Insulin Nanoemulsion Eye Drops for the Treatment of Dry Eye Disease in Sjögren’s Disease: A Randomized Clinical Trial Phase I/II
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Insulin Eyedrops
2.3. Patients
2.4. Outcome Measures
2.5. Statistical Methods
3. Results
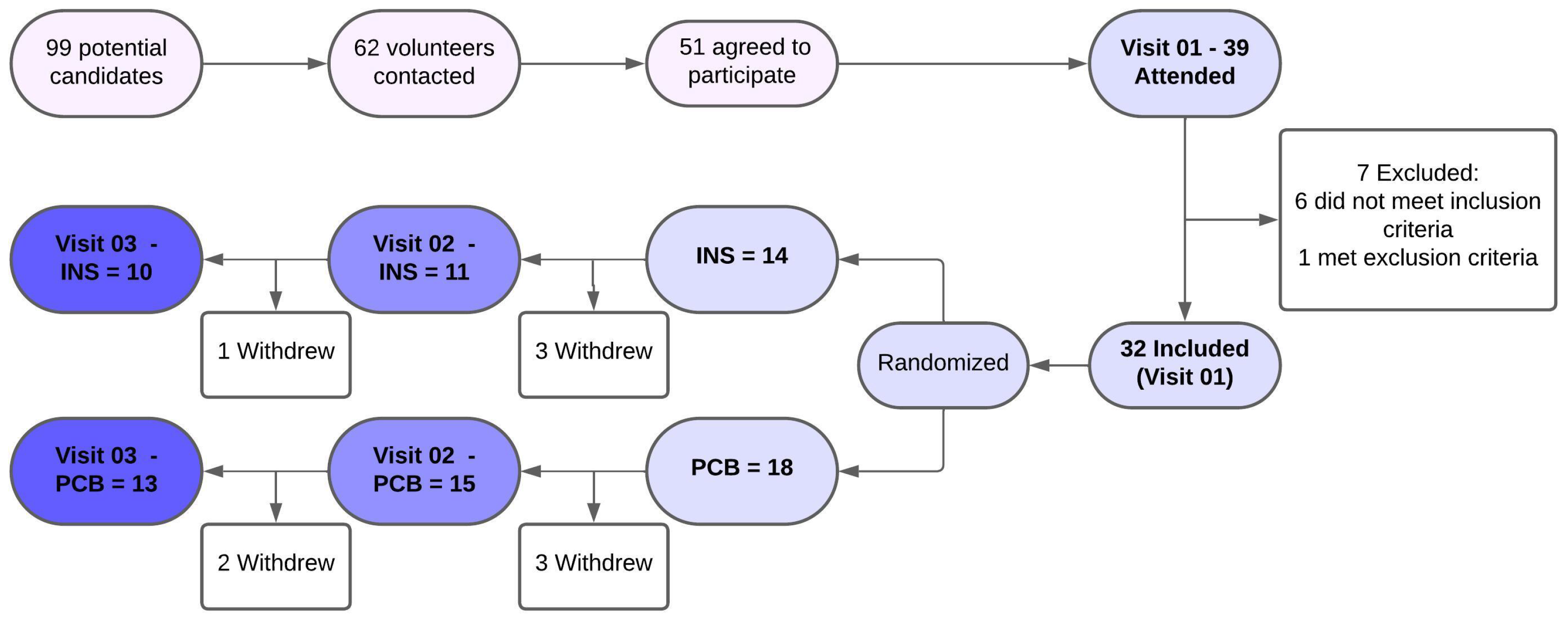
3.1. Follow-Up
3.2. Overall Analysis
3.3. Safety Results
3.4. Efficacy Results
3.5. Volunteers’ Adherence to the Protocol
4. Discussion
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DED | Dry eye disease |
| SjD | Sjögren’s disease |
| IU | International unit |
| INS | Insulin |
| PCB | Placebo |
| OSDI | Ocular Surface Disease Index |
| ST | Schirmer Test |
| TEAEs | Treatment-emergent adverse events |
| IOP | Intraocular pressure |
| TBUT | Tear Breakup Time |
| CF | Corneal staining with fluorescein |
| LG | Conjunctival staning with 1% Lissamine Green |
| SD | Standard Deviation |
| IQR | Interquartile range |
| CI | Confidence Interval |
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [PubMed]
- Rouen, P.A.; White, M.L. Dry Eye Disease: Prevalence, Assessment, and Management. Home Healthcare Now 2018, 36, 74–83. [Google Scholar]
- Parisis, D.; Chivasso, C.; Perret, J.; Soyfoo, M.S.; Delporte, C. Current State of Knowledge on Primary Sjogren’s Syndrome, an Autoimmune Exocrinopathy. J. Clin. Med. 2020, 9, 2299. [Google Scholar] [PubMed]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar]
- Kim, M.; Lee, Y.; Mehra, D.; Sabater, A.L.; Galor, A. Dry eye: Why artificial tears are not always the answer. BMJ Open Ophthalmol. 2021, 6, e000697. [Google Scholar]
- Alves, M.; Fonseca, E.C.; Alves, M.F.; Malki, L.T.; Arruda, G.V.; Reinach, P.S.; Rocha, E.M. Dry eye disease treatment: A systematic review of published trials and a critical appraisal of therapeutic strategies. Ocul. Surf. 2013, 11, 181–192. [Google Scholar]
- Rocha, E.M.; Cunha, D.A.; Carneiro, E.M.; Boschero, A.C.; Saad, M.J.; Velloso, L.A. Identification of insulin in the tear film and insulin receptor and IGF-1 receptor on the human ocular surface. Investig. Ophthalmol. Vis. Sci. 2002, 43, 963–967. [Google Scholar]
- Cunha, D.A.; Carneiro, E.M.; Alves, M.d.C.; Jorge, A.G.; de Sousa, S.M.; Boschero, A.C.; Saad, M.J.; Velloso, L.A.; Rocha, E.M. Insulin secretion by rat lacrimal glands: Effects of systemic and local variables. Am. J. Physiol.-Endocrinol. Metab. 2005, 289, E768–E775. [Google Scholar]
