MoS2 Quantum Dot Modified Electrode: An Efficient Probe for Electrochemical Detection of Hydrazine
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
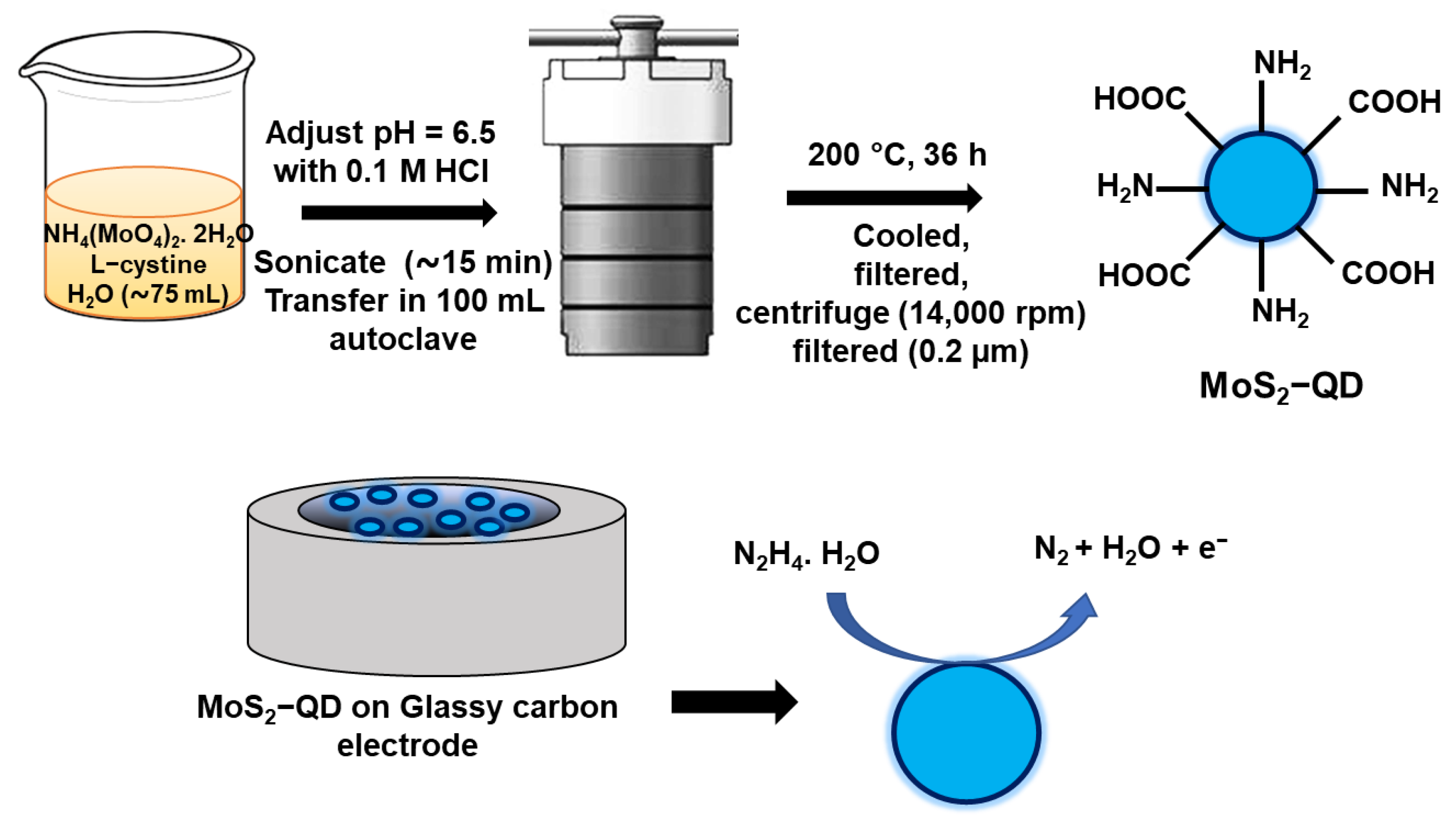
2.2. Synthesis of MoS2 Quantum Dot (MoS2 QD)
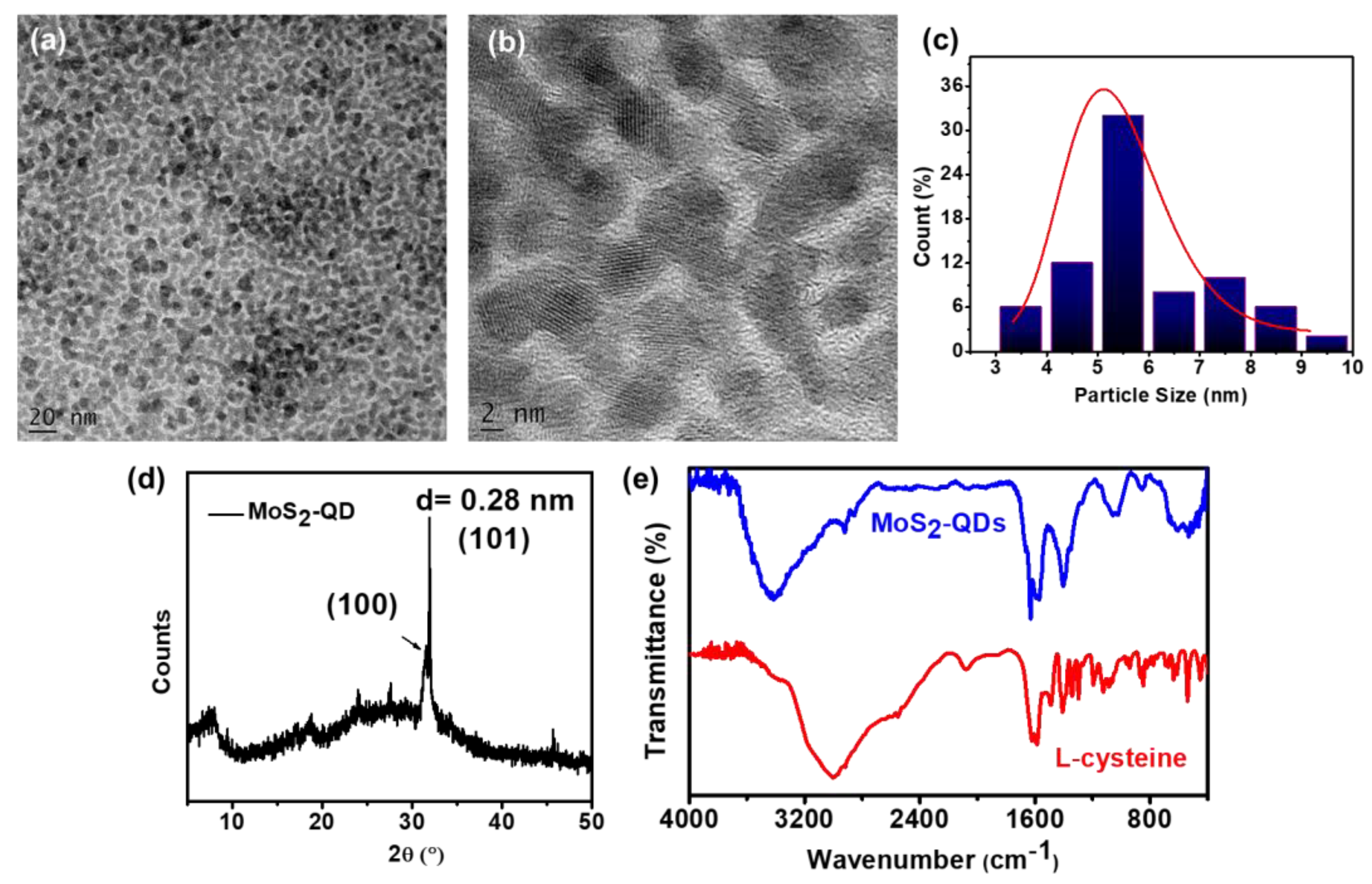
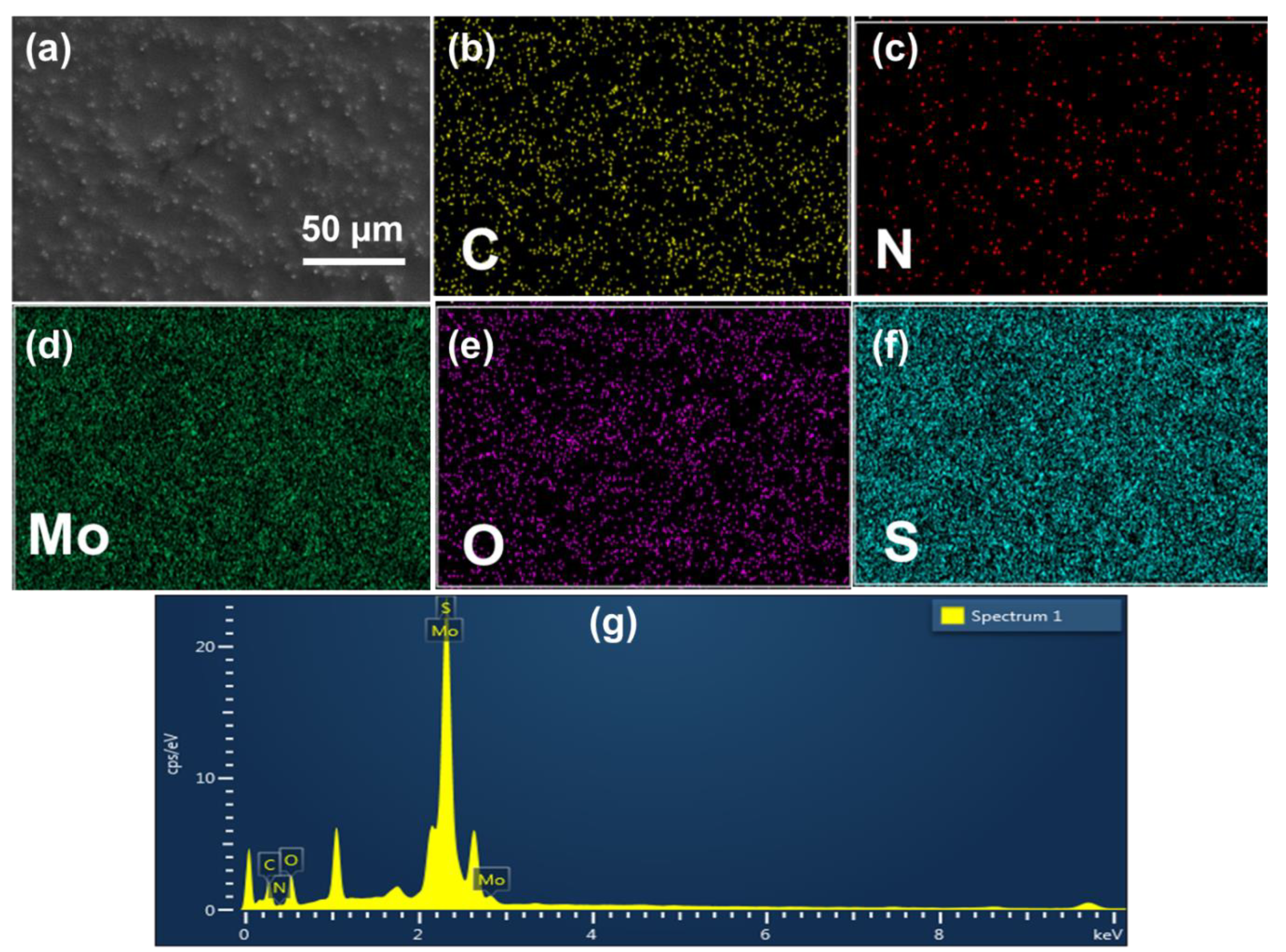
2.3. Characterizations
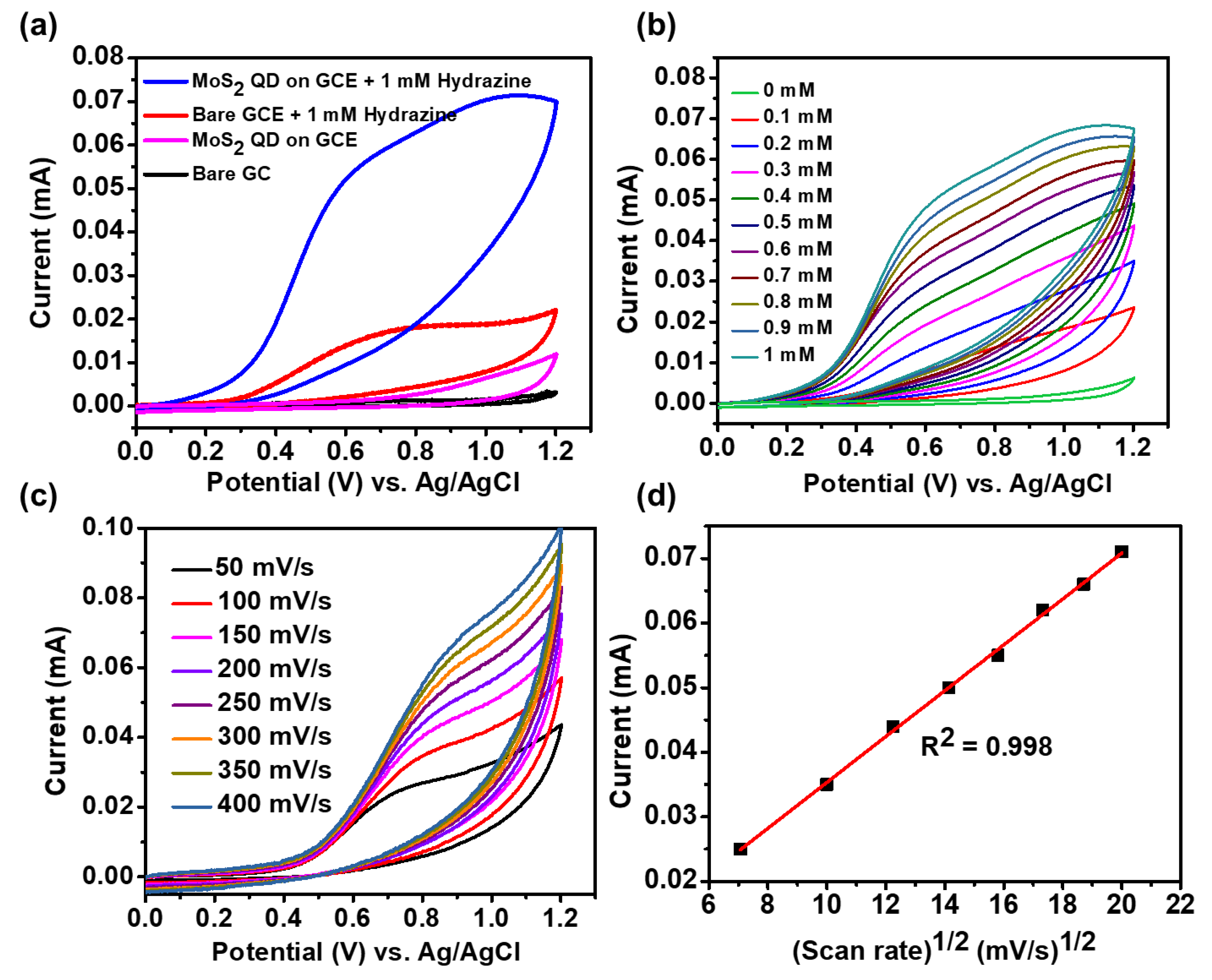
2.4. Electrochemical Characterizations
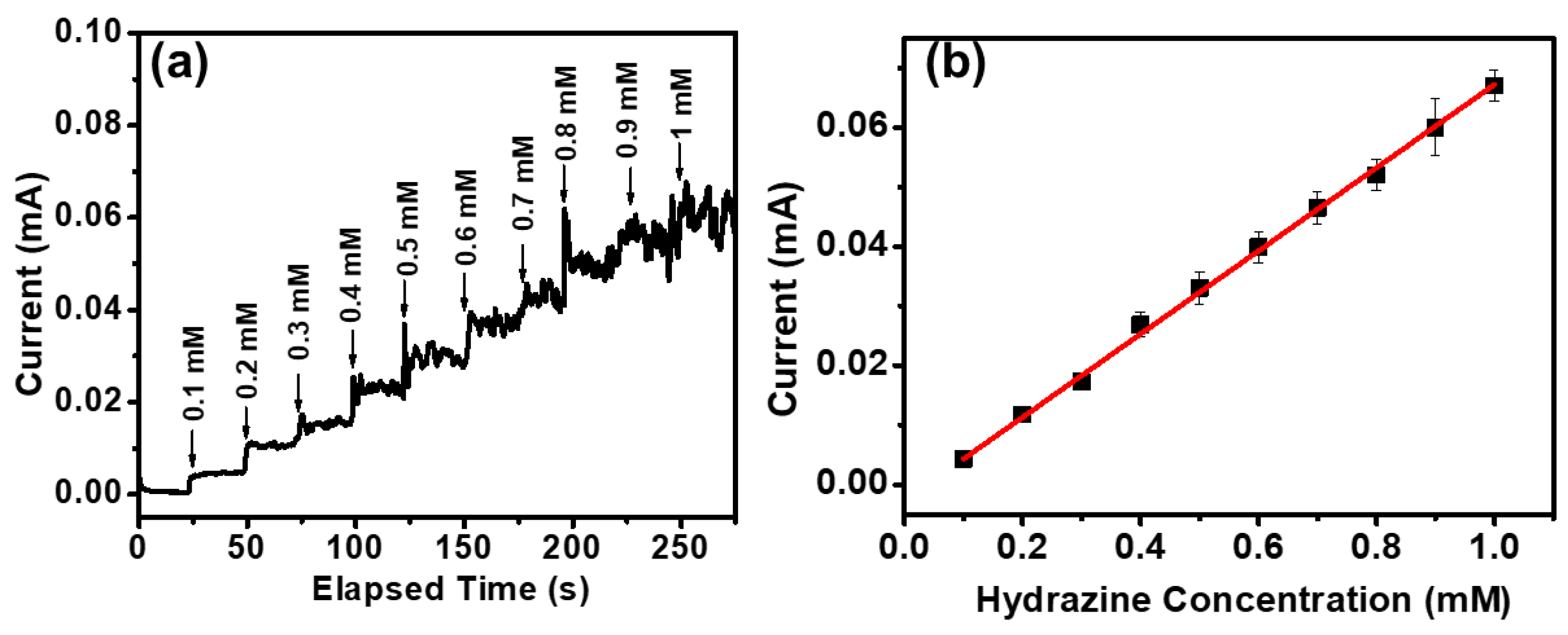
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Troyan, J.E. Properties, Production, and Uses of Hydrazine. Ind. Eng. Chem. 1953, 45, 2608–2612. [Google Scholar] [CrossRef]
- Shahid, M.M.; Rameshkumar, P.; Basirunc, W.J.; Wijayantha, U.; Chiu, W.S.; Khiew, P.S.; Huang, N.M. An electrochemical sensing platform of cobalt oxide@gold nanocubes interleaved reduced graphene oxide for the selective determination of hydrazine. Electrochim. Acta 2018, 259, 606–616. [Google Scholar] [CrossRef]
- Rahman, M.M.; Balkhoyor, H.B.; Asiri, A.M. Ultrasensitive and selective hydrazine sensor development based on Sn/ZnO nanoparticles. RSC Adv. 2016, 6, 29342–29352. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, L.; Wang, T.; Han, Q.; Xu, S. A high-performance hydrazine electrochemical sensor based on gold nanoparticles/single-walled carbon nanohorns composite film. Appl. Surf. Sci. 2016, 369, 36–42. [Google Scholar] [CrossRef]
- Avanes, A.; Hasanzadeh-Karamjavan, M.; Shokri-Jarcheloo, G. Electrocatalytic oxidation and amperometric determination of hydrazine using a carbon paste electrode modified with β-nickel hydroxide nanoplatelets. Microchim. Acta 2019, 186, 441–450. [Google Scholar] [CrossRef]
- Rees, N.V.; Compton, R.G. Carbon-free energy: A review of ammonia-and hydrazine-based electrochemical fuel cells. Energy Environ. Sci. 2011, 4, 1255–1260. [Google Scholar] [CrossRef]
