Time Course in Ocular Blood Flow and Pulse Waveform in a Case of Ocular Ischemic Syndrome with Intraocular Pressure Fluctuation
Abstract
1. Introduction
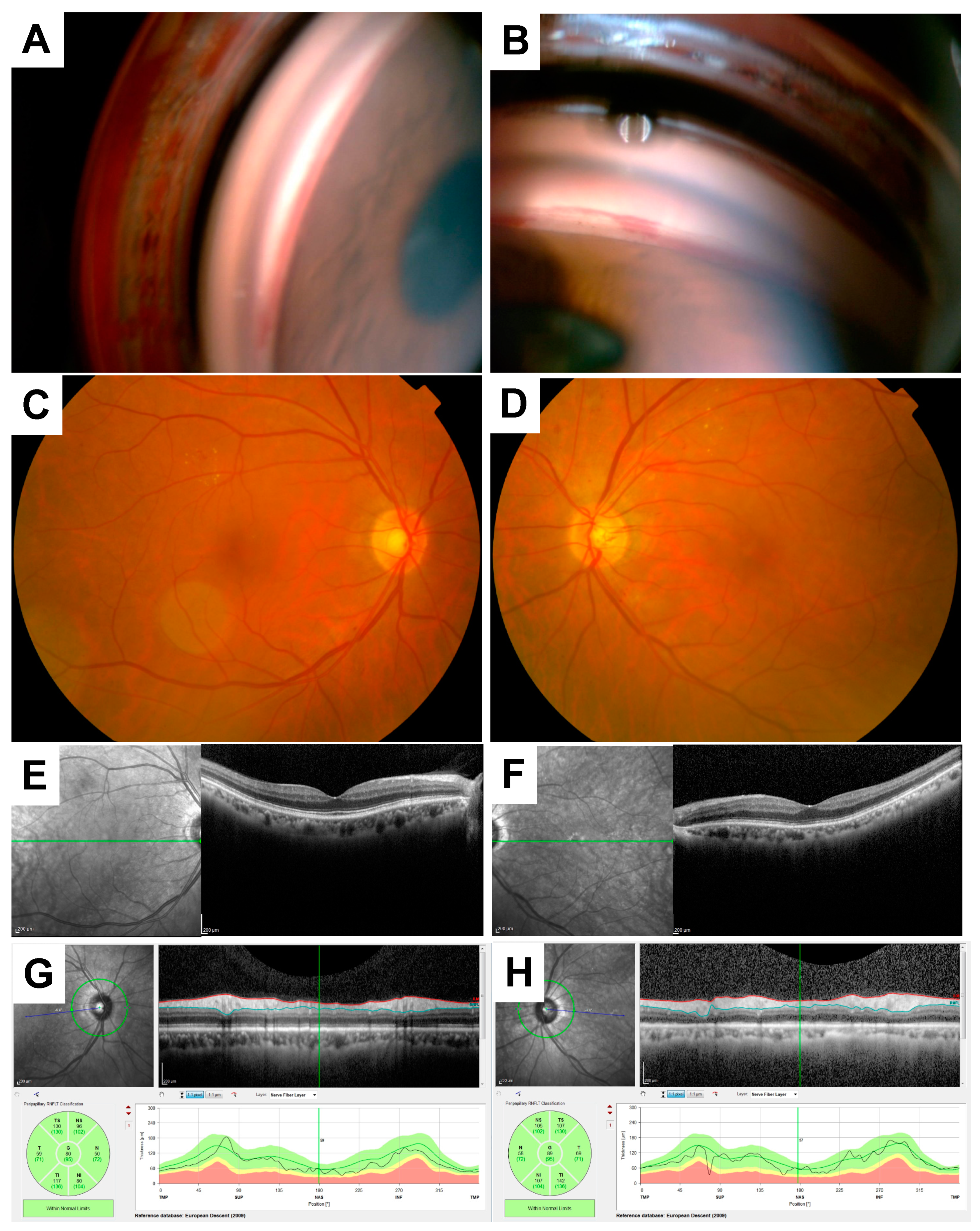
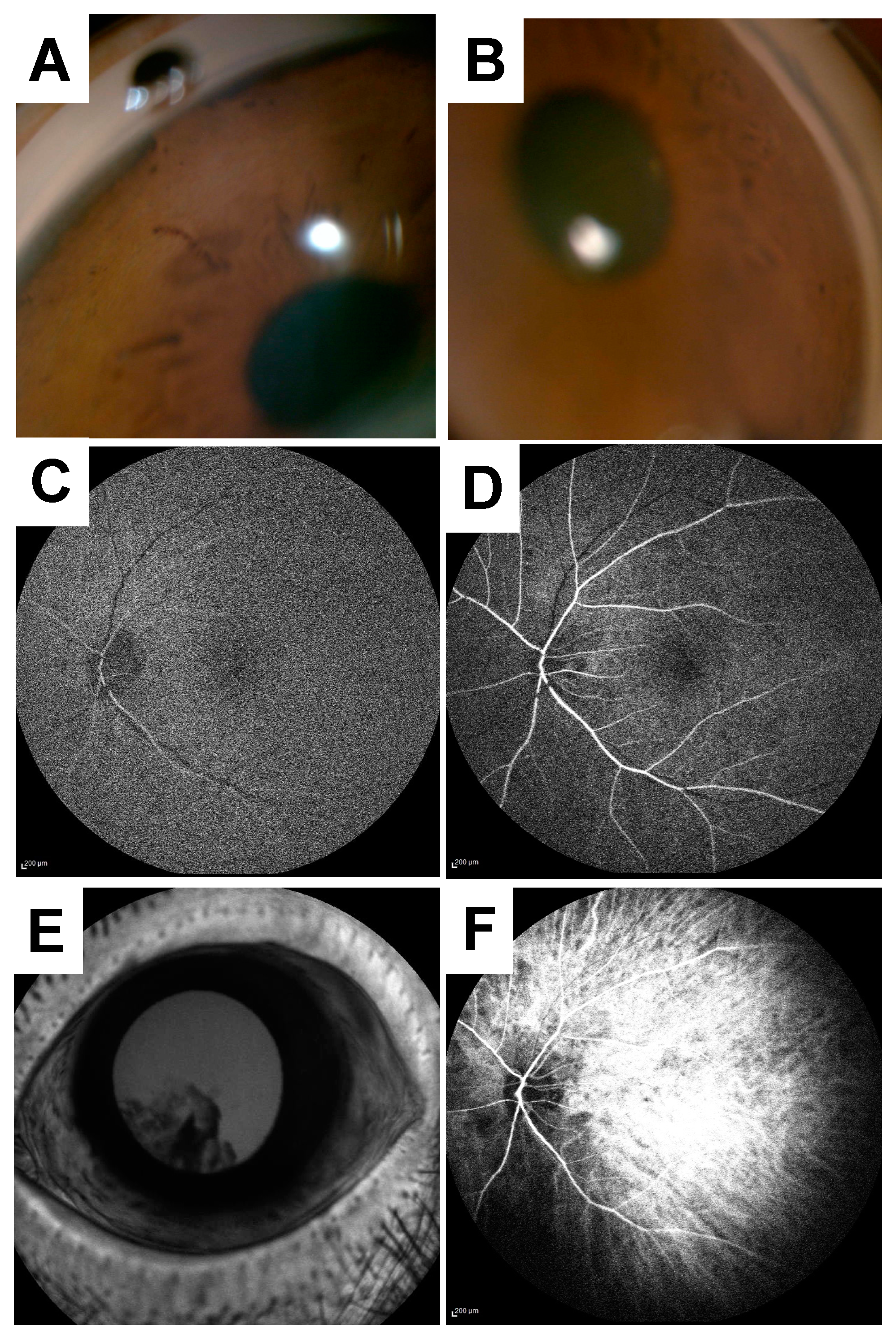
2. Case Presentation
3. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Mendrinos, E.; Machinis, T.G.; Pournaras, C.J. Ocular ischemic syndrome. Surv. Ophthalmol. 2010, 55, 2–34. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S. Blood supply of the optic nerve head and its role in optic atrophy, glaucoma, and oedema of the optic disc. Br. J. Ophthalmol. 1969, 53, 721–748. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Hayashi, M.; Yagi, F.; Sato, K.; Tomita, G.; Iwabuchi, S. Relationship between the Direction of Ophthalmic Artery Blood Flow and Ocular Microcirculation before and after Carotid Artery Stenting. J. Ophthalmol. 2016, 2016, 2530914. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Magargal, L.E. The ocular ischemic syndrome. Clinical, fluorescein angiographic and carotid angiographic features. Int. Ophthalmol. 1988, 11, 239–251. [Google Scholar] [CrossRef]
- Kim, Y.H.; Sung, M.S.; Park, S.W. Clinical Features of Ocular Ischemic Syndrome and Risk Factors for Neovascular Glaucoma. Korean J. Ophthalmol. 2017, 31, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S.; Zimmerman, M.B. Ocular arterial occlusive disorders and carotid artery disease. Ophthalmol. Retin. 2017, 1, 12–18. [Google Scholar] [CrossRef]
- Utsugi, N.; Takahashi, K.; Kishi, S. Choroidal vascular occlusion in internal carotid artery obstruction. Retina 2004, 24, 915–919. [Google Scholar] [CrossRef]
- Obana, A.; Miki, T.; Hayashi, K.; Takeda, M.; Kawamura, A.; Mutoh, T.; Harino, S.; Fukushima, I.; Komatsu, H.; Takaku, Y.; et al. Survey of complications of indocyanine green angiography in Japan. Am. J. Ophthalmol. 1994, 118, 749–753. [Google Scholar] [CrossRef]
