Predicted Visual Impact of a Small Aperture Intraocular Lens in Reducing Higher Order Aberrations in Post-Radial Keratotomy Patients
Abstract
1. Introduction
2. Materials and Methods
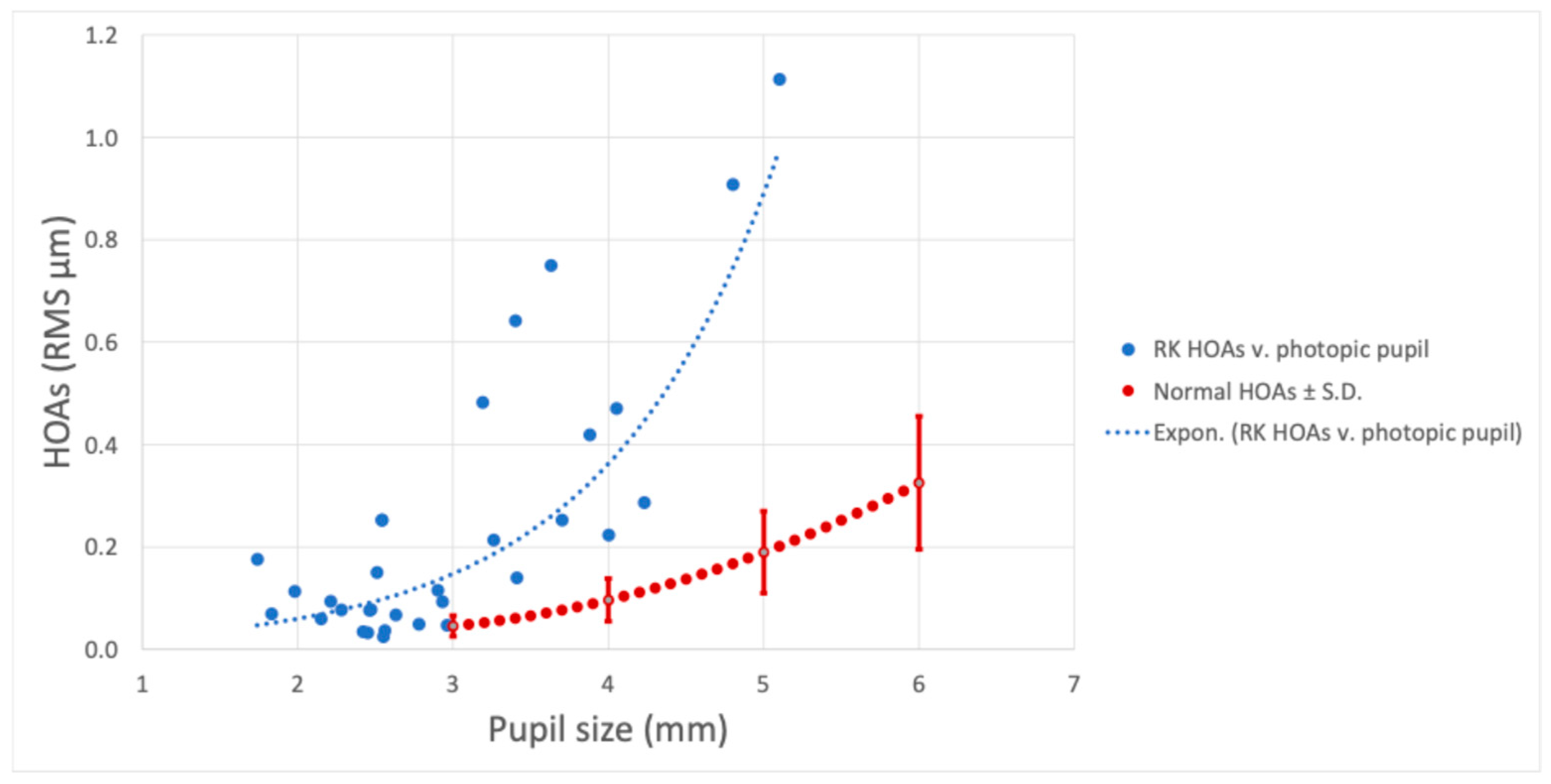
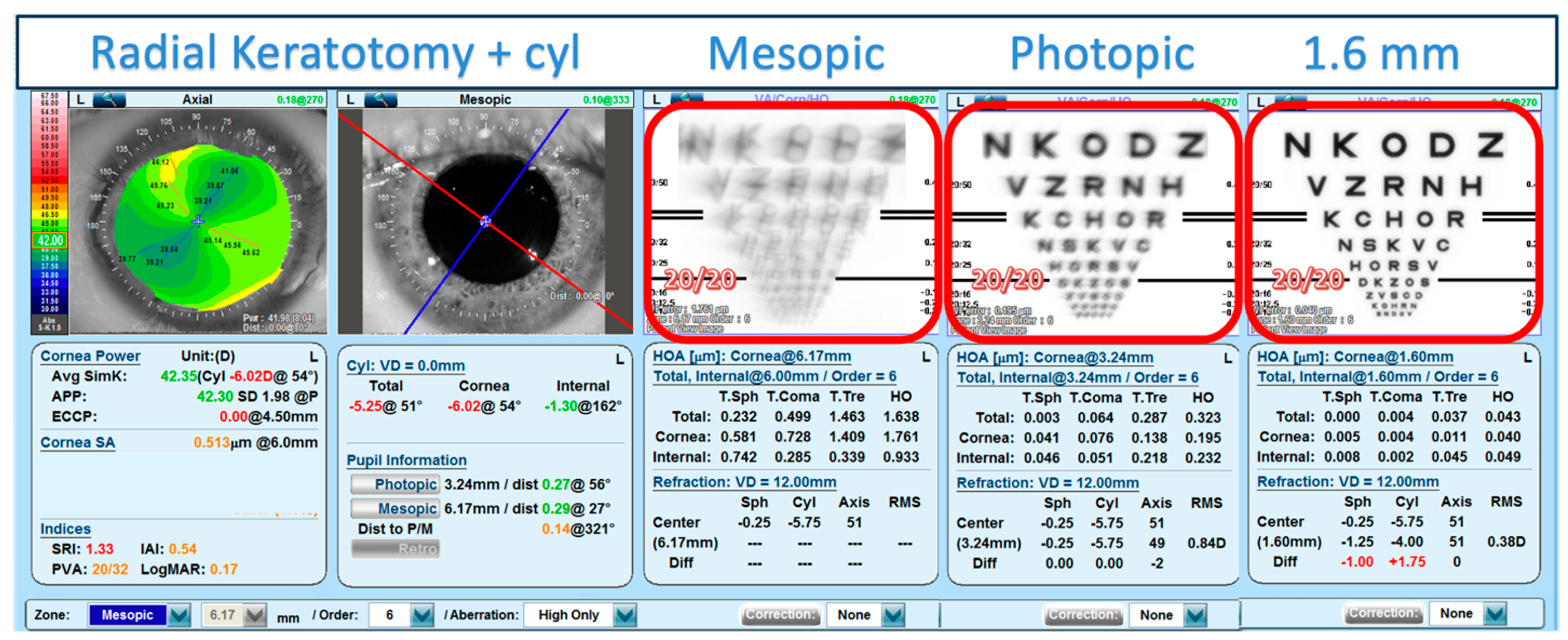
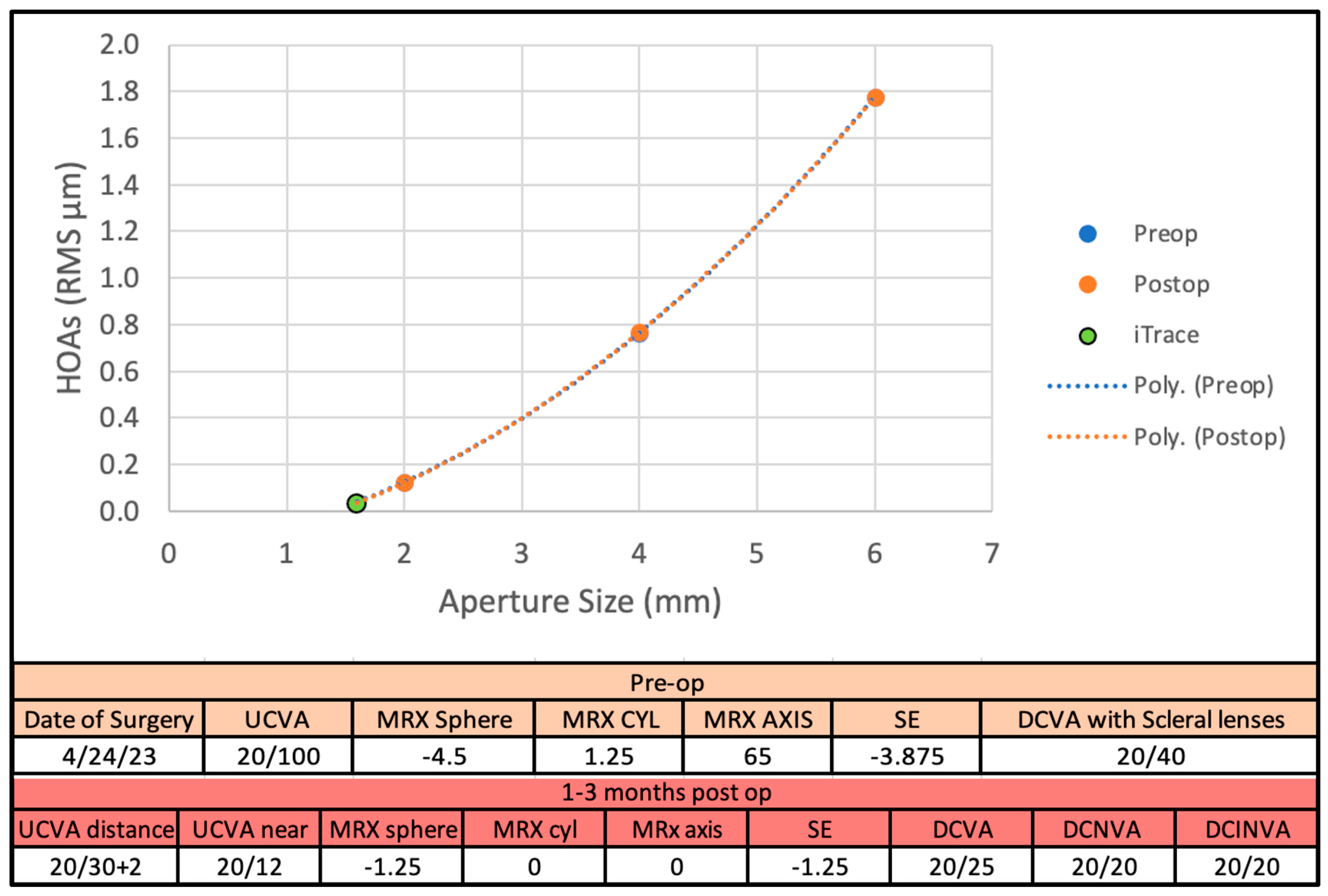
3. Results
3.1. Main Results
3.2. Figures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schneider, D.M.; Draghic, T.; Murthy, R.K. Combined myopia and astigmatism surgery. Review of 350 cases. J. Cataract Refract. Surg. 1992, 18, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Koh, S.; Inoue, R.; Maeda, N.; McDonald, M.; Nishida, K. What Happens 20 to 30 years After Radial Keratotomy? Case Series. Eye Contact Lens 2024, 50, 329–331. [Google Scholar] [CrossRef]
- Waring, G.O.; Lynn, M.J.; McDonnell, P.J. Results of the prospective evaluation of radial keratotomy (PERK) study 10 years after surgery. Arch. Ophthalmol. 1994, 112, 1298–1308. [Google Scholar] [CrossRef]