- Cunha, D.A.; Alves, M.C.; Stoppiglia, L.F.; Jorge, A.G.; Módulo, C.M.; Carneiro, E.M.; Boschero, A.C.; Saad, M.J.; Velloso, L.A.; Rocha, E.M. Extra-pancreatic insulin production in rat lachrymal gland after streptozotocin-induced islet beta-cells destruction. Biochim. Biophys. Acta 2007, 1770, 1128–1135. [Google Scholar]
- Módulo, C.M.; Jorge, A.G.; Dias, A.C.; Braz, A.M.; Bertazolli-Filho, R.; Jordão, A.A., Jr.; Marchini, S.J.; Rocha, E.M. Influence of insulin treatment on the lacrimal gland and ocular surface of diabetic rats. Endocrine 2009, 36, 161–168. [Google Scholar] [PubMed]
- Dias, A.C.; Batista, T.M.; Roma, L.P.; Módulo, C.M.; Malki, L.T.; Dias, L.C.; Alves, M.; Reinach, P.S.; Carneiro, E.M.; Rocha, E.M. Insulin replacement restores the vesicular secretory apparatus in the diabetic rat lacrimal gland. Arq. Bras. Oftalmol. 2015, 78, 158–163. [Google Scholar]
- Zagon, I.S.; Sassani, J.W.; McLaughlin, P.J. Insulin treatment ameliorates impaired corneal reepithelialization in diabetic rats. Diabetes 2006, 55, 1141–1147. [Google Scholar] [PubMed]
- Zagon, I.S.; Klocek, M.S.; Sassani, J.W.; McLaughlin, P.J. Use of topical insulin to normalize corneal epithelial healing in diabetes mellitus. Arch. Ophthalmol. 2007, 125, 1082–1088. [Google Scholar]
- Bastion, M.; Ling, K.P. Topical insulin for healing of diabetic epithelial defects?: A retrospective review of corneal debridement during vitreoretinal surgery in Malaysian patients. Med. J. Malays. 2013, 68, 209. [Google Scholar]
- Cruz-Cazarim, E.L.C.; Cazarim, M.S.; Ogunjimi, A.T.; Petrilli, R.; Rocha, E.M.; Lopez, R.F.V. Prospective insulin-based ophthalmic delivery systems for the treatment of dry eye syndrome and corneal injuries. Eur. J. Pharm. Biopharm. 2019, 140, 1–10. [Google Scholar]
- Sullivan, D.A.; Rocha, E.M.; Aragona, P.; Clayton, J.A.; Ding, J.; Golebiowski, B.; Hampel, U.; McDermott, A.M.; Schaumberg, D.A.; Srinivasan, S.; et al. TFOS DEWS II Sex, Gender, and Hormones Report. Ocul. Surf. 2017, 15, 284–333. [Google Scholar]
- Gaudana, R.; Jwala, J.; Boddu, S.H.; Mitra, A.K. Recent perspectives in ocular drug delivery. Pharm. Res. 2009, 26, 1197. [Google Scholar]
- Fai, S.; Ahem, A.; Mustapha, M.; Noh, U.K.M. Randomized Controlled Trial of Topical Insulin for Healing Corneal Epithelial Defects Induced During Vitreoretinal Surgery in Diabetics. Asia-Pac. J. Ophthalmol. 2017, 6, 418–424. [Google Scholar]