- Choudhary, G.; Hansen, H. Human health perspective of environmental exposure to hydrazines: A review. Chemosphere 1998, 37, 801–843. [Google Scholar] [CrossRef]
- Sha, R.; Jones, S.S.; Vishnu, N.; Soundiraraju, B.; Badhulika, S. A Novel Biomass Derived Carbon Quantum Dots for Highly Sensitive and Selective Detection of Hydrazine. Electroanalysis 2018, 30, 2228–2232. [Google Scholar] [CrossRef]
- Kannan, P.K.; Moshkalev, S.A.; Rout, C.S. Electrochemical sensing of hydrazine using multilayer graphene nanobelts. RSC Adv. 2016, 6, 11329–11334. [Google Scholar] [CrossRef]
- Rastakhiz, N.; Kariminik, A.; Soltani-Nejad, V.; Roodsaz, S. Simultaneous Determination of Phenylhydrazine, Hydrazine, and Sulfite Using a Modified Carbon Nanotube Paste Electrode. Int. J. Electrochem. Sci. 2010, 5, 1203–1212. [Google Scholar]
- Erdemir, S.; Malkondu, S. A colorimetric and fluorometric probe for hydrazine through subsequent ring-opening and closing reactions: Its environmental applications. Microchem. J. 2020, 152, 104375–104381. [Google Scholar] [CrossRef]
- Guria, U.N.; Manna, S.K.; Maiti, K.; Samanta, S.K.; Ghosh, A.; Datta, P.; Mandal, D.; Mahapatra, A.K. A xanthene-based novel colorimetric and fluorometric chemosensor for the detection of hydrazine and its application in the bio-imaging of live cells. New J. Chem. 2021, 45, 15869–15875. [Google Scholar] [CrossRef]
- Liu, C.; Liu, K.; Tian, M.; Lin, W. A ratiometric fluorescent probe for hydrazine detection with large fluorescence change ratio and its application for fluorescence imaging in living cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 212, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-A.; Park, J.-H.; Shin, H.-S. Sensitive determination of hydrazine in water by gas chromatography–mass spectrometry after derivatization with ortho-phthalaldehyde. Anal. Chim. Acta 2013, 769, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.; Karimi, M.A. Flow injection chemiluminescence determination of hydrazine by oxidation with chlorinated isocyanurates. Talanta 2002, 58, 785–792. [Google Scholar] [CrossRef]
