhang, H.H.; Nan, X.L.; Chen, G.Z.; Li, X.M. Exploring skid resistance over time: Steel slag as a pavement aggregate-comparative study and morphological analysis. J. Clean. Prod. 2024, 464, 142779. [Google Scholar] [CrossRef]
- Cui, P.D.; Ma, T.; Wu, S.P.; Xu, G.J.; Wang, F.S. Texture characteristic and its enhancement mechanism in stone mastic asphalt incorporating steel slag. Constr. Build. Mater. 2023, 369, 130440. [Google Scholar] [CrossRef]
- Jiao, W.X.; Sha, A.M.; Liu, Z.Z.; Jiang, W.; Hu, L.Q.; Qin, W. Analytic investigations of snow melting efficiency and temperature field of thermal conductive asphalt concrete combined with electrical-thermal system. J. Clean. Prod. 2023, 399, 136622. [Google Scholar] [CrossRef]
- Nicula, L.M.; Manea, D.L.; Simedru, D.; Cadar, O.; Dragomir, M.L.; Ardelean, I.; Corbu, O. Potential role of GGBS and ACBFS blast furnace slag at 90 days for application in rigid concrete pavements. Materials 2023, 16, 5902. [Google Scholar] [CrossRef]
- Pooni, J.; Robert, D.; Giustozzi, F.; Gunasekara, C.; Setunge, S. A review on soil stabilisation of unsealed road pavements from an Australian perspective. Road Mater. Pavement 2023, 24, 1005–1049. [Google Scholar] [CrossRef]
- Pasetto, M.; Baliello, A.; Giacomello, G.; Pasquini, E. Mechanical feasibility of asphalt materials for pavement solar collectors: Small-Scale laboratory characterization. Appl. Sci. 2023, 13, 358. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, X.C.; Fu, P.F.; Lei, B.L.; Shi, J.J.; Xu, M. Synergetic mechanism of multiple industrial solid Waste-Based geopolymer binder for soil stabilization: Optimization using D-Optimal mixture design. Processes 2024, 12, 436. [Google Scholar] [CrossRef]
- Ozsoy, M.; Firat, S. Numerical analysis of road layers made with steel slag. J. Polytech. Politek. Derg. 2023, 26, 1661–1673. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Wang, X.C.; Zhang, Y.; Zhao, J.; Jiang, A.Q. Preparation, mechanism, and application of silica fume-steel slag composite cementitious material based on the D-optimal mixture method. Constr. Build. Mater. 2023, 408, 133638. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Jiang, T.H.; Li, S.Y.; Wang, W.S. Engineering properties and environmental impact of soil mixing with steel slag applied in subgrade. Appl. Sci. 2023, 13, 1574. [Google Scholar] [CrossRef]
- Alex, T.C.; Mucsi, G.; Venugopalan, T.; Kumar, S. BOF steel slag: Critical assessment and integrated approach for utilization. J. Sustain. Metall. 2021, 7, 1407–1424. [Google Scholar] [CrossRef]
- Chen, Z.M.; Li, R.; Zheng, X.M.; Liu, J.X. Carbon sequestration of steel slag and carbonation for activating RO phase. Cement Concrete Res. 2021, 139, 106271. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, W.W. Spectroscopic analysis of activated carbon mixed with steel slag composite material in sintering flue gas of desulfurization and denitration. Spectrosc. Spect. Anal. 2020, 40, 1195–1200. [Google Scholar]
- Wang, C.; Wang, W.Q.; Sardans, J.; Singla, A.; Zeng, C.S.; Lai, D.; Penuelas, J. Effects of steel slag and biochar amendments on CO2, CH4, and N2O flux, and rice productivity in a subtropical Chinese paddy field. Environ. Geochem. Health 2019, 41, 1419–1431. [Google Scholar] [CrossRef]
- Shao, Y.X.; El-Baghdadi, A.; He, Z.; Mucci, A.; Fournier, B. Carbon Dioxide-Activated steel slag for Slag-Bonded wallboard application. J. Mater. Civ. Eng. 2015, 27, 04014119. [Google Scholar] [CrossRef]
- Mahoutian, M.; Chaallal, O.; Shao, Y.X. Pilot production of steel slag masonry blocks. Can. J. Civ. Eng. 2018, 45, 537–546. [Google Scholar] [CrossRef]