- Dasrilsyah, A.M.; Halim, W.H.W.A.; Mustapha, M.; Tang, S.F.; Kaur, B.; Ong, E.Y.; Bastion, M.L.C. Randomized Clinical Trial of Topical Insulin Versus Artificial Tears for Healing Rates of Iatrogenic Corneal Epithelial Defects Induced During Vitreoretinal Surgery in Diabetics. Cornea 2023, 42, 1395–1403. [Google Scholar]
- Azmi, N.A.; Bastion, M.C. Short-term results of trial of topical insulin for treatment of dry eyes in diabetics. Eye Contact Lens 2020, 46 (Suppl. 1), S25–S32. [Google Scholar]
- Tahmaz, V.; Menghesha, L.; Stern, M.E.; Holtick, U.; Scheid, C.; Steven, P. Insulin eye drops for severe refractory chronic ocular graft-versus-host disease. Bone Marrow Transplant. 2024, 59, 1031–1033. [Google Scholar] [PubMed]
- Restrepo-Jimenez, P.; Molano-Gonzalez, N.; Anaya, J.M. Geoepidemiology of Sjogren’s syndrome in Latin America. Jt. Bone Spine 2019, 86, 620–626. [Google Scholar]
- Garcia, D.M.; Oliveira, F.R.; Módulo, C.M.; Faustino, J.; Barbosa, A.P.; Alves, M.; Rocha, E.M. Is Sjögren’s syndrome dry eye similar to dry eye caused by other etiologies? Discriminating different diseases by dry eye tests. PLoS ONE 2018, 13, e0208420. [Google Scholar]
- Oliveira, F.R.; Motta, A.C.F.; Módulo, C.M.; Garcia, D.M.; Chiorini, J.A.; Louzada-Junior, P.; Rocha, E.M. Clinical and laboratory evaluation of sicca complaints: Distinctive aspects of primary, secondary and non-Sjogren syndrome. Adv. Rheumatol. 2022, 62, 23. [Google Scholar]
- Marzola, M.M.; Gutierrez, D.R.; Cintra, B.C.; Murashima, A.d.A.B.; Dalmolin, L.F.; Garcia, D.M.; Lopez, R.F.V.; de Oliveira, F.R.; Rocha, E.M. Insulin nanoemulsion eye drops for the treatment of dry eye disease in Sjögren’s Disease: A randomized clinical trial phase I/II. In Proceedings of the 10th International Conference on the Tear Film & Ocular Surface: Basic Science and Clinical Relevance, Venice, Italy, 31 October–2 November 2024. [Google Scholar]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann. Rheum. Dis. 2017, 76, 9–16. [Google Scholar]
- Chauhan, S.; Jhawat, V.; Singh, R.P.; Yadav, A. Topical delivery of insulin using novel organogel formulations: An approach for the management of diabetic wounds. Burns 2024, 50, 1068–1082. [Google Scholar]
- Atikah, A.; Suzana, M.; Wan Haslina, W.A.H.; Norshamsiah, M.D.; Mushawiahti, M.; Birinder, K.S.S.; Tang, S.F.; Bastion, M.L.C. Randomized controlled trial on effects of topical insulin compared to artificial tears and normal saline on tear inflammatory mediator levels and clinical parameters in diabetics with dry eye disease. Cont. Lens Anterior Eye 2024, 48, 102346. [Google Scholar] [PubMed]
