Gaze Dispersion During a Sustained-Fixation Task as a Proxy of Visual Attention in Children with ADHD
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. Eye Movement Recordings
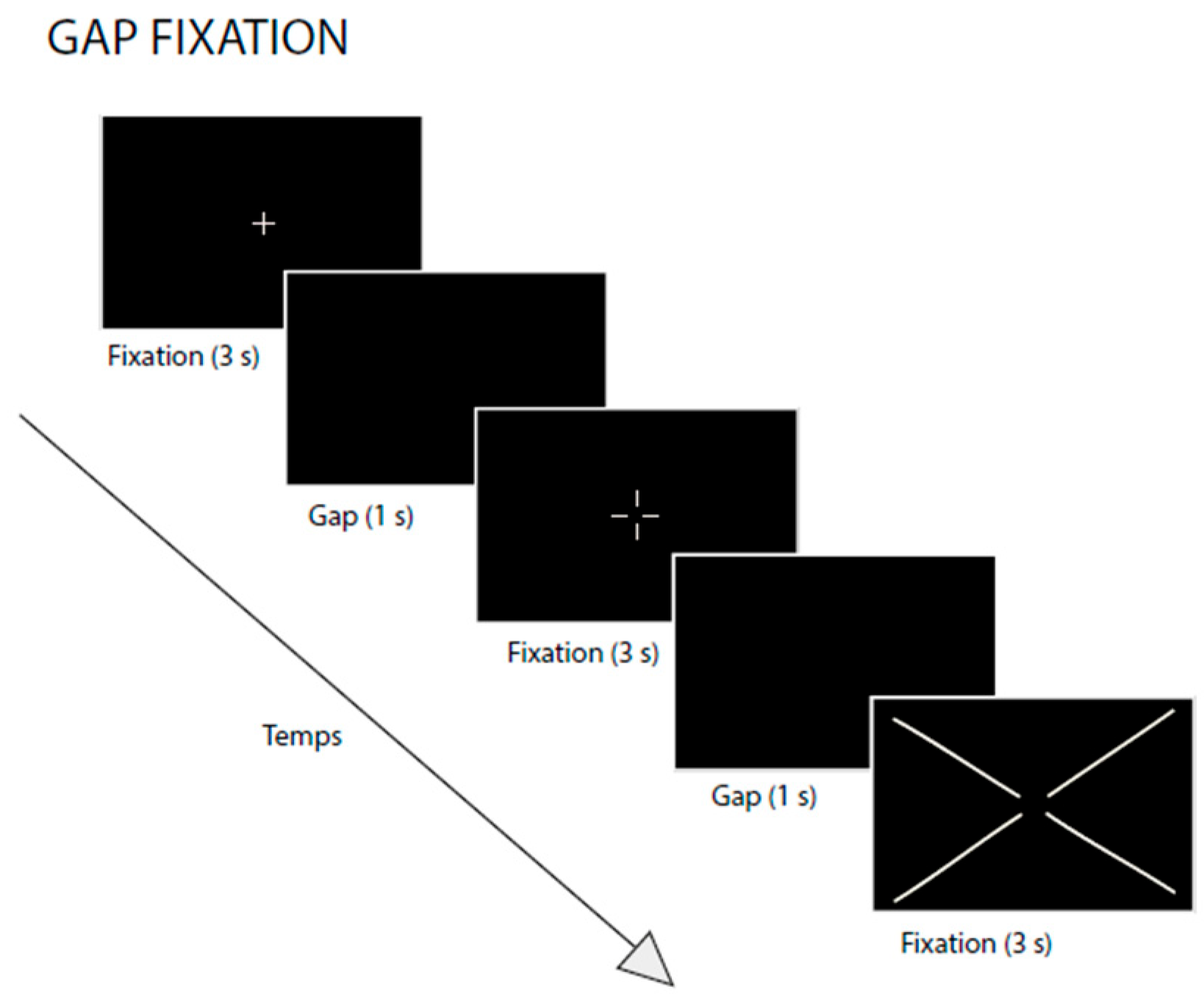
2.3. Fixation Task
2.4. Data Analysis
2.5. Statistical Analysis
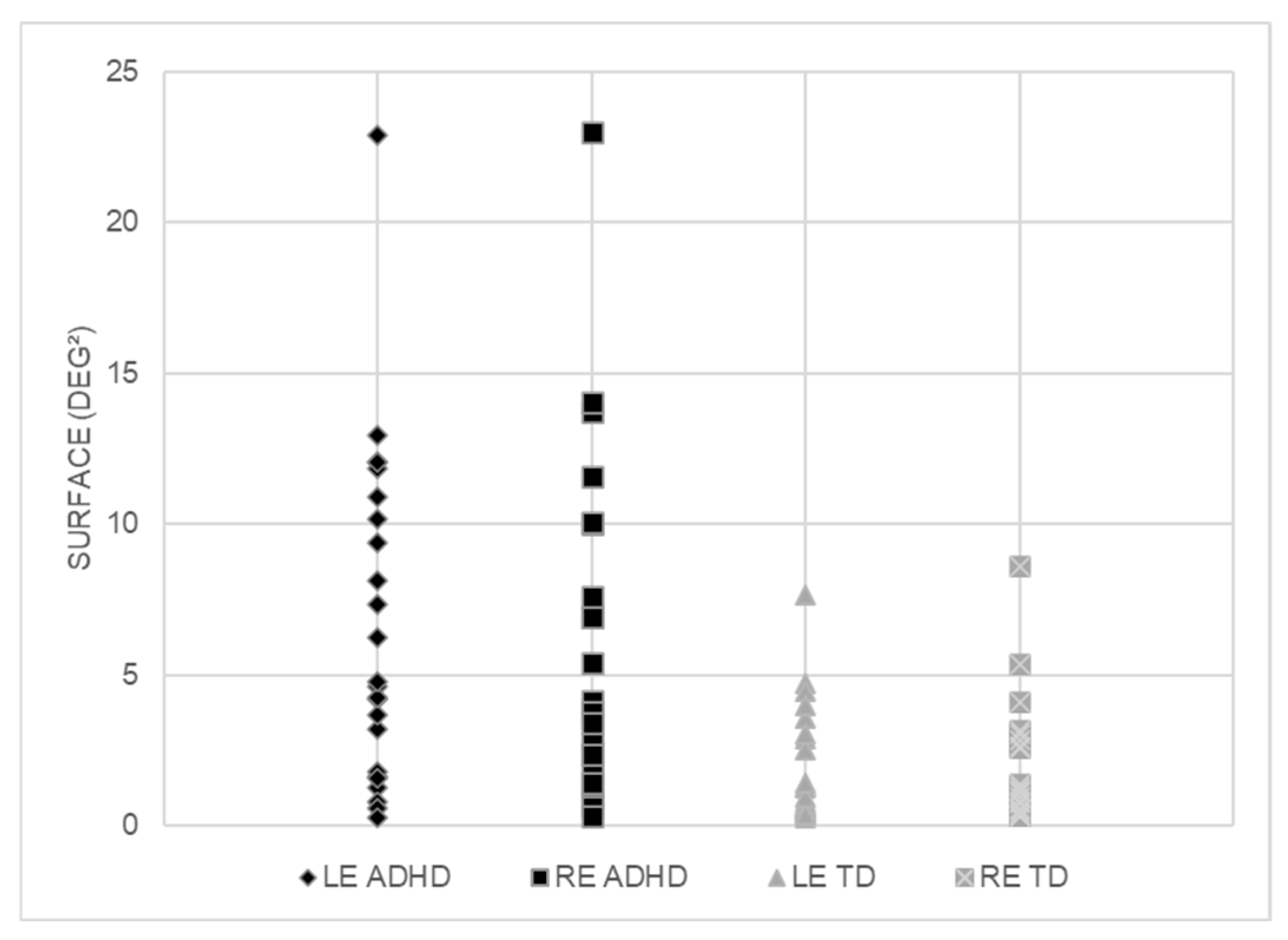
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Bucci, M.P.; Thierry, E.; Roux, S.; Demailly, C. Eye Movements and Postural Control in Children; Biomarkers of Neurodevelopmental Disorders. J. Pediatr. Neuropsychol. 2024, 10, 231–242. [Google Scholar] [CrossRef]
- Willcutt, E.G.; Doyle, A.E.; Nigg, J.T.; Faraone, S.V.; Pennington, B.F. Validity of the Executive Function Theory of Attention-Deficit/Hyperactivity Disorder: A Meta-Analytic Review. Biol. Psychiatry 2010, 57, 1336–1346. [Google Scholar] [CrossRef]
- Wang, H.E.; Reid, D. Balance Function in Children with Attention Deficit Hyperactivity Disorder. Percept. Mot. Skills 2003, 96, 1109–1121. [Google Scholar]
- Castellanos, F.X.; Giedd, J.N.; Marsh, W.L.; Hamburger, S.D.; Vaituzis, A.C.; Dickstein, D.P.; Rapoport, J.L. Quantitative Brain Magnetic Resonance Imaging in Attention-Deficit Hyperactivity Disorder. Arch. Gen. Psychiatry 1996, 53, 607–616. [Google Scholar] [CrossRef]
- Valera, E.M.; Faraone, S.V.; Murray, K.E.; Seidman, L.J. Meta-Analysis of Structural Imaging Findings in Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2007, 61, 1361–1369. [Google Scholar] [CrossRef]
- Ivanov, I.; Murrough, J.W.; Bansal, R.; Hao, X.; Peterson, B.S. Cerebellar Morphology and the Effects of Stimulant Medications in Youths with Attention Deficit Hyperactivity Disorder. Neuropsychopharmacology 2010, 35, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Durston, S. Differential Patterns of Striatal Activation in Young Children with and without ADHD. Biol. Psychiatry 2003, 53, 871–878. [Google Scholar] [CrossRef]