- Yannuzzi, L.A.; Rohrer, K.T.; Tindel, L.J.; Sobel, R.S.; Costanza, M.A.; Shields, W.; Zang, E. Fluorescein angiography complication survey. Ophthalmology 1986, 93, 611–617. [Google Scholar] [CrossRef]
- Aizawa, N.; Nitta, F.; Kunikata, H.; Sugiyama, T.; Ikeda, T.; Araie, M.; Nakazawa, T. Laser speckle and hydrogen gas clearance measurements of optic nerve circulation in albino and pigmented rabbits with or without optic disc atrophy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7991–7996. [Google Scholar] [CrossRef]
- Hashimoto, R.; Sugiyama, T.; Ubuka, M.; Maeno, T. Autoregulation of Optic Nerve Head Blood Flow Induced by Elevated Intraocular Pressure during Vitreous Surgery. Curr. Eye Res. 2016, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Suzuma, K.; Fukazawa, Y.; Yamada, Y.; Tsuiki, E.; Fujikawa, A.; Kitaoka, T. Retinal blood flow levels measured by laser speckle flowgraphy in patients who received intravitreal bevacizumab injection for macular edema secondary to central retinal vein occlusion. Retin. Cases Brief. Rep. 2014, 8, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Iwase, T.; Ra, E.; Yamamoto, K.; Kaneko, H.; Ito, Y.; Terasaki, H. Differences of Retinal Blood Flow Between Arteries and Veins Determined by Laser Speckle Flowgraphy in Healthy Subjects. Medicine 2015, 94, e1256. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, R.; Hirota, A.; Maeno, T. Choroidal blood flow impairment demonstrated using laser speckle flowgraphy in a case of commotio retinae. Am. J. Ophthalmol. Case Rep. 2016, 4, 30–34. [Google Scholar] [CrossRef]
- Saito, W.; Hashimoto, Y.; Hirooka, K.; Ishida, S. Changes in choroidal blood flow velocity in patients diagnosed with central serous chorioretinopathy during follow-up for pachychoroid pigment epitheliopathy. Am. J. Ophthalmol. Case Rep. 2020, 18, 100651. [Google Scholar] [CrossRef]