- Quitério, M.; Simões, S.; Ascenso, A.; Carvalheiro, M.; Leandro, A.P.; Correia, I.; Viana, A.S.; Faísca, P.; Ascensão, L.; Molpeceres, J.; et al. Development of a topical insulin polymeric nanoformulation for skin burn regeneration: An experimental approach. Int. J. Mol. Sci. 2021, 22, 4087. [Google Scholar]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar]
- Prigol, A.M.; Tenório, M.B.; Matschinske, R.; Gehlen, M.L.; Skare, T. Translation and validation of ocular surface disease index to Portuguese. Arq. Bras. Oftalmol. 2013, 75, 24–28. [Google Scholar]
- Aynsley, T.R. The use of insulin in the treatment of corneal ulcers. Br. J. Ophthalmol. 1945, 29, 361–363. [Google Scholar] [PubMed]
- Cuartero-Martínez, A.; Hermelo-Vidal, G.; Castro-Balado, A.; Gómez-García, Á.; González-Barcia, M.; Otero-Espinar, F.J.; Fernández-Ferreiro, A.; Mondelo-García, C. Stability of insulin eye drops in the treatment of refractory corneal ulcers. Farm. Hosp. 2022, 46, 335–339. [Google Scholar] [PubMed]
- Vicario-de-la-Torre, M.; Puebla-García, V.; Ybañez-García, L.; López-Cano, J.J.; González-Cela-Casamayor, M.A.; Brugnera, M.; Burgos-Blasco, B.; Díaz-Valle, D.; Gegúndez-Fernández, J.A.; Benítez-Del-Castillo, J.M.; et al. Topical insulin eye drops: Stability and safety of two compounded formulations for treating persistent corneal epithelial defects. Pharmaceutics 2024, 16, 580. [Google Scholar] [CrossRef]
- Dana, R.; Bradley, J.L.; Guerin, A.; Pivneva, I.; Stillman, I.Ö.; Evans, A.M.; Schaumberg, D.A. Estimated prevalence and incidence of dry eye disease based on coding analysis of a large, all-age United States health care system. Am. J. Ophthalmol. 2019, 202, 47–54. [Google Scholar]
- Wolffsohn, J.S.; Semp, D.A.; Dutta, D.; Jones, L.; Craig, J.P. TFOS ambassadors. Clinical practice patterns in the management of dry eye disease: A TFOS international survey 2023–2024. Ocul. Surf. 2025, 36, 164–172. [Google Scholar] [PubMed]
- Anghel, L.A.; Farcas, A.M.; Oprean, R.N. An overview of the common methods used to measure treatment adherence. Med. Pharm. Rep. 2019, 92, 117–122. [Google Scholar]
- Novack, G.D.; Asbell, P.; Barabino, S.; Bergamini, M.V.W.; Ciolino, J.B.; Foulks, G.N.; Goldstein, M.; Lemp, M.A.; Schrader, S.; Woods, C.; et al. TFOS DEWS II Clinical Trial Design Report. Ocul. Surf. 2017, 15, 629–649. [Google Scholar]