- Barathi, P.; Kumar, A.S. Quercetin tethered pristine-multiwalled carbon nanotube modified glassy carbon electrode as an efficient electrochemical detector for flow injection analysis of hydrazine in cigarette tobacco samples. Electrochim. Acta 2014, 135, 1–10. [Google Scholar] [CrossRef]
- Rana, U.; Paul, N.D.; Mondal, S.; Chakraborty, C.; Malik, S. Water soluble polyaniline coated electrode: A simple and nimble electrochemical approach for ascorbic acid detection. Synth. Met. 2014, 192, 43–49. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Hosseinzadeh, R.; Afshar, A.A.; Varma, R.S.; Jang, H.W.; Shokouhimehr, M. Electrochemical Detection of Hydrazine by Carbon Paste Electrode Modified with Ferrocene Derivatives, Ionic Liquid, and CoS2-Carbon Nanotube Nanocomposite. ACS Omega 2021, 6, 4641–4648. [Google Scholar] [CrossRef]
- Liu, T.; Guo, Y.; Zhang, Z.; Miao, Z.; Zhang, X.; Su, Z. Fabrication of hollow CuO/PANI hybrid nanofibers for non-enzymatic electrochemical detection of H2O2 and glucose. Sens. Actuators B 2019, 286, 370–376. [Google Scholar] [CrossRef]
- Dou, B.; Xu, L.; Jiang, B.; Yuan, R.; Xiang, Y. Aptamer-Functionalized and Gold Nanoparticle Array-Decorated Magnetic Graphene Nanosheets Enable Multiplexed and Sensitive Electrochemical Detection of Rare Circulating Tumor Cells in Whole Blood. Anal. Chem. 2019, 91, 10792–10799. [Google Scholar] [CrossRef]
- Kalaivani, A.; Narayanan, S.S. Fabrication of CdSe quantum dots @ nickel hexacyanoferrate core–shell nanoparticles modified electrode for the electrocatalytic oxidation of hydrazine. J. Mater. Sci. Mater. Electron. 2018, 29, 20146–20155. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, J.; Hou, H.; You, T. Electrochemical detection of hydrazine based on electrospun palladium nanoparticle/carbon nanofibers. Electroanalysis 2009, 21, 1869–1874. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, X.; Zheng, J. Facile synthesis of three-dimensional porous Au@Pt core-shell nanoflowers supported on graphene oxide for highly sensitive and selective detection of hydrazine. Chem. Eng. J. 2017, 327, 431–440. [Google Scholar] [CrossRef]
- Rao, D.; Sheng, Q.; Zheng, J. Preparation of flower-like Pt nanoparticles decorated chitosan-grafted graphene oxide and its electrocatalysis of hydrazine. Sens. Actuators B 2016, 236, 192–200. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Zhang, L.; Zhang, H.; Li, C.M.; Lei, Y. Preparation of TiO2-Pt hybrid nanofibers and their application for sensitive hydrazine detection. Nanoscale 2011, 3, 1149–1157. [Google Scholar] [CrossRef]
- Saengsookwaow, C.; Rangkupan, R.; Chailapakul, O.; Rodthongkum, N. Nitrogen-doped graphene-polyvinylpyrrolidone/gold nanoparticles modified electrode as a novel hydrazine sensor. Sens. Actuators B 2016, 227, 524–532. [Google Scholar] [CrossRef]
- Rani, G.; Kumar, M. Amperometric Determination of Hydrazine Based on Copper Oxide Modified Screen Printed Electrode. Sens. Transducers 2018, 223, 22–25. [Google Scholar]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Krishnan, S. Review—Electrochemical Sensors for Large and Small Molecules in Biofluids. J. Electrochem. Soc. 2020, 167, 167505. [Google Scholar] [CrossRef]
- Luo, X.; Morrin, A.; Killard, A.J.; Smyth, M.R. Application of Nanoparticles in Electrochemical Sensors and Biosensors. Electroanalysis 2006, 18, 319–326. [Google Scholar] [CrossRef]
- Hanoglu, S.B.; Man, E.; Harmanci, D.; Ruzgar, S.T.; Sanli, S.; Keles, N.A.; Ayden, A.; Tuna, B.G.; Duzgun, O.; Ozkan, O.F.; et al. Magnetic Nanoparticle-Based Electrochemical Sensing Platform Using Ferrocene-Labelled Peptide Nucleic Acid for the Early Diagnosis of Colorectal Cancer. Biosensors 2022, 12, 736. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical biosensing based on noble metal nanoparticles. Microchim. Acta 2012, 177, 245–270. [Google Scholar] [CrossRef]