- Kemp, J.R.; Martinez, C.E.; Klyce, S.D.; Coorpender, S.J.; McDonald, M.B.; Lucci, L.; Lynn, M.J.; Waring, G.O. Diurnal fluctuations in corneal topography 10 years after radial keratotomy in the Prospective Evaluation of Radial Keratotomy Study. J. Cataract Refract. Surg. 1999, 25, 904–910. [Google Scholar] [CrossRef]
- Savini, G.; Hoffer, K.J. Intraocular lens power calculation in eyes with previous corneal refractive surgery. Eye Vis. 2018, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Alio, J.L.; Abdelghany, A.A.; Abdou, A.A.; Maldonado, M.J. Cataract surgery on the previous corneal refractive surgery patient. Surv. Ophthalmol. 2016, 61, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.E.; Applegate, R.A.; Klyce, S.D.; McDonald, M.B.; Medina, J.P.; Howland, H.C. Effect of pupillary dilation on corneal optical aberrations after photorefractive keratectomy. Arch. Ophthalmol. 1998, 116, 1053–1062. [Google Scholar] [CrossRef]
- Salmon, T.O.; van de Pol, C. Normal-eye Zernike coefficients and root-mean-square wavefront errors. J. Cataract Refract. Surg. 2006, 32, 2064–2074. [Google Scholar] [CrossRef]
- Campbell, F.W.; Gregory, A.H. Effect of size of pupil on visual acuity. Nature 1960, 187, 1121–1123. [Google Scholar] [CrossRef]
- Marcos, S.; Moreno, E.; Navarro, R. The depth-of-field of the human eye from objective and subjective measurements. Vision Res. 1999, 39, 2039–2049. [Google Scholar] [CrossRef]
- Narang, P.; Agarwal, A. Reply: Pinhole pupilloplasty after previous radial keratotomy. J. Cataract Refract. Surg. 2021, 47, 1383. [Google Scholar] [CrossRef]
- Trindade, C.C.; Trindade, B.C.; Trindade, F.C.; Werner, L.; Osher, R.; Santhiago, M.R. New pinhole sulcus implant for the correction of irregular corneal astigmatism. J. Cataract Refract. Surg. 2017, 43, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Artal, P. Optical modeling of a corneal inlay in real eyes to increase depth of focus: Optimum centration and residual defocus. J. Cataract Refract. Surg. 2012, 38, 270–277. [Google Scholar] [CrossRef]
- AcuFocus IC8 Apthera IOL. Hydrophobic Acrylic Small Aperture Intraocular Lens. Directions for Use. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf21/P210005C.pdf (accessed on 12 January 2024).