- Tsai, M.J.; Fu, Y.S.; Lin, Y.H.; Huang, Y.B.; Wu, P.C. The Effect of Nanoemulsion as a Carrier of Hydrophilic Compound for Transdermal Delivery. PLoS ONE 2014, 9, e102850. [Google Scholar]
- Ali, F.R.; Shoaib, M.H.; Ali, S.A.; Yousuf, R.I.; Siddiqui, F.; Raja, R.; Jamal, H.S.; Saleem, M.T.; Ahmed, K.; Imtiaz, M.S.; et al. A nanoemulsion based transdermal delivery of insulin: Formulation development, optimization, In-Vitro permeation across Strat-M® membrane and its pharmacokinetic/pharmacodynamic evaluation. J. Drug Deliv. Sci. Technol. 2022, 71, 103338. [Google Scholar]
- Chatzidaki, M.D.; Mitsou, E. Advancements in Nanoemulsion-Based Drug Delivery Across Different Administration Routes. Pharmaceutics 2025, 17, 337. [Google Scholar] [CrossRef] [PubMed]
| No | ||
|---|---|---|
| Characteristcs | Insulin 20 IU/mL (n = 10) | Placebo (n = 13) |
| Age, mean (SD) y | 55.30 (14.84) | 59.15 (13.28) |
| Female, No. (%) | 10 (100) | 13 (100) |
| Race or ethnicity, No. (%) | ||
| White | 7 (70) | 9 (69.23) |
| Black | 3 (30) | 4 (30.77) |
| Duration of Sjd, means (SD) y | 8.80 (5.51) | 12.17 (10.69) |
| IOP, mean (SD), mmHg | Visit 01 (n = 4) | Visit 01 (n = 9) |
| OD: 13.50 (3.42) | OD: 13.67 (2.60) | |
| OS: 14.00 (3.37) | OS: 13.33 (2.00) | |
| Insulin | |||||
|---|---|---|---|---|---|
| Index | 20 IU/mL | Placebo | |||
| (n = 4) | (n = 5) | p-Value | |||
| Headache | Visit 02 | 1 | 0 | >0.05 | |
| Visit 03 | 0 | 1 | >0.05 | ||
| Dizziness | Visit 02 | 0 | 1 | >0.05 | |
| Visit 03 | 0 | 1 | >0.05 | ||
| Insomnia | Visit 02 | 0 | 0 | >0.05 | |
| Visit 03 | 0 | 0 | >0.05 | ||
| Appetite disturbance | Visit 02 | 0 | 0 | >0.05 | |
| Visit 03 | 0 | 1 | >0.05 | ||
| Ocular pruritus | Visit 02 | 3 | 2 | >0.05 | |
| Visit 03 | 3 | 3 | >0.05 | ||
| Ocular burning | Visit 02 | 2 | 1 | >0.05 | |
| Visit 03 | 3 | 2 | >0.05 | ||
| Visual blurring | Visit 02 | 3 | 1 | >0.05 | |
| Visit 03 | 4 | 1 | 0.046 * | ||
| Dry eye | Visit 02 | 0 | 0 | >0.05 | |
| Visit 03 | 0 | 0 | >0.05 | ||
| Others * | Visit 02 | 0 | 1 | >0.05 | |
| Visit 03 | 1 | 0 | >0.05 | ||
| IOP, mean (SD), mmHg | OD: 12.25 (4.50) | OD: 11.67 (2.12) | >0.05 | ||
| Visit 02 | OS: 12.50 (4.72) | (n = 9) | OS: 11.11 (2.52) | ||
| Visit 03 | OD: 13.75 (5.56) | (n = 9) | OD: 13.78 (1.92) | >0.05 | |
| OS: 13.25 (4.99) | OS: 12.67 (1.80) | ||||
| Insulin 20 IU/mL (n = 10) | Placebo (n = 13) | Mixed Model (p-Value) | ||||||
|---|---|---|---|---|---|---|---|---|
| Index | Time Point | Value (LSM) | 95% CI | Value (LSM) | 95% CI | Group | Time | Group × Time |