- Priya, C.; Sivasankari, G.; Narayanan, S.S. Electrochemical behavior of Azure A/gold nanoclusters modified electrode and its application as non-enzymatic hydrogen peroxide sensor. Colloids Surf. B Biointerfaces 2012, 97, 90–96. [Google Scholar] [CrossRef]
- Gopalan, S.A.; Anantha-Iyengar, G.; Shin-Won, K.; Shanmugasundaram, K.; Kwang-Pill, L. One Pot Synthesis of New Gold Nanoparticles Dispersed Poly(2-aminophenyl boronic acid) Composites for Fabricating an Affinity-Based Electrochemical Detection of Glucose. Sci. Adv. Mater. 2014, 6, 1356–1364. [Google Scholar]
- Safavi, A.; Maleki, N.; Tajabadi, F.; Farjami, E. High electrocatalytic effect of palladium nanoparticle arrays electrodeposited on carbon ionic liquid electrode. Electrochem. Commun. 2007, 9, 1963–1968. [Google Scholar] [CrossRef]
- Jeykumari, D.R.S.; Narayanan, S.S. Bienzyme Based Biosensing Platform Using Functionalized Carbon Nanotubes. J. Nanosci. Nanotechnol. 2008, 9, 5411–5416. [Google Scholar] [CrossRef]
- Roushani, M.; Shamsipur, M.; Rajabi, H.R. Highly selective detection of dopamine in the presence of ascorbic acid and uric acid using thioglycolic acid capped CdTe quantum dots modified electrode. J. Electroanal. Chem. 2014, 712, 19–24. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, Y.; Ai, S.; Chen, Q.; Zhu, X.; Liu, X.; Zhu, L. Sensitivity and selectivity determination of BPA in real water samples using PAMAM dendrimer and CoTe quantum dots modified glassy carbon electrode. J. Hazard. Mater. 2010, 174, 236–243. [Google Scholar] [CrossRef]