- Zhang, R.; Murray, S.B.; Duval, C.J.; Wang, D.J.J.; Jann, K. Functional Connectivity and Complexity Analyses of Resting-State fMRI in Pre-Adolescents Demonstrating the Behavioral Symptoms of ADHD. Psychiatry Res. 2024, 334, 115794. [Google Scholar] [CrossRef] [PubMed]
- Leigh, R.J.; Zee, D.S. The Neurology of Eye Movements; Oxford University Press: Oxford, UK, 2015; ISBN 978-0-19-996928-9. [Google Scholar]
- Munoz, D.P.; Armstrong, I.T.; Hampton, K.A.; Moore, K.D. Altered Control of Visual Fixation and Saccadic Eye Movements in Attention-Deficit Hyperactivity Disorder. J. Neurophysiol. 2003, 90, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Rommelse, N.N.J.; Van Der Stigchel, S.; Sergeant, J.A. A Review on Eye Movement Studies in Childhood and Adolescent Psychiatry. Brain Cogn. 2008, 68, 391–414. [Google Scholar] [CrossRef]
- Caldani, S.; Razuk, M.; Septier, M.; Barela, J.; Delorme, R.; Acquaviva, E.; Bucci, M. The Effect of Dual Task on Attentional Performance in Children with ADHD. Front. Integr. Neurosci. 2019, 12, 67. [Google Scholar] [CrossRef]
- Bucci, M.P.; Stordeur, C.; Septier, M.; Acquaviva, E.; Peyre, H.; Delorme, R. Oculomotor Abnormalities in Children with Attention-Deficit/Hyperactivity Disorder Are Improved by Methylphenidate. J. Child Adolesc. Psychopharmacol. 2017, 27, 274–280. [Google Scholar] [CrossRef]
- Chamorro, Y.; Betz, L.T.; Philipsen, A.; Kambeitz, J.; Ettinger, U. The Eyes Have It: A Meta-Analysis of Oculomotor Inhibition in Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2022, 7, 1090–1102. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.N.; Bowe, S.J.; Spencer-Smith, M.; Mellahn, O.J.; Perrykkad, K.; Bellgrove, M.A.; Johnson, B.P. Oculomotor Deficits in Attention Deficit Hyperactivity Disorder (ADHD): A Systematic Review and Comprehensive Meta-Analysis. Neurosci. Biobehav. Rev. 2021, 131, 1198–1213. [Google Scholar] [CrossRef]