- Li, H.W.; Tang, Z.G.; Li, N.; Cui, L.P.; Mao, X.Z. Mechanism and process study on steel slag enhancement for CO2 capture by seawater. Appl. Energy 2020, 276, 115515. [Google Scholar] [CrossRef]
- Zhu, H.J.; Ma, M.Y.; He, X.Y.; Zheng, Z.Q.; Su, Y.; Yang, J.; Zhao, H. Effect of wet-grinding steel slag on the properties of Portland cement: An activated method and rheology analysis. Constr. Build. Mater. 2021, 286, 122823. [Google Scholar] [CrossRef]
- Li, H.W.; Zhang, R.J.; Wang, T.Y.; Wu, Y.; Xu, R.; Wang, Q.; Tang, Z.G. Performance evaluation and environment risk assessment of steel slag enhancement for seawater to capture CO2. Energy 2022, 238, 121861. [Google Scholar] [CrossRef]
- Chen, J.; Xing, Y.; Wang, Y.; Zhang, W.B.; Guo, Z.F.; Su, W. Application of iron and steel slags in mitigating greenhouse gas emissions: A review. Sci. Total Environ. 2022, 844, 157041. [Google Scholar] [CrossRef] [PubMed]
- Shibata, E.; Sato, T.; Mori, K. Desiliconization rate of molten high carbon iron by slags containing FeO. Tetsu Hagane 1999, 85, 639–644. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Di, Z.C.; Cao, Y.; Yang, F.L.; Cheng, F.Q.; Zhang, K. Studies on steel slag as an oxygen carrier for chemical looping combustion. Fuel 2018, 226, 618–626. [Google Scholar] [CrossRef]
- Yao, D.; Yu, H.; Chen, X.; Yu, X.; Yao, J.; Zheng, X.; Zhang, C.; Gong, L. Effect of surface modification on properties of steel slag aggregate and mixture. Constr. Build. Mater. 2023, 402, 133058. [Google Scholar] [CrossRef]
- Zhao, X.R.; Sheng, Y.P.; Lv, H.L.; Jia, H.C.; Liu, Q.L.; Ji, X.; Xiong, R.; Meng, J.D. Laboratory investigation on road performances of asphalt mixtures using steel slag and granite as aggregate. Constr. Build. Mater. 2022, 315, 125655. [Google Scholar] [CrossRef]
- Jonczy, I.; Grzesik, B.; Wieczorek, A.N.; Gerle, A.; Nuckowski, P.; Staszu, M. Characteristics of the phase and chemical composition of blast furnace slag in terms of the possibility of its economic use. Gospod. Surowcami Min. 2022, 38, 153–172. [Google Scholar] [CrossRef]
- Sha, A.M.; Lou, B.W.; Barbieri, D.M.; Hoff, I. Microwave heating as an innovative road maintenance technology: Aging effect on binder and feasibility evaluation. Materials 2022, 15, 316. [Google Scholar] [CrossRef]
- Liu, M.Y.; Wang, X.C.; Deng, Y.Y.; Guo, Y.C.; Zhao, J.; Li, M.X. Study on microwave deicing efficiency of Microwave-Absorbing concrete pavements and its influencing factors. Materials 2022, 15, 8923. [Google Scholar] [CrossRef] [PubMed]
- Azadgoleh, M.A.; Mohammadi, M.M.; Ghodrati, A.; Sharifi, S.S.; Palizban, S.; Ahmadi, A.; Vahidi, E.; Ayar, P. Characterization of contaminant leaching from asphalt pavements: A critical review of measurement methods, reclaimed asphalt pavement, porous asphalt, and waste-modified asphalt mixtures. Water Res. 2022, 219, 118584. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Yang, C.; Zou, Y.X.; Wang, F.S.; Zhou, X.J.; Barbieri, D.M.; Wu, S.P. Compaction procedures and associated environmental impacts analysis for application of steel slag in road base layer. Sustainability 2021, 13, 4396. [Google Scholar] [CrossRef]
- Li, H.B.; Tong, Y.F.; Zhang, H.B.; Zhang, X.S.; Duan, J.K. Study on road performance of cement fly ash stabilized steel Slag-Concrete recycled macadam. Materials 2021, 14, 7530. [Google Scholar] [CrossRef]
- Paul, D.; Suresh, M.; Pal, M. Utilization of fly ash and glass powder as fillers in steel slag asphalt mixtures. Case Stud. Constr. Mater. 2021, 15, e00672. [Google Scholar] [CrossRef]
- Zhou, M.K.; Cheng, X.; Chen, X. Studies on the volumetric stability and mechanical properties of Cement-Fly-Ash-Stabilized steel slag. Materials 2021, 14, 495. [Google Scholar] [CrossRef] [PubMed]