- Kalaivani, A.; Narayanan, S.S. Simultaneous Determination of Adenine and Guanine Using Cadmium Selenide Quantum Dots-Graphene Oxide Nanocomposite Modified Electrode. J. Nanosci. Nanotechnol. 2015, 15, 4697–4705. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, G.; Ji, Y.; Liu, M.; Feng, Y.; Zhang, Z.; Fang, B. Enhancement in analytical hydrazine based on gold nanoparticles deposited on ZnO-MWCNTs films. Sens. Actuators B 2010, 150, 247–253. [Google Scholar] [CrossRef]
- Li, J.; Xie, H.; Chen, L. A sensitive hydrazine electrochemical sensor based on electrodeposition of gold nanoparticles on choline film modified glassy carbon electrode. Sens. Actuators B 2011, 153, 239–245. [Google Scholar] [CrossRef]
- Qureshi, S.; Asif, M.; Sajid, H.; Gilani, M.A.; Ayub, K.; Mahmood, T. First-principles study for electrochemical sensing of neurotoxin hydrazine derivatives via h-g-C3N4 quantum dot. Surf. Interfaces 2022, 30, 101913–101922. [Google Scholar] [CrossRef]
- Centane, S.; Sekhosana, E.K.; Matshitse, R.; Nyokong, T. Electrocatalytic activity of a push-pull phthalocyanine in the presence of reduced and amino functionalized graphene quantum dots towards the electrooxidation of hydrazine. J. Electroanal. Chem. 2018, 820, 146–160. [Google Scholar] [CrossRef]
- Chen, W.; Wang, H.; Tang, H.; Yang, C.; Guan, X.; Li, Y. Amperometric sensing of hydrazine by using single gold nanopore electrodes filled with Prussian Blue and coated with polypyrrole and carbon dots. Microchim. Acta 2019, 186, 350–356. [Google Scholar] [CrossRef]
- Huang, H.; Camarada, M.B.; Wang, D.; Liao, X.; Xiong, J.; Hong, Y. MoS2 quantum dots and titanium carbide co-modified carbon nanotube heterostructure as electrode for highly sensitive detection of zearalenone. Microchim. Acta 2022, 189, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Bobde, Y.; Ghosh, B.; Chakraborty, C. Targeted Bioimaging of Cancer Cells Using Free Folic Acid-Sensitive Molybdenum Disulfide Quantum Dots through Fluorescence “Turn-Off”. ACS Appl. Bio. Mater. 2021, 4, 2839–2849. [Google Scholar] [CrossRef]