- Goldberg, M.E.; Bisley, J.W.; Powell, K.D.; Gottlieb, J. The Role of the Frontal Eye Fields in the Control of Attention. Ann. N. Y. Acad. Sci. 1986, 462, 193–206. [Google Scholar]
- Mountcastle, V.B.; Andersen, R.A.; Motter, B.C. The Influence of Attentive Fixation upon the Excitability of the Light-Sensitive Neurons of the Posterior Parietal Cortex. J. Neurosci. 1981, 1, 1218–1225. [Google Scholar] [CrossRef]
- Munoz, D.P.; Wurtz, R.H. Role of the Rostral Superior Colliculus in Active Visual Fixation and Execution of Express Saccades. J. Neurophysiol. 1992, 67, 1000–1002. [Google Scholar] [CrossRef]
- Reilly, J.L.; Lencer, R.; Bishop, J.R.; Keedy, S.K.; Sweeney, J.A. Pharmacological Treatment Effects on Eye Movement Control. Brain Cogn. 2008, 68, 415–435. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Conde, S.; Macknik, S.L.; Hubel, D.H. The Role of Fixational Eye Movements in Visual Perception. Nat. Rev. Neurosci. 2004, 5, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Thaler, L.; Schütz, A.C.; Goodale, M.A.; Gegenfurtner, K.R. What Is the Best Fixation Target? The Effect of Target Shape on Stability of Fixational Eye Movements. Vision Res. 2013, 76, 31–42. [Google Scholar] [CrossRef]
- Crossland, M.D.; Rubin, G.S. The Use of an Infrared Eyetracker to Measure Fixation Stability. Optom. Vis. Sci. 2002, 79, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.U.; Saker, S.; Wilde, C.; Sahni, A.; Rubin, G.S. Reference Clinical Database for Fixation Stability Metrics in Normal Subjects Measured with the MAIA Microperimeter. Transl. Vis. Sci. Technol. 2016, 5, 6. [Google Scholar] [CrossRef]
- Falck-Ytter, T.; Pettersson, E.; Bölte, S.; D’Onofrio, B.; Lichtenstein, P.; Kennedy, D.P. Difficulties Maintaining Prolonged Fixation and Attention-Deficit/Hyperactivity Symptoms Share Genetic Influences in Childhood. Psychiatry Res. 2020, 293, 113384. [Google Scholar] [CrossRef]