- Sugiyama, T. Basic Technology and Clinical Applications of the Updated Model of Laser Speckle Flowgraphy to Ocular Diseases. Photonics 2014, 1, 220–234. [Google Scholar] [CrossRef]
- Shinohara, Y.; Kashima, T.; Akiyama, H.; Shimoda, Y.; Li, D.; Kishi, S. Evaluation of Fundus Blood Flow in Normal Individuals and Patients with Internal Carotid Artery Obstruction Using Laser Speckle Flowgraphy. PLoS ONE 2017, 12, e0169596. [Google Scholar] [CrossRef]
- Costa, V.P.; Harris, A.; Anderson, D.; Stodtmeister, R.; Cremasco, F.; Kergoat, H.; Lovasik, J.; Stalmans, I.; Zeitz, O.; Lanzl, I.; et al. Ocular perfusion pressure in glaucoma. Acta Ophthalmol. 2014, 92, e252–e266. [Google Scholar] [CrossRef]
- Hashimoto, R.; Sugiyama, T.; Masahara, H.; Sakamoto, M.; Ubuka, M.; Maeno, T. Impaired Autoregulation of Blood Flow at the Optic Nerve Head during Vitrectomy in Patients with Type 2 Diabetes. Am. J. Ophthalmol. 2017, 181, 125–133. [Google Scholar] [CrossRef]
- Hashimoto, R.; Sugiyama, T.; Ubuka, M.; Maeno, T. Impairment of autoregulation of optic nerve head blood flow during vitreous surgery in patients with hypertension and hyperlipidemia. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 2227–2235. [Google Scholar] [CrossRef]
- Akahori, T.; Iwase, T.; Yamamoto, K.; Ra, E.; Terasaki, H. Changes in Choroidal Blood Flow and Morphology in Response to Increase in Intraocular Pressure. Invest. Ophthalmol. Vis. Sci. 2017, 58, 5076–5085. [Google Scholar] [CrossRef]
- Riva, C.E.; Titze, P.; Hero, M.; Petrig, B.L. Effect of acute decreases of perfusion pressure on choroidal blood flow in humans. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1752–1760. [Google Scholar]