- Grabner, G.; Ang, R.E.; Vilupuru, S. The Small-Aperture IC-8 Intraocular Lens: A New Concept for Added Depth of Focus in Cataract Patients. Am. J. Ophthalmol. 2015, 160, 1176–1184.e1171. [Google Scholar] [CrossRef] [PubMed]
- Dick, H.B.; Piovella, M.; Vukich, J.; Vilupuru, S.; Lin, L.; Investigators, C. Prospective multicenter trial of a small-aperture intraocular lens in cataract surgery. J. Cataract Refract. Surg. 2017, 43, 956–968. [Google Scholar] [CrossRef] [PubMed]
- IC-8® Apthera™ IOL. Summary of Safety and Effectiveness. Data (SSED). Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf21/P210005B.pdf (accessed on 12 January 2024).
- van den Berg, R.M.; van den Berg, A.; Rocha, K.M.; de Barros, M.F.; Dodhia, M.; Shahid, M.; Klyce, S.D. Prediction of the small aperture intraocular lens on visual acuity in patients with keratoconus. J. Cataract Refract. Surg. 2024, 50, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E.; Klyce, S.D. Quantitative descriptors of corneal topography: A clinical study. Arch Ophthalmol. 1991, 109, 349–353. [Google Scholar] [CrossRef]
- Dawson, V.J.; Patnaik, J.L.; Ifantides, C.; Miller, D.C.; Lynch, A.M.; Christopher, K.L. Comparison of refractive prediction for intraoperative aberrometry and Barrett True K no history formula in cataract surgery patients with prior radial keratotomy. Acta Ophthalmol. 2021, 99, e844–e851. [Google Scholar] [CrossRef]
- Leite de Pinho Tavares, R.; de Almeida Ferreira, G.; Ghanem, V.C.; Ghanem, R.C. IOL Power Calculation After Radial Keratotomy Using the Haigis and Barrett True-K Formulas. J. Refract. Surg. 2020, 36, 832–837. [Google Scholar] [CrossRef]
- Curado, S.X.; Hida, W.T.; Vilar, C.M.C.; Ordones, V.L.; Chaves, M.A.P.; Tzelikis, P.F. Intraoperative Aberrometry Versus Preoperative Biometry for IOL Power Selection After Radial Keratotomy: A Prospective Study. J. Refract. Surg. 2019, 35, 656–661. [Google Scholar] [CrossRef]
- Turnbull, A.M.J.; Crawford, G.J.; Barrett, G.D. Methods for Intraocular Lens Power Calculation in Cataract Surgery after Radial Keratotomy. Ophthalmology 2020, 127, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Klyce, S.D.; Smolek, M.K.; McDonald, M.B. Disparity between keratometry-style readings and corneal power within the pupil after refractive surgery for myopia. Cornea 1997, 16, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Savini, G.; Hoffer, K.J.; Schiano-Lomoriello, D.; Barboni, P. Intraocular lens power calculation using a Placido disk-Scheimpflug tomographer in eyes that had previous myopic corneal excimer laser surgery. J. Cataract Refract. Surg. 2018, 44, 935–941. [Google Scholar] [CrossRef]
- Applegate, R.A.; Howland, H.C.; Sharp, R.P.; Cottingham, A.J.; Yee, R.W. Corneal aberrations and visual performance after radial keratotomy. J. Refract. Surg. 1998, 14, 397–407. [Google Scholar] [CrossRef]
- Yang, L.W.Y.; Ong, H.S.; Chiam, N.; Mehta, J.S. Centration and Stability of Small-Aperture Intraocular Lens in Aberrated Eyes. J. Refract. Surg. 2022, 38, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Ang, R.E. Small-aperture intraocular lens tolerance to induced astigmatism. Clin. Ophthalmol. 2018, 12, 1659–1664. [Google Scholar] [CrossRef]
- Ang, R.E. Visual Performance of a Small-Aperture Intraocular Lens: First Comparison of Results After Contralateral and Bilateral Implantation. J. Refract. Surg. 2020, 36, 12–19. [Google Scholar] [CrossRef]