- Hassoumi, A.; Peysakhovich, V.; Hurter, C. Improving Eye-Tracking Calibration Accuracy Using Symbolic Regression. PLoS ONE 2019, 14, e0213675. [Google Scholar] [CrossRef]
- Hafed, Z.M.; Krauzlis, R.J. Similarity of Superior Colliculus Involvement in Microsaccade and Saccade Generation. J. Neurophysiol. 2012, 107, 1904–1916. [Google Scholar] [CrossRef]
- Wurtz, R.H.; Albano, J.E. Visual-Motor Function of the Primate Superior Colliculus. Annu. Rev. Neurosci. 1980, 3, 189–226. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Riggio, L.; Dascola, I.; Umiltá, C. Reorienting Attention across the Horizontal and Vertical Meridians: Evidence in Favor of a Premotor Theory of Attention. Neuropsychologia 1987, 25, 31–40. [Google Scholar] [CrossRef]
- Aflalo, J.; Caldani, S.; Acquaviva, E.; Moscoso, A.; Delorme, R.; Bucci, M.P. Pilot Study to Explore Poor Visual Searching Capabilities in Children with ADHD: A Tablet-Based Computerized Test Battery Study. Nord. J. Psychiatry 2023, 77, 491–497. [Google Scholar] [CrossRef]
- Serpa, E.; Alecka, M.; Ceple, I.; Luneau, K. The Impact of Eye Dominance on Fixation Stability in School-Aged Children. J. Eye Mov. Res. 2023, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Caldani, S.; Steg, S.; Lefebvre, A.; Atzori, P.; Peyre, H.; Delorme, R.; Bucci, M.P. Oculomotor Behavior in Children with Autism Spectrum Disorders. Autism Int. J. Res. Pract. 2020, 24, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Bucci, M.P.; Goulème, N.; Dehouck, D.; Stordeur, C.; Acquaviva, E.; Septier, M.; Lefebvre, A.; Gerard, C.-L.; Peyre, H.; Delorme, R. Interactions between Eye Movements and Posture in Children with Neurodevelopmental Disorders. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2018, 71, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Moiroud, L.; Rateaux, M.; Libersa, C. Cas Clinique—Apport de l’eye Tracking dans le Suivi du Trouble du Déficit de L’attention avec ou sans Hyperactivité. Available online: https://www.edimark.fr/revues/images-en-ophtalmologie/vol-xix-n-2-copy-copy/apport-de-leye-tracking-dans-le-suivi-du-trouble-du-deficit-de-lattention-avec-ou-sans-hyperactivite (accessed on 24 June 2025).