- Roy, S.; Ganeshan, S.K.; Pal, S.; Chakraborty, C. Targeted enhancement of electrochromic memory in Fe(II) based metallo-supramolecular polymer using molybdenum disulfide quantum dots. Sol. Energy Mater. Sol. Cells 2022, 234, 111487–111497. [Google Scholar] [CrossRef]
- Li, F.; Li, J.; Cao, Z.; Lin, X.; Li, X.; Fang, Y.; An, X.; Fu, Y.; Jin, J.; Li, R. MoS2 quantum dot decorated RGO: A designed electrocatalyst with high active site density for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 21772–21778. [Google Scholar] [CrossRef]
- Kabel, J.; Sharma, S.; Acharya, A.; Zhang, D.; Yap, Y.K. Molybdenum Disulfide Quantum Dots: Properties, Synthesis, and Applications. C 2021, 7, 45. [Google Scholar] [CrossRef]
- Yang, X.; Jia, Q.; Duan, F.; Hu, B.; Wang, M.; He, L.; Song, Y.; Zhang, Z. Multiwall carbon nanotubes loaded with MoS2 quantum dots and MXene quantum dots: Non–Pt bifunctional catalyst for the methanol oxidation and oxygen reduction reactions in alkaline solution. Appl. Surf. Sci. 2019, 464, 78–87. [Google Scholar] [CrossRef]
- Li, L.; Guo, Z.; Wang, S.; Li, D.; Hou, X.; Wang, F.; Yang, Y.; Yang, X. Facile synthesis of MoS2 quantum dots as fluorescent probes for sensing of hydroquinone and bioimaging. Anal. Methods 2019, 11, 3307–3313. [Google Scholar] [CrossRef]
- Haldar, D.; Dinda, D.; Saha, S.K. High selectivity in water-soluble MoS2 quantum dots for sensing nitro explosives. J. Mater. Chem. C 2016, 4, 6321–6326. [Google Scholar] [CrossRef]
- Jayasri, D.; Narayanan, S.S. Amperometric determination of hydrazine at manganese hexacyanoferrate modified graphite–wax composite electrode. J. Hazard. Mater. 2007, 144, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Miranzadeh, L.; Hallaj, R. Amperometric and voltammetric detection of hydrazine using glassy carbon electrodes modified with carbon nanotubes and catechol derivatives. Talanta 2008, 75, 147–156. [Google Scholar] [CrossRef]