- Sitko, J.; Bialy, W.; Ivanova, T.N. Analysis of impact the refining technology on the mechanical strength cast steel. Mater. Today Proc. 2021, 38, 1279–1283. [Google Scholar] [CrossRef]
- Cui, P.D.; Wu, S.P.; Xiao, Y.; Liu, Q.T.; Wang, F.S. Hazardous characteristics and variation in internal structure by hydrodynamic damage of BOF slag-based thin asphalt overlay. J. Hazard. Mater. 2021, 412, 125344. [Google Scholar] [CrossRef]
- Zhang, H.N.; Wei, C.; Dong, J.P. Inhibition kinetics of chromium leaching by calcite coating on the surface of stainless steel slag via the Gas-Solid accelerated carbonation process. Waste Biomass Valorization 2021, 12, 475–485. [Google Scholar] [CrossRef]
- Yang, T.Y.; Chen, M.Z.; Wu, S.P. Removal effect of basic oxygen furnace slag porous asphalt concrete on copper and zinc in road runoff. Materials 2021, 14, 5327. [Google Scholar] [CrossRef]
- Lee, M.Y.; Kang, J.H.; Hwang, D.G.; Yoon, Y.S.; Yoo, M.S.; Jeon, T.W. Environmental assessment of recycling (EAoR) for safe recycling of steelmaking slag in the republic of korea: Applications, leaching test, and toxicity. Sustainability 2021, 13, 8805. [Google Scholar] [CrossRef]
- Gong, J.S.; Baek, I.W.; Kim, J.G.; Song, Y.S.; Kim, T.H. A pilot test for the utilization of road subsoil of the tertiary mudstone in pohang basin. J. Korean Geosynth. Soc. 2021, 20, 1–10. [Google Scholar] [CrossRef]
- Jing, H.J.; Zhang, J.H.; Gao, M.; Liu, Q.; Wolowiec-Korecka, E. Base performances of Cement-Stabilized magnesium Slag-Aeolian sand mixture. Acta Montan. Slovaca 2021, 26, 427–443. [Google Scholar] [CrossRef]
- Wei, C.Y.; Wang, J.; Li, P.R.; Wu, B.D.; Liu, H.H.; Jiang, Y.B.; Huang, T.Y. A new strategy for sponge city construction of urban roads: Combining the traditional functions with landscape and drainage. Water 2021, 13, 3469. [Google Scholar] [CrossRef]
- Wang, F.S.; Xie, J.; Wu, S.P.; Li, J.S.; Barbieri, D.M.; Zhang, L. Life cycle energy consumption by roads and associated interpretative analysis of sustainable policies. Renew. Sustain. Energy Rev. 2021, 141, 110823. [Google Scholar] [CrossRef]
- Lou, B.W.; Liu, Z.Z.; Sha, A.M.; Jia, M.; Li, Y.P. Microwave absorption ability of steel slag and road performance of asphalt mixtures incorporating steel slag. Materials 2020, 13, 663. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yang, C.; Zhang, L.L.; Zhou, X.J.; Wu, S.P.; Ye, Q.S. Investigation of the physic-chemical properties and toxic potential of Basic Oxygen Furnace Slag (BOF) in asphalt pavement constructed after 15 years. Constr. Build. Mater. 2020, 238, 117630. [Google Scholar] [CrossRef]
- Kuo, Y.M.; Huang, K.L.; Wang, J.W.; Tsai, C.H.; Lin, S.L. An alternative approach to reclaim spent nickel-metal hydride batteries. Environ. Prog. Sustain. 2020, 39, e13433. [Google Scholar] [CrossRef]
- Patel, S.; Pai, R.R.; Bakare, M.D.; Shahu, J.T. Field evaluation of road pavement constructed with waste materials through nondestructive testing. Mater. Today Proc. 2020, 28, 1254–1260. [Google Scholar] [CrossRef]
- Lind, B.B.; Fällman, A.M.; Larsson, L.B. Environmental impact of ferrochrome slag in road construction. Waste Manag. 2001, 21, 255–264. [Google Scholar] [CrossRef]
- Milad, A.; Ali, A.; Yusoff, N. A review of the utilisation of recycled waste material as an alternative modifier in asphalt mixtures. Civ. Eng. J. 2020, 6, 42–60. [Google Scholar] [CrossRef]
- Bonoli, A.; Degli Esposti, A.; Magrini, C. A case study of industrial symbiosis to reduce GHG emissions: Performance analysis and LCA of asphalt concretes made with RAP aggregates and steel slags. Front. Mater. 2020, 7, 572955. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).