- Kiyota, N.; Shiga, Y.; Ichinohasama, K.; Yasuda, M.; Aizawa, N.; Omodaka, K.; Honda, N.; Kunikata, H.; Nakazawa, T. The Impact of Intraocular Pressure Elevation on Optic Nerve Head and Choroidal Blood Flow. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3488–3496. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.J.; Shin, J.Y.; Yu, H.G. Oral Administration of Cilostazol Increases Ocular Blood Flow in Patients with Diabetic Retinopathy. Korean J. Ophthalmol. 2017, 31, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Higashide, T.; Nagata, A.; Sugiyama, K. Effects of ripasudil, a rho kinase inhibitor, on blood flow in the optic nerve head of normal rats. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Kohmoto, R.; Sugiyama, T.; Kojima, S.; Ueki, M.; Ikeda, T. Optic Nerve Blood Flow Change Induced Ripasudil Added to Prostaglandin Analogues in Primary Open Angle Glaucoma. EC Ophthalmol. 2017, 4, 640–647. [Google Scholar]
- Perrott, R.L.; North, R.V.; Drasdo, N.; Ahmed, K.A.; Owens, D.R. The influence of plasma glucose upon pulsatile ocular blood flow in subjects with type II diabetes mellitus. Diabetologia 2001, 44, 700–705. [Google Scholar] [CrossRef]
- Mees, B.; Wagner, S.; Ninci, E.; Tribulova, S.; Martin, S.; van Haperen, R.; Kostin, S.; Heil, M.; de Crom, R.; Schaper, W. Endothelial nitric oxide synthase activity is essential for vasodilation during blood flow recovery but not for arteriogenesis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1926–1933. [Google Scholar] [CrossRef]
- Yang, J.; Ruan, F.; Zheng, Z. Ripasudil Attenuates Lipopolysaccharide (LPS)-Mediated Apoptosis and Inflammation in Pulmonary Microvascular Endothelial Cells via ROCK2/eNOS Signaling. Med. Sci. Monit. 2018, 24, 3212–3219. [Google Scholar] [CrossRef]
- Hamidi Shishavan, M.; Henning, R.H.; van Buiten, A.; Goris, M.; Deelman, L.E.; Buikema, H. Metformin Improves Endothelial Function and Reduces Blood Pressure in Diabetic Spontaneously Hypertensive Rats Independent from Glycemia Control: Comparison to Vildagliptin. Sci. Rep. 2017, 7, 10975. [Google Scholar] [CrossRef]