- Hafed, Z.M.; Lovejoy, L.P.; Krauzlis, R.J. Modulation of Microsaccades in Monkey during a Covert Visual Attention Task. J. Neurosci. 2011, 31, 15219–15230. [Google Scholar] [CrossRef] [PubMed]
| ADHD Group (N = 22) | TD Group (N = 24) | |
|---|---|---|
| Age (years) | 8.75 ± 0.96 | 8.76 ± 1.27 |
| ADHD-RS total score | 38± 4.5 | 4.6 ± 1.3 |
| WISC-V subtest scores: | ||
| Verbal comprehension | 95 ± 6 | |
| Visual spatial | 92 ± 4 | |
| Fluid reasoning | 93 ± 7 | |
| Working memory | 94 ± 8 | |
| Processing speed | 98 ± 5 | |
| Similarities | 10.9 ± 0.5 | 10 ± 2 |
| Matrix reasoning | 11.1 ± 2 | 11.5 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moiroud, L.; Moscoso, A.; Acquaviva, E.; Michel, A.; Delorme, R.; Bucci, M.P. Gaze Dispersion During a Sustained-Fixation Task as a Proxy of Visual Attention in Children with ADHD. Vision 2025, 9, 76. https://doi.org/10.3390/vision9030076
Moiroud L, Moscoso A, Acquaviva E, Michel A, Delorme R, Bucci MP. Gaze Dispersion During a Sustained-Fixation Task as a Proxy of Visual Attention in Children with ADHD. Vision. 2025; 9(3):76. https://doi.org/10.3390/vision9030076
Chicago/Turabian StyleMoiroud, Lionel, Ana Moscoso, Eric Acquaviva, Alexandre Michel, Richard Delorme, and Maria Pia Bucci. 2025. "Gaze Dispersion During a Sustained-Fixation Task as a Proxy of Visual Attention in Children with ADHD" Vision 9, no. 3: 76. https://doi.org/10.3390/vision9030076
APA StyleMoiroud, L., Moscoso, A., Acquaviva, E., Michel, A., Delorme, R., & Bucci, M. P. (2025). Gaze Dispersion During a Sustained-Fixation Task as a Proxy of Visual Attention in Children with ADHD. Vision, 9(3), 76. https://doi.org/10.3390/vision9030076