| ST (*), mm | Visit 01 | 3.8 | 1.1–9.9 | 3.3 | 1.1–7.8 | |||
| Visit 02 | 4.6 | 1.5–11.6 | 5.8 | 2.3–13.0 | ||||
| Visit 03 | 4.5 | 1.4–11.3 | 4.0 | 1.4–9.1 | 0.993 | 0.242 | 0.590 | |
| OSDI score | Visit 01 a,b | 47.2 | 31.4–63.0 | 64.8 | 51.0–78.7 | |||
| Visit 02 a | 26.9 | 11.1–42.7 | 37.2 | 23.4–51.1 | ||||
| Visit 03 b | 29.5 | 13.7–45.3 | 27.1 | 13.2–40.9 | 0.261 | 0.0002 | 0.301 | |
| CF score | Visit 01 | 3.8 | 1.2–6.4 | 6.5 | 4.2–8.7 | |||
| Visit 02 | 5.2 | 2.6–7.8 | 4.8 | 2.5–7.0 | ||||
| Visit 03 | 4.9 | 2.3–7.5 | 5.4 | 3.1–7.6 | 0.568 | 0.933 | 0.0069 | |
| LG score | Visit 01 | 5.4 | 3.0–7.8 | 8.4 c | 6.3–10.5 | |||
| Visit 02 | 3.7 | 1.3–6.1 | 3.2 c | 1.1–5.3 | ||||
| Visit 03 | 5.0 | 2.6–7.4 | 5.7 | 3.6–7.8 | 0.407 | 0.0006 | 0.1074 | |
| TBUT test (*), s | Visit 01 | 6.1 | 3.1–11.1 | 3.5 | 1.8–2.0 | |||
| Visit 02 | 3.1 | 1.4–6.1 | 3.1 | 1.6–5.6 | ||||
| Visit 03 | 3.0 | 1.3–5.8 | 3.1 | 1.6–5.6 | 0.643 | 0.075 | 0.221 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzola, M.M.; Gutierrez, D.R.; Cintra, B.C.; Murashima, A.d.A.B.; Dalmolin, L.F.; Garcia, D.M.; Lopez, R.F.V.; Oliveira, F.R.; Rocha, E.M. Insulin Nanoemulsion Eye Drops for the Treatment of Dry Eye Disease in Sjögren’s Disease: A Randomized Clinical Trial Phase I/II. Vision 2025, 9, 54. https://doi.org/10.3390/vision9030054
Marzola MM, Gutierrez DR, Cintra BC, Murashima AdAB, Dalmolin LF, Garcia DM, Lopez RFV, Oliveira FR, Rocha EM. Insulin Nanoemulsion Eye Drops for the Treatment of Dry Eye Disease in Sjögren’s Disease: A Randomized Clinical Trial Phase I/II. Vision. 2025; 9(3):54. https://doi.org/10.3390/vision9030054
Chicago/Turabian StyleMarzola, Mateus Maia, Diego Rocha Gutierrez, Beatriz Carneiro Cintra, Adriana de Andrade Batista Murashima, Luciana Facco Dalmolin, Denny Marcos Garcia, Renata Fonseca Vianna Lopez, Fabiola Reis Oliveira, and Eduardo Melani Rocha. 2025. "Insulin Nanoemulsion Eye Drops for the Treatment of Dry Eye Disease in Sjögren’s Disease: A Randomized Clinical Trial Phase I/II" Vision 9, no. 3: 54. https://doi.org/10.3390/vision9030054
APA StyleMarzola, M. M., Gutierrez, D. R., Cintra, B. C., Murashima, A. d. A. B., Dalmolin, L. F., Garcia, D. M., Lopez, R. F. V., Oliveira, F. R., & Rocha, E. M. (2025). Insulin Nanoemulsion Eye Drops for the Treatment of Dry Eye Disease in Sjögren’s Disease: A Randomized Clinical Trial Phase I/II. Vision, 9(3), 54. https://doi.org/10.3390/vision9030054