| Electrode Materials | Sensitivity μAmM−1cm−2 | LOD | Linear Range | Reference |
|---|---|---|---|---|
| GO-Chitosan-Pt | 104.6 | 3.6 μM | 20 μM–10 mM | [24] |
| Carbon QDs | 151.5 | 39.7 μM | 125–1125 μM | [8] |
| Mn-hexacyanoferrate-graphite–wax | 0.4753 | 6.65 μM | ~33 μM–8 mM | [53] |
| MWCNT/Chlorogenic acid | 4.1 | - | 2.5 μM–5 mM | [54] |
| β-nickel hydroxide nanoplatelets/CPE | 1.33 | 0.28 μM | 1–1300 μM | [5] |
| Pt NPs/TiO2NSs/GCE | 187.4 | 2 μM | 20–900 μM | [25] |
| CuO/CNT/SPE | 70.72 | 5 μM | 5–50 μM | [27] |
| Carbon QDs | 151.5 | 39.7 μM | 125–1125 μM | [8] |
| Au@Pt-NFs/GO/GCE | 1695.3 | 0.43 μM | 0.8–429 μM | [23] |
| ferrocene-derivative/ionic liquid/CoS2-CNT/CPE | 0.073 | 0.015 μM | 0.03–500 μM | [18] |
| N-doped Graphene-PVP/AuNPs/SPE | 1.37 | 0.07 μM | 2–500 μM | [26] |
| MoS2-QD on GCE | 990 | 34.8 μM | 100–1000 μM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, S.; Sharma, S.; Chappanda, K.N.; Chakraborty, C. MoS2 Quantum Dot Modified Electrode: An Efficient Probe for Electrochemical Detection of Hydrazine. Designs 2023, 7, 13. https://doi.org/10.3390/designs7010013
Roy S, Sharma S, Chappanda KN, Chakraborty C. MoS2 Quantum Dot Modified Electrode: An Efficient Probe for Electrochemical Detection of Hydrazine. Designs. 2023; 7(1):13. https://doi.org/10.3390/designs7010013
Chicago/Turabian StyleRoy, Susmita, Sarda Sharma, Karumbaiah N. Chappanda, and Chanchal Chakraborty. 2023. "MoS2 Quantum Dot Modified Electrode: An Efficient Probe for Electrochemical Detection of Hydrazine" Designs 7, no. 1: 13. https://doi.org/10.3390/designs7010013
APA StyleRoy, S., Sharma, S., Chappanda, K. N., & Chakraborty, C. (2023). MoS2 Quantum Dot Modified Electrode: An Efficient Probe for Electrochemical Detection of Hydrazine. Designs, 7(1), 13. https://doi.org/10.3390/designs7010013