- Charman, W.N. Pinholes and presbyopia: Solution or sideshow? Ophthalmic Physiol. Opt. 2019, 39, 1–10. [Google Scholar] [CrossRef]
- Son, H.S.; Khoramnia, R.; Mayer, C.; Labuz, G.; Yildirim, T.M.; Auffarth, G.U. A pinhole implant to correct postoperative residual refractive error in an RK cataract patient. Am. J. Ophthalmol. Case Rep. 2020, 20, 100890. [Google Scholar] [CrossRef]
- Langer, J.; Shajari, M.; Kreutzer, T.; Priglinger, S.; Mayer, W.J.; Mackert, M.J. Predictability of Refractive Outcome of a Small-Aperture Intraocular Lens in Eyes with Irregular Corneal Astigmatism. J. Refract. Surg. 2021, 37, 312–317. [Google Scholar] [CrossRef]
- Kanclerz, P.; Khoramnia, R.; Atchison, D. Applications of the pinhole effect in clinical vision science. J. Cataract Refract. Surg. 2024, 50, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Khoo, L.W.; Koshy, Z. Posterior Segment Visualization in Eyes with Small-Aperture Intraocular Lens. J. Refract. Surg. 2019, 35, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Borkenstein, A.F.; Kormilina, T.K.; Fitzek, H.; Rattenberger, J.; Kothleitner, G.; Charry, F.E.M.; Borkenstein, E.M. Incorrectly Focused Neodymium:Yttrium-Aluminum-Garnet (Nd:YAG) Laser Beam Leads to Massive Destructive Effects in Small-Aperture (Pinhole) Intraocular Lenses. Ophthalmol. Ther. 2024, 13, 2745–2758. [Google Scholar] [CrossRef]
- Bucur, J.; Werner, L.; Kohnen, T. Carbon bursts inside a small aperture intraocular lens after Nd:YAG laser capsulotomy. Am. J. Ophthalmol. Case Rep. 2024, 36, 102129. [Google Scholar] [CrossRef]
- Hwang, S.H. Contrast sensitivity and visual function after implantation of a small-aperture intraocular lens. Ophthalmology 2020, 127, 178–185. [Google Scholar]
- Manzanera, S.; Webb, K.; Artal, P. Adaptation to Brightness Perception in Patients Implanted with a Small Aperture. Am. J. Ophthalmol. 2019, 197, 36–44. [Google Scholar] [CrossRef]

| Sex (F/M) | 13/10 |
| Age (years) | 65 ± 7 (49–77) * |
| RK incisions | 7.7 ± 2.6 (4–16) * |
| CDVA (LogMar) | 0.21 ± 0.16 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Berg, R.M.; DeVaro, S.; Rocha, K.M.; Fetrin de Barros, M.; Klyce, S.D. Predicted Visual Impact of a Small Aperture Intraocular Lens in Reducing Higher Order Aberrations in Post-Radial Keratotomy Patients. Vision 2025, 9, 46. https://doi.org/10.3390/vision9020046
van den Berg RM, DeVaro S, Rocha KM, Fetrin de Barros M, Klyce SD. Predicted Visual Impact of a Small Aperture Intraocular Lens in Reducing Higher Order Aberrations in Post-Radial Keratotomy Patients. Vision. 2025; 9(2):46. https://doi.org/10.3390/vision9020046
Chicago/Turabian Stylevan den Berg, Roberta M., Sarah DeVaro, Karolinne Maia Rocha, Marcela Fetrin de Barros, and Stephen D. Klyce. 2025. "Predicted Visual Impact of a Small Aperture Intraocular Lens in Reducing Higher Order Aberrations in Post-Radial Keratotomy Patients" Vision 9, no. 2: 46. https://doi.org/10.3390/vision9020046
APA Stylevan den Berg, R. M., DeVaro, S., Rocha, K. M., Fetrin de Barros, M., & Klyce, S. D. (2025). Predicted Visual Impact of a Small Aperture Intraocular Lens in Reducing Higher Order Aberrations in Post-Radial Keratotomy Patients. Vision, 9(2), 46. https://doi.org/10.3390/vision9020046