- Nagasato, D.; Mitamura, Y.; Semba, K.; Akaiwa, K.; Nagasawa, T.; Yoshizumi, Y.; Tabuchi, H.; Kiuchi, Y. Correlation between optic nerve head circulation and visual function before and after anti-VEGF therapy for central retinal vein occlusion: Prospective, interventional case series. BMC Ophthalmol. 2016, 16, 36. [Google Scholar] [CrossRef] [PubMed]

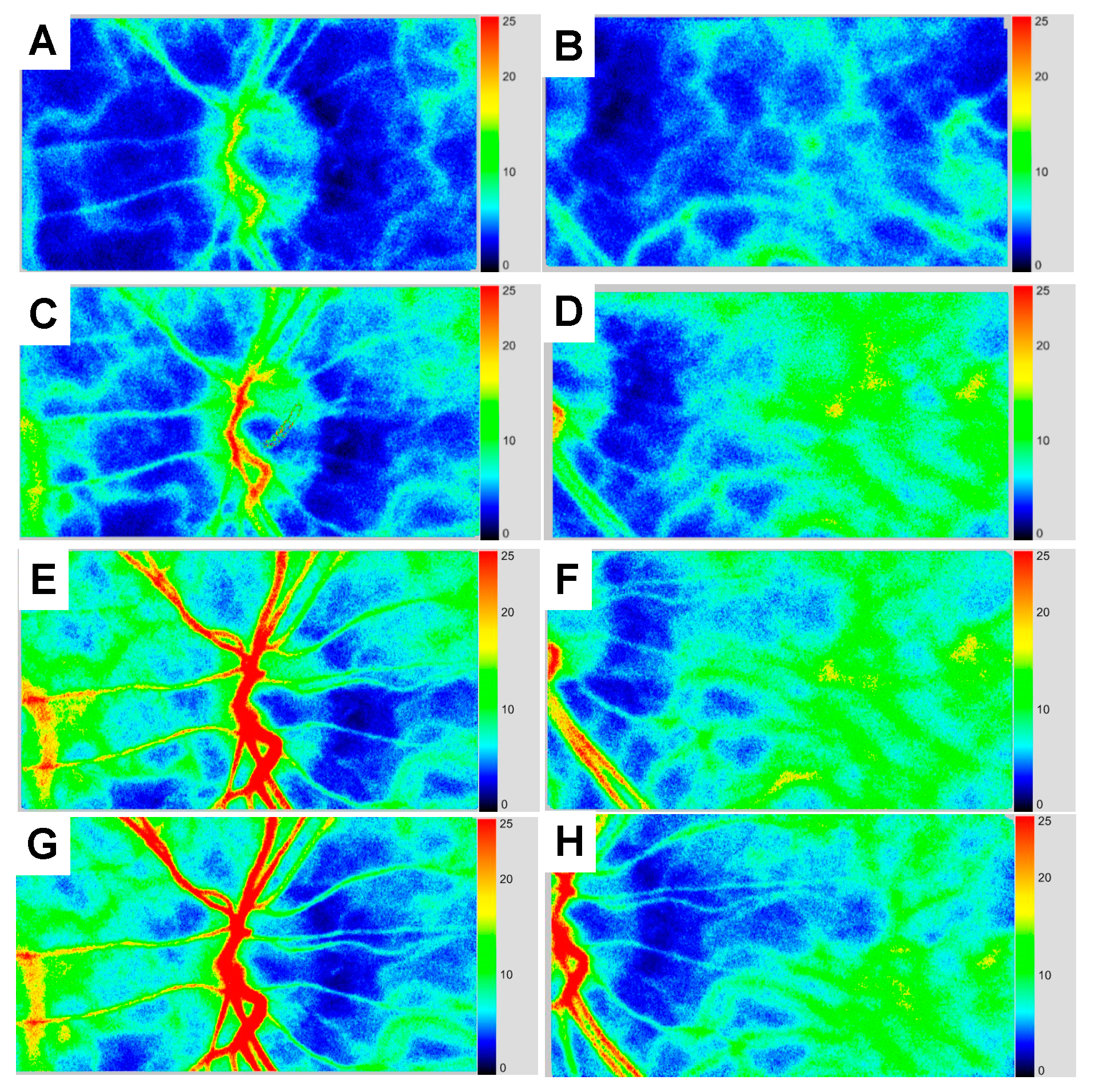

| MBR in the ONH Vessel (AU) | MBR in the ONH Tissue (AU) | MBR in the Choroid (AU) | BOS in the Entire ONH (AU) | BOS in the Choroid (AU) | |
|---|---|---|---|---|---|
| Baseline | 15.0 | 7.5 | 6.7 | 47.1 | 47.2 |
| 4 days | 15.4 | 7.7 | 5.1 | 58.2 | 49.0 |
| 2 weeks | 19.5 | 8.4 | 8.7 | 69.2 | 61.0 |
| 3 months | 20.4 | 8.5 | 9.0 | 74.3 | 65.4 |
| 5 months | 22.2 | 8.4 | 8.7 | 67.0 | 64.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamazaki, R.; Hashimoto, R.; Masahara, H.; Sakamoto, M.; Maeno, T. Time Course in Ocular Blood Flow and Pulse Waveform in a Case of Ocular Ischemic Syndrome with Intraocular Pressure Fluctuation. Vision 2020, 4, 31. https://doi.org/10.3390/vision4020031
Yamazaki R, Hashimoto R, Masahara H, Sakamoto M, Maeno T. Time Course in Ocular Blood Flow and Pulse Waveform in a Case of Ocular Ischemic Syndrome with Intraocular Pressure Fluctuation. Vision. 2020; 4(2):31. https://doi.org/10.3390/vision4020031
Chicago/Turabian StyleYamazaki, Ryo, Ryuya Hashimoto, Hidetaka Masahara, Masashi Sakamoto, and Takatoshi Maeno. 2020. "Time Course in Ocular Blood Flow and Pulse Waveform in a Case of Ocular Ischemic Syndrome with Intraocular Pressure Fluctuation" Vision 4, no. 2: 31. https://doi.org/10.3390/vision4020031
APA StyleYamazaki, R., Hashimoto, R., Masahara, H., Sakamoto, M., & Maeno, T. (2020). Time Course in Ocular Blood Flow and Pulse Waveform in a Case of Ocular Ischemic Syndrome with Intraocular Pressure Fluctuation. Vision, 4(2), 31. https://doi.org/10.3390/vision4020031





